
A retrospective study of clinicopathological characteristics and prognostic factors of Krukenberg tumor with gastric origin
Introduction
Gastric cancer (GC) is a common malignant tumor of the digestive system. According to Global Cancer Statistics 2020, the incidence and mortality rate rank fifth and fourth, respectively, among global malignancies, with 43.9% and 48.6% occurring in China (1). Metastasis is the main feature of GC development and the main cause of death (2-4), and female GC patients, especially young women, are prone to ovarian metastasis (5). The German pathologist Friedrich Krukenberg first reported GC with ovarian metastasis in 1896, naming the tumor after himself: Krukenberg tumor (KT) (6). KT is a secondary ovarian tumor which stems from a wide range of sources, such as the stomach, colon and rectum, breast, oesophagus, gallbladder, and appendix, but GC is the most common origin (7,8). Ovarian metastasis occurs in approximately 5–10% with female GC and has also been reported in 33–41% of autopsies (9). KT has mostly been reported in case reports (10-13), with an overall survival (OS) of less than 1 year. As described in case reports and clinical analysis, the main pathological type of KT is signet ring cell carcinoma. Ovarian metastatic surgery in some patients can improve the prognosis. Tumor markers may be related to the prognosis. However, due to the limited samples, the clinicopathological features and prognostic factors of KTs from GC are still uncertain. Young women, in particular, are asymptomatic until the cancer is quite advanced, which seriously affects the quality of life and survival time. The optimal management of the disease is not fully understood (14), and there are no adequate prognostic factors and treatment guidelines for patients diagnosed with KT of gastric origin.
We have established a database of GC covering 130 patients with KTs of GC. The clinicopathological features included age, tumor location, tumor markers, mismatch repair (MMR), human epidermal growth factor receptor 2 (HER-2) status, estrogen receptor (ER)/progesterone receptor (RP), P53 etc. We use univariate and multivariate analysis to explore the prognostic predictors of GC ovarian metastasis in order to improve knowledge of the clinical diagnosis and treatment, this study retrospectively analyzed the clinical and pathological data and investigated the clinicopathological and prognostic features of KTs. We presented the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-464/rc).
Methods
Patient information and data
This was a retrospective study of KTs derived from gastric origin. Data from 130 patients with GC combined with ovarian metastasis from January 2011 to September 2021 were collected from Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine. The diagnosis of GC was confirmed by gastroscopy or pathology of surgical specimen, and KT of gastric origin was confirmed by imaging or pathology. It had been reported that the clinicopathological features of patients, such as demographics, some laboratory indicators, treatment data, and pathological features, were related to the prognosis of patients with GC. Although some risk factors had been reported in the literature, the clinicopathological features of KTs from GC were still largely uncertain. So the clinical data collection of patients in this study included the basic information of patients, such as age, menstrual status, clinical manifestations; characteristics of primary GC and ovarian metastases; treatment-related information, including surgery and chemotherapy regimen; peripheral blood-related indicators such as serum albumin, hemoglobin, tumor markers, etc., which we would introduce in detail afterwards. Univariate and multivariate analysis were performed on clinical factors that may affect ovarian metastasis from GC. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Ren Ji Hospital (No. KY2021-183-B). Individual consent for this retrospective analysis was waived.
Pathology sections were reviewed by a pathologist applying the 2019 WHO classification of tumors of the digestive system (15). Immunohistochemical criteria for HER-2, P53, ER, PR, MLH1, PMS2, MSH2, MSH6 were determined through optical microscopy, and positive cells were counted in five randomly selected fields of view in each section by light microscopy. Positive cells ≤10% were scored as 0, 11–50% were considered as 1, 51–75% as 2, and >75% as 3. The intensity was graded as follows: 0 points (no staining), 1 point (light-brown staining), 2 points (brown staining), and 3 points (dark-brown staining). The final score was obtained by summing the percentage of positive cells and their intensity: 1–2 were classified as “1+”, 3–4 as “2+”, and “5–6” as “3+” (16). The tumor-node-metastasis (TNM) staging of initially diagnosed GC was evaluated in accordance with the TNM classification of cancer, 8th edition, of the American Joint Committee on Cancer (AJCC) (17).
