
Development of a novel combined nomogram model integrating Rad-score, age and ECOG to predict the survival of patients with hepatocellular carcinoma treated by transcatheter arterial chemoembolization
Introduction
Liver cancer is a major human health challenge worldwide. Related reports estimate that liver cancer will affect more than 1 million people per year by 2025 (1). Primary liver carcinoma, most of which is hepatocellular carcinoma (HCC), is now the second leading cause of cancer death worldwide (2). Half of the annual number of new cases of HCC are in China, which is associated with the high rate of hepatitis B virus infection (3,4). According to the Barcelona Clinic Liver Cancer (BCLC) staging system for unresectable HCC, especially for predominantly intermediate-advanced HCC, transcatheter arterial chemoembolization (TACE) has become a routine and standard treatment option (1,5). However, due to disease progression and a high recurrence rate, the prognosis of patients after TACE is not promising, with limited OS of 11–20 months (6). HCC is temporally and spatially heterogeneous, and several factors impact the prognosis of patients. Currently, studies have shown that tumor size, tumor number, pathological grading, staging classification, microvascular invasion (MVI), and various biomarkers correlate with patient prognostic outcomes (7-10). However, this traditional prognostic model, due to its invasive examination, existing comorbidities, and geographical differences in staging classification make its clinical application limited, with the decreased granularity in predicting outcomes, lower accuracy, specificity, and sensitivity, and cannot obtain information on tumor heterogeneity and provide patients with accurate prognostic information to the extent that it affects the clinical decision-making treatment process of patients, resulting in poor prognosis and reduced OS (8,11-13). In addition, Traditional imaging features, although capable of observing disease-related progression, are poorly effective in predicting prognosis. In recent years, radiomics, as an emerging new non-invasive technology, has solved the problem of difficult quantitative assessment due to tumor heterogeneity. Radiomics extracts a large number of high-throughput features from traditional images to describe major diseases such as tumors, promoting comprehensive exploration within tumors, and predicting tumor pathological characteristics, treatment effects, and survival quality, thereby improving the accuracy of diagnosis and prognosis prediction (14-16). Therefore, preoperative assessment of patient response to TACE and clarification of its therapeutic effects are important and can help to provide individualized follow-up treatment strategies, thus improving the OS rate of HCC patients. Clinical oncologists have always aimed to provide individualized treatment strategies and prognostic prediction for their patients (17). Nomograms transform complex regression equations into simple and visual graphs, making the results of prediction models more readable and personalized to calculate the survival rate of tumor patients, which has greater value. This advantage has led to more attention and application of nomograms in medical research and clinical practice (18). Based on this consideration, we report the prediction of survival after TACE in HCC patients based on a preoperative computed tomography (CT) image-based radiomics nomogram. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-548/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (No. KY2021002). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Patients
We selected 70 consecutive patients (59 females and 11 males) with a first clinical diagnosis of HCC who underwent TACE between January 2013 and July 2019 at our institution. The inclusion criteria were as follows: (I) HCC confirmed according to the European Association for the Study of the Liver criteria or histopathological testing; (II) underwent TACE with postoperative follow-up of at least 3 months; (III) postoperative CT-enhanced scan; (IV) ECOG score ≤1; and (V) Child-Pugh grade A or B. The exclusion criteria were as follows: (I) extrahepatic or lymph node metastasis; (II) received any other treatment, such as hepatectomy or liver transplantation; (III) Child-Pugh grade C; (IV) diagnosed with other malignant tumors; (V) incomplete clinical or follow-up data; and (VI) patients with missing CT imaging data or poor image quality, which were not conducive to radiomics analysis. The follow-up endpoint was defined according to the guidelines of the American Association for the Study of Liver Diseases (19). The primary endpoint was OS, defined as the time from the first TACE procedure to death. Patients were routinely followed at 4–6 weeks after surgery and then every 3–6 months thereafter. A total of 45 patients had endpoint events after TACE.
Patient clinical baseline information
Pre-TACE clinical baseline information was collected, which included age, sex, and ECOG score.
CT image acquisition
CT is currently the most widely used imaging modality in radiomics research, with high density resolution imaging characteristics (20). Scanning was performed using a Philips Brilliance iCT machine, and all patients were scanned before the TACE procedure.
Region of interest mapping and feature extraction
All images were derived from a picture archiving and communication system in Digital Imaging and Communications in Medicine (DICOM) format and transferred to 3D Slicer (version 4.11.20210226). Two physicians with ≥10 years clinical experience in radiology manually drew the regions of interest (ROIs) on CT images using 3D Slicer software and feature extraction was performed on the outlined ROIs. A total of 851 features were extracted.