Clinicopathological data included clinical manifestations, laboratory findings, imaging reports, pathology and immunohistochemistry (IHC) reports, and treatment characteristics (Table 1). Ovarian metastasis was defined as synchronous if detected within 6 months of the initial diagnosis of GC and metachronous if detected more than 6 months after the diagnosis. Baseline data were collected before treatment commenced. Patients with infection, cirrhosis, autoimmune disease, hematologic disorders, courses of steroid or aspirin use, or other conditions that could confound coagulation, the neutrophil count, lymphocyte count, or platelet count were excluded. The pretreatment neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) values were calculated according to the following formulas: SII = neutrophil cell count (×109/L) × platelet cell count (×109/L)/lymphocyte count (×109/L).
Table 1
| Characteristics | N (%) |
|---|---|
| Age (years) (n=130) | |
| ≤40 | 53 (40.8) |
| >40 | 77 (59.2) |
| Transfer mode (n=130) | |
| Synchronous | 82 (63.1) |
| Metachronous | 48 (36.9) |
| Interval time (months) (n=130) | |
| ≤6 | 82 (63.1) |
| >6, ≤12 | 9 (6.9) |
| >12 | 39 (30.0) |
| Menstrual status (n=130) | |
| Pre-menopausal | 89 (68.5) |
| Post-menopausal | 41 (31.5) |
| Peritoneal metastasis (n=130) | |
| Yes | 89 (68.5) |
| No | 41 (31.5) |
| First symptoms (n=130) | |
| Digestive symptoms | 81 (62.3) |
| Ovarian metastasis symptoms | 17 (13.1) |
| Both | 12 (9.2) |
| Other | 20 (15.4) |
| MMR detection (n=40) | |
| pMMR | 39 (97.5) |
| dMMR | 1 (2.5) |
| Pathology of gastric (n=130) | |
| Poorly differentiated adenocarcinoma | 60 (46.2) |
| Signet ring cell carcinoma | 30 (23.1) |
| Mixed adenocarcinoma | 38 (29.2) |
| Other | 2 (1.5) |
| HER-2 of gastric (n=111) | |
| Negative | 109 (98.2) |
| Positive | 2 (1.8) |
| Tumor location (n=130) | |
| Cardiac and fundus | 10 (7.7) |
| Gastric body | 74 (56.9) |
| Gastric antrum | 30 (23.1) |
| More than two locations | 16 (12.3) |
| Grade (n=130) | |
| Well differentiated | 1 (0.8) |
| Moderately differentiated | 0 (0.0) |
| Poorly differentiated | 128 (98.5) |
| Unknow | 1 (0.8) |
| ER/PR of gastric (n=11) | |
| 0 | 10 (91.0) |
| 3+ | 1 (9.0) |
| ER/PR of ovarian (n=46) | |
| 0 | 40 (87.0) |
| 1+–3+ | 6 (13.0) |
| P53 testing (n=64) | |
| 0 | 18 (28.1) |
| 1+ | 28 (43.8) |
| 2+ | 8 (12.5) |
| 3+ | 10 (15.6) |
| Ovarian metastases (n=130) | |
| Unilateral | 38 (29.2) |
| Bilateral | 92 (70.8) |
| Pathology of ovarian (n=88) | |
| Poorly differentiated adenocarcinoma | 43 (48.9) |
| Signet ring cell carcinoma | 22 (25.0) |
| Mixed adenocarcinoma | 20 (22.7) |
| Other | 3 (3.4) |
| HER-2 of ovarian (n=43) | |
| Negative | 43 (100.0) |
| Positive | 0 (0.0) |
KTs, Krukenberg tumors; MMR, mismatch repair; pMMR, proficient MMR; dMMR, deficient MMR; HER-2, human epidermal growth factor receptor 2; ER, estrogen receptor; PR, progesterone receptor.
Receiver operating characteristic (ROC) curves were generated to set thresholds for NLR, PLR, and SII, while the categorical variable was 1-year survival (≥12 vs. <12 months). According to the literature, the median OS of patients with stage IV GC is less than 12 months (18), and because only five patients in our study had a follow-up of less than 12 months, the choice of 12 months was feasible. Patients were divided into high and low- groups according to the cut-off values of NLR, PLR, and SII. The normal ranges of these indicators are as follows: carcinoembryonic antigen (CEA; 0–5 µg/L), cancer antigen 125 (CA-125; 0–35 U/mL), CA-199 (0–35 U/mL), CA-724 (0–6.9 U/mL), D-dimer (0–0.5 µg/mL), and fibrinogen (2–4 g/L).
Follow-up
Patients were followed up through outpatient follow-up visits, medical record reviews, and telephone call backs, and the follow-up period was from January 2011 to December 2021. The OS time was defined as the interval between the diagnosis of KT to death or the last follow-up.
Statistical analysis
Statistical analysis was performed using SPSS 26.0 software. Clinical data of GC patients with metastatic ovarian were collected and reviewed retrospectively. Descriptive statistics were used to describe the clinicopathological characteristics. OS was estimated using the Kaplan-Meier method, the log-rank test was used to compare the survival rates between groups. Significant variables in the univariate analysis were entered into multivariate analysis using the Cox regression model. The 95% confidence interval (CI) of the hazard ration (HR) for significant effects were also reported. When the two-tailed P value <0.05 was considered as a statistically significant difference.
Results
Clinicopathological characteristics of KT
The clinicopathologic characteristics of KT are listed in Table 1, and representative imaging data are shown in Figure 1. Of all patients, 89 (68.5%) were premenopausal, 90 (69.2%) had GC metastasis at the time of initial diagnosis, and 82 patients (63.1%) had synchronous ovarian metastases. The most common initial clinical symptoms were digestive symptoms (62.3%, n=81), followed by ovarian metastasis symptoms (13.1%, n=17), and 9.2% (n=12) of patients had both types of symptoms. The median age at KT diagnosis was 41 (range, 23–73) years, with a mean age of 45.59 years. The mean interval between ovarian metastasis and primary GC was 12 (range, 0–132) months, while 56.9% of patients had gastric tumors located in the gastric body and 70.8% (n=92) had bilateral ovarian metastases. The most predominant site of extraovarian metastasis was the peritoneum (68.5%, n=89).
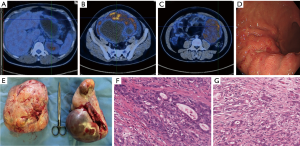
Histopathological findings showed that primary GC was predominantly poorly differentiated (98.5%, 128/130), of which 23.1% were signet ring cell carcinoma, 46.2% were poorly differentiated adenocarcinoma, and 29.2% were mixed adenocarcinoma (poorly differentiated adenocarcinoma with signet ring cell carcinoma). Among the pathological types of ovarian metastases, 25.0% were signet ring cell carcinoma, 48.9% were poorly differentiated adenocarcinoma, and 22.7% were mixed adenocarcinoma. For IHC, seven patients underwent Epstein-Barr virus (EBV) detection and all were negative. A total of 40 patients underwent MMR, of which only one showed deficient MMR (dMMR) [MLH1(−), PMS2(−), MSH2(+), MSH6(+)] and the rest had proficient MMR (pMMR). HER-2 testing was performed on 111 patients, of which only two were positive by IHC 3+ or 2+ combined with FISH amplification, and the HER-2 positive rate was 1.8%. Forty-three cases of ovarian metastases were negative for HER-2. Eleven patients had ER/RP detection of primary gastric lesions, of which only one was ER/PR positive (IHC, 3+).
Treatment and survival analysis
A total of 67.7% of patients (n=88) underwent resection of the primary tumors, of which 59 underwent radical surgery and 29 underwent palliative surgery. A total of 64.6% of patients (n=84) underwent oophorectomy for metastatic tumors. Overall, 85.4% of patients (n=111) received chemotherapy for ovarian metastases, including patients after resection of metastases and those who did not have surgery. The chemotherapy regimen included fluorouracil, oxaliplatin/cisplatin, and docetaxel for 1 to 10 cycles.
The follow-up duration ranged from 3 to 62 months. A total of 118 patients (90.8%, 118/130) completed follow-up, and the 12 patients lost to follow-up were not included in the survival analysis. Univariate analysis of survival is shown in Table 2. Among all 118 patients, the median OS from the diagnosis of KT was 13.0 (95% CI: 10.59–15.41; range, 1–62.0) months, and there were 94 deaths.