Data pre-processing and normalization
Due to the different instrument settings and acquisition parameters, the images needed to be pre-processed before feature extraction, and all image radiomics features needed to be normalized.
Feature selection and radiomics signature building
We reduced the dimensionality of the extracted radiomics features by the LASSO-COX regression model, optimized the penalty parameters by 10-fold cross validation, and selected the lambda min with the smallest error; the simplest model within a range of variance, to achieve the dimensionality reduction of the features, and building the radiomics signature. The most useful features with non-zero coefficients were filtered and multiplied with their coefficients, then summed. Finally, a constant term was added, and the result was the radiomics (Rad)-score for each patient.
Statistics analysis
The statistical analysis was performed with GraphPad Prism version 8.3.0 and R version 3.5.1 (http://www.R-project.org). Clinical baseline information, including sex, age, and ECOG score, were included in the univariate analysis first using the chi-square test, and then clinical factors with P<0.05 were included in the multivariate Cox regression analysis. Clinical factors with P<0.05 in the multivariate analysis were included in the clinical modeling. Radiomics score (Rad-score) were compared with two independent samples t-tests using Fisher’s exact probability method for categorical variables. The Rad-score was added to the clinical model to create a radiomics nomogram, and from this, a combination model was created. The Kaplan-Meier method was used to describe the survival curves of patients after TACE and were compared using the log-rank test. Predictive performance was assessed by ROC curves for each model. The area under the ROC curve (AUC) and its 95% CI were obtained, as well as accuracy, sensitivity, and specificity. C-index (including calculated its 95% CI) and calibration curves were plotted to assess the predictive accuracy of the nomogram. All statistical tests used in this study were two-sided, and differences were considered statistically significant at P<0.05.
Results
Analysis of the patient clinical baseline data
We retrospectively analyzed 70 patients who met the inclusion criteria. Three clinical characteristics were included in this study, namely age, sex, and ECOG score. The univariate analysis of patients who reached endpoint events and those who did not is shown in Table 1. P values for age and ECOG score were <0.05, but sex did not differ significantly.
Table 1
| Variable | Estimate | SE | z | Wald | P | HR (95% CI) |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | – | – | – | – | – | – |
| Female | 0.547 | 0.374 | 1.463 | 2.14 | 0.143 | 1.728 (0.830–3.598) |
| Age, years | 0.039 | 0.014 | 2.707 | 7.326 | 0.007** | 1.040 (1.011–1.070) |
| <50 | – | – | – | – | – | – |
| 50–64 | 0.745 | 0.383 | 1.947 | 3.791 | 0.052 | 2.106 (0.995–4.459) |
| ≥65 | 1.029 | 0.422 | 2.435 | 5.93 | 0.015* | 2.798 (1.222–6.403) |
| ECOG | ||||||
| 0 | – | – | – | – | – | – |
| 1 | 0.936 | 0.321 | 2.919 | 8.522 | 0.004** | 2.551 (1.360–4.783) |
*P<0.05, **P<0.01. TACE, Transcatheter Arterial Chemoembolization; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
Construction and validation of the radiomics signature
A total of 851 radiomics features were extracted from the ROIs. They were included in the LASSO Cox regression model to select the most significant features for survival prediction. The coefficients of the radiomics features at different lambda values are shown in Figure 1A. The data were cross-validated 10-fold, and the results are shown in Figure 1B. At lambda =0.191, the error of the model was minimized, and the number of features with non-zero coefficients was 3 (Table 2).

Table 2
| Selected radiomics feature | Coefficient |
|---|---|
| Feature 1: wavelet.HLL.ngtdm.Contrast | 1.683 |
| Feature 2: wavelet.HLL.glrlm.ShortRunLowGrayLevelEmphasis | 2.538 |
| Feature 3: wavelet.HLL.gldm.SmallDependenceLowGrayLevelEmphasis | −0.158 |
The radiomics signatures were calculated, and the formula was Rad_score = 1.68316825496*Feature1 + 2.5374949*Feature2 − 0.1579961*Feature3 (Figure 1C).