Table 2
| Characteristics | N | OS | P |
|---|---|---|---|
| Baseline characteristics | |||
| Age (n=118) | 0.478 | ||
| >40 years | 70 | 15 | |
| ≤40 years | 48 | 12 | |
| Pathological type (n=116) | 0.425 | ||
| Poorly differentiated adenocarcinoma | 54 | 13 | |
| Signet ring cell carcinoma | 26 | 8 | |
| Mixed adenocarcinoma | 36 | 16 | |
| T stage (n=81) | 0.140 | ||
| T2–3 | 29 | 21 | |
| T4 | 52 | 13 | |
| Nerve invasion (n=74) | 0.154 | ||
| No | 37 | 18 | |
| Yes | 37 | 15 | |
| Size of gastric foci (n=79) | 0.031* | ||
| ≥5 cm | 48 | 12 | |
| <5 cm | 31 | 17 | |
| Ki-67 (n=90) | 0.312 | ||
| ≥50% | 45 | 15 | |
| <50% | 45 | 15 | |
| P53 (n=57) | 0.967 | ||
| 0–1+ | 39 | 15 | |
| 2+–3+ | 18 | 16 | |
| Menstrual status (n=118) | 0.034* | ||
| Pre-menopausal | 81 | 15 | |
| Post-menopausal | 37 | 9 | |
| TNM stage (n=118) | 0.414 | ||
| II | 6 | 21 | |
| III | 32 | 15 | |
| IV | 80 | 12 | |
| M stage (n=118) | 0.193 | ||
| 0 | 38 | 15 | |
| 1 | 80 | 10 | |
| Vascular invasion (n=74) | 0.565 | ||
| No | 34 | 13 | |
| Yes | 40 | 17 | |
| Number of lymph node metastases (n=66) | 0.016* | ||
| >6 | 46 | 12 | |
| ≤6 | 20 | 21 | |
| Tumour location (n=118) | 0.855 | ||
| Cardiac and fundus | 10 | 11 | |
| Gastric body | 64 | 12 | |
| Gastric antrum | 29 | 15 | |
| ≥ Two locations | 15 | 14 | |
| Characteristics of metastases | |||
| Transfer mode (n=118) | 0.287 | ||
| Synchronous | 73 | 12 | |
| Metachronous | 45 | 15 | |
| Metastatic site (n=118) | 0.923 | ||
| Unilateral | 35 | 12 | |
| Bilateral | 83 | 13 | |
| Peritoneal metastasis (n=118) | 0.000* | ||
| Yes | 79 | 10 | |
| No | 39 | 25 | |
| Interval time (n=45) | 0.025* | ||
| >6 months, ≤12 months | 9 | 6 | |
| >12 months | 36 | 15 | |
| Size of ovarian (n=112) | 0.013* | ||
| >5 cm | 84 | 16 | |
| ≤5 cm | 28 | 10 | |
| Treatment characteristics | |||
| Gastrectomy (n=118) | 0.000* | ||
| Yes | 79 | 16 | |
| No | 39 | 8 | |
| Chemotherapy (n=118) | 0.000* | ||
| Yes | 103 | 15 | |
| No | 15 | 5 | |
| Oophorectomy (n=118) | 0.000* | ||
| Yes | 75 | 16 | |
| No | 43 | 8 | |
| Chemotherapy drugs (n=103) | 0.049* | ||
| Two drugs | 66 | 16 | |
| ≥ Three drugs | 37 | 14 | |
| Peripheral blood index | |||
| Hemoglobin (n=118) | 0.35 | ||
| >100 g/L | 78 | 14 | |
| ≤100 g/L | 40 | 12 | |
| CEA (n=115) | 0.511 | ||
| >5 µg/L | 34 | 11 | |
| ≤5 µg/L | 81 | 15 | |
| CA-199 (n=115) | 0.499 | ||
| >35 U/mL | 47 | 14 | |
| ≤35 U/mL | 68 | 12 | |
| Platelet count (n=118) | 0.008* | ||
| >300×109/L | 28 | 10 | |
| ≤300×109/L | 90 | 14 | |
| D-dimer (n=82) | 0.003* | ||
| >0.5 µg/mL | 51 | 9 | |
| ≤0.5 µg/mL | 31 | 16 | |
| Fibrinogen (n=113) | 0.000* | ||
| >4 g/L | 27 | 8 | |
| ≤4 g/L | 86 | 15 | |
| Pretreatment PLR (n=118) | 0.009* | ||
| >172.8 | 63 | 10 | |
| ≤172.8 | 55 | 16 | |
| Albumin (n=118) | 0.006* | ||
| >35 g/L | 103 | 14 | |
| ≤35 g/L | 15 | 6 | |
| CA-125 (n=109) | 0.000* | ||
| >35 U/mL | 66 | 10 | |
| ≤35 U/mL | 43 | 21 | |
| CA-724 (n=85) | 0.751 | ||
| >6.9 U/mL | 47 | 11 | |
| ≤6.9 U/mL | 38 | 12 | |
| Pretreatment NLR (n=118) | 0.311 | ||
| >2.9 | 51 | 10 | |
| ≤2.9 | 67 | 15 | |
| Pretreatment SII (n=118) | 0.013* | ||
| >449.9 | 80 | 11 | |
| ≤449.9 | 38 | 21 |
*, P<0.05. KTs, Krukenberg tumors; TNM, tumor-node-metastasis; CEA, carcinoembryonic antigen; CA, cancer antigen; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; SII, systemic immune-inflammation index; OS, overall survival.