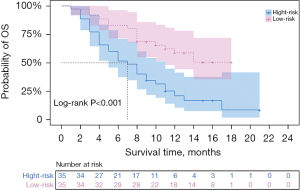
Rad-score was also an independent risk factor for OS after TACE in HCC patients (HR =2.178; 95% CI: 1.762–4.193; P<0.001) as determined by univariate and multivariate Cox analysis, with an AUC of 0.772 (95% CI: 0.661–0.882). We performed a validation of the predictive effect of the Rad-score. Patients were divided into a low risk group (cut-off value ≤0.68) and high risk group (cut-off value >0.68) according to the cut-off value of the Rad-score. Kaplan-Meier survival analysis was used to explore the correlation between Rad-score and OS, and the log-rank test was applied to compare the survival curves between the high and low risk groups if there was a significant difference. Survival times were 7 months for the high-risk group and 12 months for the low-risk group (P<0.001) (Table 3). Kaplan-Meier survival analysis showed that patients in the high-risk group had significantly lower OS than those in the low-risk group (P<0.001) (Figure 2).
Table 3
| Total | High risk | Low risk | P value | |
|---|---|---|---|---|
| Rad-score | 9.00 (5.00–13.75) | 7.00 (4.00–11.00) | 12.00 (8.00–15.00) | <0.001 |
OS, overall survival; Rad-score, radiomics score.
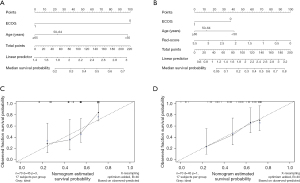
Development of the predictive nomogram
Nomograms are commonly used to estimate prognosis in oncology and medicine and give a pictorial representation of a complex mathematical formula (21). According to the univariate analysis, age and ECOG score were relevant risk factors for OS. They were included in the multivariate Cox regression analysis, and the results showed that both age (age ≥65 years) (HR =2.411, 95% CI: 1.039–5.595, P<0.05) and ECOG score (ECOG score =1) (HR =2.167, 95% CI: 1.135–4.139, P<0.05) were independent risk predictors for OS (Table 4). The radiomics nomogram was constructed by the 3 independent risk predictors described above to predict patient survival, while the models that incorporated age and ECOG score were developed and presented as the clinical nomogram (Figure 3). The nomogram enabled individualized prediction of patients with advanced HCC after TACE, and the higher the calculated total score, the higher the probability of survival after surgery, and the higher the OS.
Table 4
| Variable | Estimate | SE | z | Wald | P | HR (95% CI) |
|---|---|---|---|---|---|---|
| ECOG | ||||||
| 0 | – | – | – | – | – | – |
| 1 | 0.773 | 0.33 | 2.342 | 5.486 | 0.019* | 2.167 (1.135–4.139) |
| Age, years | ||||||
| <50 | – | – | – | – | – | – |
| ≥65 | 0.88 | 0.43 | 2.048 | 4.196 | 0.041* | 2.411 (1.039–5.595) |
| 50–64 | 0.556 | 0.394 | 1.41 | 1.989 | 0.158 | 1.744 (0.805–3.778) |
*P<0.05. HCC, hepatocellular carcinoma; OS, overall survival; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
Performance of the radiomics nomogram
The AUCs of the ROC curves for the clinical nomogram, Rad-score, and radiomics nomogram were 0.747 (95% CI: 0.627–0.866), 0.772 (95% CI: 0.661–0.882), and 0.801 (95% CI: 0.693–0.909), respectively, which indicated that the ROC curves constructed by the radiomics nomogram had better predictive performance. Combined clinical factors and Rad-score together had better predictive performance, and the introduction of Rad-score into the clinical model increased its predictive performance. The sensitivity of the clinical nomogram, Rad-score, and radiomics nomogram was 0.867, 0.644 and 0.822, respectively, and the specificity of the clinical nomogram, Rad-score, and radiomics nomogram was 0.520, 0.800 and 0.720, respectively (Figure 4 and Table 5). The C-indexes of the clinical nomogram, Rad-score, and radiomics nomogram plot were 0.669, 0.679, and 0.700, respectively (Table 6). The calibration curves of the clinical and radiomics nomograms are shown in Figure 3C,3D.
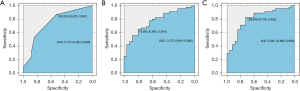
Table 5
| Clinical | Rad-score | Radiomics nomogram | |
|---|---|---|---|
| AUC (95% CI) | 0.747 (0.627−0.866) | 0.772 (0.661−0.882) | 0.801 (0.693−0.909) |
| Sensitivity (95% CI) | 0.867 (0.725−0.945) | 0.644 (0.487−0.777) | 0.822 (0.674−0.915) |
| Specificity (95% CI) | 0.520 (0.318−0.717) | 0.800 (0.587−0.924) | 0.720 (0.674−0.915) |
Rad-score, radiomics score; AUC, area under the curve.
Table 6
| Model | C-index |
|---|---|
| Clinical | 0.669 |
| Rad-score | 0.679 |
| Radiomics nomogram | 0.700 |
Rad-score, radiomics score.
Discussion
TACE therapy has become a routine treatment modality for patients with unresectable and intermediate to advanced HCC (1,5,22), where hepatic artery embolization leads to tumor ischemia and subsequent necrosis, inhibiting tumor development (23,24). However, predicting the prognosis of patients is important and has implications for clinical decision-making due to individual differences and the temporal and spatial heterogeneity of HCC (25-27), the high recurrence rate of local tumors, low OS after TACE, and variations in prognosis (28-31).
In recent years, computer-aided technology has been widely used in the treatment and prognostic prediction of diseases in hospital (32,33). Radiomics, as a non-invasive computer-aided technology, was first proposed by Lambin in 2012 (34), which can mine high-throughput features from images and perform quantitative analysis of the features to provide comprehensive information about the interior of tumors that cannot be observed by the naked eyes. This helps physicians to develop individualized treatment strategies and advance toward precision medicine (35-37). Nomograms containing multiple risk factors have been used to predict the diagnostic and prognostic aspects of tumors. In recent years, Huang et al. developed a radiomics index into a nomogram along with clinical risk factors that performed better in predicting disease-free survival in early-stage non-small cell lung cancer (38). Li et al. developed a radiomics signature for the pretreatment prediction of OS and time to progression for patients with advanced HCC treated with lapatinib plus TACE (39). Tang et al. established comprehensive radiomics signatures for predicting survival in patients with combined HCC and cholangiocarcinoma (40). In this study, we developed a radiomics nomogram that accommodated preclinical baseline information (age and ECOG score) and radiomics scores to predict the OS of patients with advanced HCC after TACE. The radiomics signatures consisted of 3 significant radiomics features screened by LASSO regression, and the Rad-score was calculated. We classified patients into low and high-risk groups according to the Rad-score cut-off, and a significant difference was found in the prediction of median survival time between the 2 groups. When our radiomics nomogram was combined with clinical risk factors, it was significantly more effective in predicting OS after TACE in HCC patients, with an AUC of 0.801, indicating the incremental value of the radiomics nomogram in predicting OS in these patients.
There were some limitations to our study. First, due to the small patient sample size and limited follow-up information, no validation of the model using a separate cohort was performed, and the performance assessment of the model is subject to some bias, which is the focus of our further study later stages. Second, selection bias was unavoidable because this was a single-center retrospective study. Third, our sample size was small, and more samples are needed to optimize our model.
In conclusion, radiomics provides a new method to extract important tumor features from clinical images in a non-invasive and more accurate manner. This provides information on the patients’ disease and may aid in developing personalized and precise treatment strategies.
Acknowledgments
Funding: This work was supported by the Luzhou Municipal People’s Government-Southwest Medical University Science and Technology Strategic Cooperation Fund (No. 2020LZXNYDJ12); Sichuan Provincial Medical Research Youth Innovation Project Program (No. Q17080); Sichuan Provincial Medical Research Project Program (No. S21004).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-548/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-548/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-548/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Affiliated Hospital of Southwest Medical University (No. KY2021002). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Wallace MC, Preen D, Jeffrey GP, et al. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol 2015;9:765-79. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol 2019;70:893-903. [Crossref] [PubMed]
- Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol 2015;62:1187-95. [Crossref] [PubMed]
- Piñero F, Dirchwolf M, Pessôa MG. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells 2020;9:1370. [Crossref] [PubMed]
- Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol 2019;26:1474-93. [Crossref] [PubMed]
- Casadei-Gardini A, Orsi G, Caputo F, et al. Developments in predictive biomarkers for hepatocellular carcinoma therapy. Expert Rev Anticancer Ther 2020;20:63-74. [Crossref] [PubMed]
- Marrero JA, Kudo M, Bronowicki JP. The challenge of prognosis and staging for hepatocellular carcinoma. Oncologist 2010;15:23-33. [Crossref] [PubMed]
- Zang Y, Long P, Wang M, et al. Development and validation of prognostic nomograms in patients with hepatocellular carcinoma: a population-based study. Future Oncol 2021;17:5053-66. [Crossref] [PubMed]
- Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329-38. [Crossref] [PubMed]
- Tsilimigras DI, Pawlik TM. Prognostication in hepatocellular carcinoma: is it a burden or a ticket? Br J Surg 2021;108:337-9. [Crossref] [PubMed]
- Avanzo M, Wei L, Stancanello J, et al. Machine and deep learning methods for radiomics. Med Phys 2020;47:e185-202. [Crossref] [PubMed]
- Park HJ, Park B, Lee SS. Radiomics and Deep Learning: Hepatic Applications. Korean J Radiol 2020;21:387-401. [Crossref] [PubMed]
- Cannella R, Sartoris R, Grégory J, et al. Quantitative magnetic resonance imaging for focal liver lesions: bridging the gap between research and clinical practice. Br J Radiol 2021;94:20210220. [Crossref] [PubMed]
- Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 2008;26:1364-70. [Crossref] [PubMed]
- Park SY. Nomogram: An analogue tool to deliver digital knowledge. J Thorac Cardiovasc Surg 2018;155:1793. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Peng Q. Prediction of survival benefit in patients with hepatocellular carcinoma after transcatheter arterial chemoembolization based on CT radiomics signatures. Guangzhou: Jinan University, 2019.
- Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol 2015;16:e173-80. [Crossref] [PubMed]
- Chang Y, Jeong SW, Young Jang J, et al. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci 2020;21:8165. [Crossref] [PubMed]
- Kong C, Zhao Z, Chen W, et al. Prediction of tumor response via a pretreatment MRI radiomics-based nomogram in HCC treated with TACE. Eur Radiol 2021;31:7500-11. [Crossref] [PubMed]
- Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 2016;64:1090-8. [Crossref] [PubMed]
- Furuta M, Ueno M, Fujimoto A, et al. Whole genome sequencing discriminates hepatocellular carcinoma with intrahepatic metastasis from multi-centric tumors. J Hepatol 2017;66:363-73. [Crossref] [PubMed]
- Xue R, Li R, Guo H, et al. Variable Intra-Tumor Genomic Heterogeneity of Multiple Lesions in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:998-1008. [Crossref] [PubMed]
- Huang A, Zhao X, Yang XR, et al. Circumventing intratumoral heterogeneity to identify potential therapeutic targets in hepatocellular carcinoma. J Hepatol 2017;67:293-301. [Crossref] [PubMed]
- Lewis S, Hectors S, Taouli B. Radiomics of hepatocellular carcinoma. Abdom Radiol (NY) 2021;46:111-23. [Crossref] [PubMed]
- Fransvea E, Paradiso A, Antonaci S, et al. HCC heterogeneity: molecular pathogenesis and clinical implications. Cell Oncol 2009;31:227-33. [PubMed]
- Friemel J, Rechsteiner M, Frick L, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res 2015;21:1951-61. [Crossref] [PubMed]
- Tsurusaki M, Murakami T. Surgical and Locoregional Therapy of HCC: TACE. Liver Cancer 2015;4:165-75. [Crossref] [PubMed]
- Hu HT, Wang Z, Huang XW, et al. Ultrasound-based radiomics score: a potential biomarker for the prediction of microvascular invasion in hepatocellular carcinoma. Eur Radiol 2019;29:2890-901. [Crossref] [PubMed]
- Zhou LQ, Wang JY, Yu SY, et al. Artificial intelligence in medical imaging of the liver. World J Gastroenterol 2019;25:672-82. [Crossref] [PubMed]
- Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012;48:441-6. [Crossref] [PubMed]
- Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14:749-62. [Crossref] [PubMed]
- Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016;278:563-77. [Crossref] [PubMed]
- Liu Q, Li J, Liu F, et al. A radiomics nomogram for the prediction of overall survival in patients with hepatocellular carcinoma after hepatectomy. Cancer Imaging 2020;20:82. [Crossref] [PubMed]
- Huang Y, Liu Z, He L, et al. Radiomics Signature: A Potential Biomarker for the Prediction of Disease-Free Survival in Early-Stage (I or II) Non-Small Cell Lung Cancer. Radiology 2016;281:947-57. [Crossref] [PubMed]
- Li L, Kan X, Zhao Y, et al. Radiomics Signature: A potential biomarker for the prediction of survival in Advanced Hepatocellular Carcinoma. Int J Med Sci 2021;18:2276-84. [Crossref] [PubMed]
- Tang YY, Zhao YN, Zhang T, et al. Comprehensive radiomics nomogram for predicting survival of patients with combined hepatocellular carcinoma and cholangiocarcinoma. World J Gastroenterol 2021;27:7173-89. [Crossref] [PubMed]