The optimal cut-off values for NLR, PLR, and SII were defined based on the ROC curves. The areas under the curve (AUCs) for NLR, PLR, and SII were 0.608 (P=0.049; 95% CI: 0.503–0.712), 0.658 (P=0.004; 95% CI: 0.557–0.758), and 0.619 (P=0.03; 95% CI: 0.516–0.722), respectively. The optimal cut-offs for NLR, PLR, and SII were 2.9 (sensitivity 56.9%; specificity 66.1%), 172.8 (sensitivity 70.6%; specificity 58.1%), and 449.9 (sensitivity 82.4%; specificity 41.9%), respectively (Figure 2A-2C).
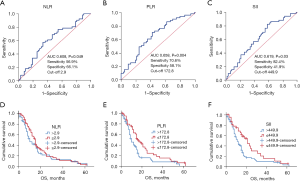
By analyzing survival for these different indices, we found OS was significantly prolonged for pre-treatment NLR ≤2.9 compared to NLR >2.9 (median survival, 15 vs. 10 months), but did not reach statistical significance (P=0.311, Figure 2D). PLR >172.8 and SII >449.9 were associated with poor OS (all P<0.05; Figure 2E,2F), while albumin >35 g/L, CA-125 ≤35 U/mL, platelet count ≤300×109/L, D-dimer ≤0.5 µg/mL, and fibrinogen ≤4 g/L were associated with better survival (all P<0.05). There was no difference in survival according to hemoglobin, CEA, CA-199, CA-724, or pretreatment NLR (all P>0.05).
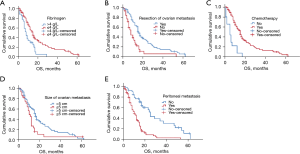
The median OS was 16 (95% CI: 14.10–17.90) vs. 8 (95% CI: 6.01–9.98) months in the oophorectomy group and no oophorectomy group (P=0.000), respectively, and 15 (95% CI: 13.03–16.97) vs. 5 (95% CI: 1.43–8.58) months in patients receiving chemotherapy vs. no chemotherapy (P=0.000). Platinum, docetaxel and fluoropyrimidine-based regimens were the main drug treatment options, and of the 103 patients who received chemotherapy, those who underwent a two-drug regimen had a better OS than those who received the three-drug regimen (16 vs. 14 months, P=0.049). Survival was higher in patients with a primary gastric tumor less than 5 cm (17 vs. 12 months, P=0.031), an ovarian tumor larger than 5 cm (16 vs. 10 months, P=0.013), no peritoneal metastasis (25 vs. 10 months, P=0.000), resection of primary gastric lesion (16 vs. 8 months, P=0.000), premenopausal (15 vs. 9 months, P=0.034), fewer than six lymph node metastases (21 vs. 12 months, P=0.016), and interval to ovarian metastasis longer than 12 months (15 vs. 6 months, P=0.025). Ovariectomy rates were significantly higher in patients with ovarian metastases >5 cm in size than in those size ≤5 cm (70.2% vs. 42.9%, P=0.009). Tumor location, age, histopathological type, nerve invasion, vascular invasion, TNM stage at initial diagnosis, metastatic site and Ki-67 and P53 had no significant relationship with survival.
In multivariate analysis, fibrinogen, size of ovarian metastatic tumor, chemotherapy after ovarian metastasis, oophorectomy, and the presence of peritoneal metastasis were independent predictors of OS (Table 3, Figure 3). It is worth mentioning that one patient with resected ovarian metastasis and gastric primary tumor survived without recurrence at 5 years of follow-up.
Table 3
| Variables | B | SE | Wald | df | P | HR | 95% CI |
|---|---|---|---|---|---|---|---|
| Oophorectomy | 0.543 | 0.244 | 4.927 | 1 | 0.026 | 1.720 | 1.066–2.778 |
| Size of ovarian metastasis | 0.592 | 0.219 | 7.334 | 1 | 0.007 | 1.808 | 1.178–2.776 |
| Fibrinogen | −0.728 | 0.242 | 9.007 | 1 | 0.003 | 0.483 | 0.300–0.777 |
| Peritoneal metastasis | 1.009 | 0.273 | 13.662 | 1 | 0.000 | 2.742 | 1.606–4.682 |
| Chemotherapy after ovarian metastasis | −1.634 | 0.339 | 23.267 | 1 | 0.000 | 0.195 | 0.101–0.379 |
KTs, Krukenberg tumors; SE, standard error; df, degrees of freedom; HR, hazard ratio; CI, confidence interval.
Discussion
The incidence of KT varies considerably according to different studies. It usually occurs with bilateral involvement, and as reported in our article, in women 15–20 years younger than the typical age at which primary ovarian cancer occurs (19,20). This may be related to the more active ovarian function and abundant ovarian blood supply in premenopausal women which provides a favourable internal environment for tumor cell metastasis and growth, and where the pathological types in young GC patients are mostly poorly differentiated adenocarcinoma and signet ring cell carcinoma (21). Despite great progress in the diagnosis and treatment of GC in recent years, the median OS is only 13.8 months, even in HER-2-positive GC patients treated with chemotherapy combined with trastuzumab-targeted therapy (22). The pathological types of KT are mostly poorly differentiated adenocarcinoma and signet ring cell carcinoma, which are mainly HER-2 negative, and in our study, the positive rate of HER-2 with KT-GC was only 1.8%, much lower than the 16.6% reported in the TOGA study. There is no suitable targeted therapy for KT, and because of its poor prognosis, there is an urgent need to find effective predictors of treatment efficacy, intervene early, and seek effective treatments.
Serum tumor markers are the focus of clinical attention, and previous laboratory reports on KT are nonspecific. Some scholars have found the detection of preoperative CA-125 testing helps in the diagnosis and treatment monitoring of KT and it has been reported that elevated CEA was associated with a poor prognosis compared with CA-199 and CA-125 (23-25). In our study, OS was significantly shorter in patients with elevated CA-125 compared to CEA and CA-199 than in patients with normal CA-125 (10 vs. 21 months). In view of the different results reported by various studies, the exact role of serum tumor markers in the diagnosis and prognosis of KT needs to be confirmed by large-scale studies.
In addition, inflammation, abnormal coagulation, and nutritional status play important roles in the occurrence and development of tumors (26-29), and neutrophils, platelets, and lymphocytes also contribute to tumor cell invasion into the peripheral blood, where they can survive and reseed in distant organs. Several prognostic scores based on inflammation and nutrition have been developed to predict survival and recurrence of cancer, such as NLR, PLR, and SII (30-32). SII is an integrated indicator based on peripheral lymphocyte, neutrophil, and platelet counts, and may better reflect the balance of host inflammatory and immune status. Shi et al. reported that compared with other systemic inflammatory indices, SII was a reliable index for predicting postoperative survival of GC (33), while Gu et al. reported a higher PLR (34), and Zhang et al. reported an elevated NLR and PLR were also reliable (35). Wu et al. found GC patients with high fibrinogen and low albumin had a poor prognosis, and that FAR was an independent prognostic factor for recurrence after radical resection in GC patients (36). However, the utility of these indices for KT-GC has not been reported. By Univariate analysis, our study showed that hypoalbuminemia, high PLR and SII, elevated platelet count, D-dimer, and fibrinogen were associated with a poor prognosis in KTs of GC origin. This hints that malnutrition and hypercoagulability play an important role in the development of the tumor. However, in the multivariate analysis, only fibrinogen was an independent prognostic factor. Fibrinogen is an extracellular matrix protein involved in the formation of blood clots and in tumor angiogenesis and metastasis (37), indicating attention should be paid to the coagulation status of KT patients in clinical practice. Previous studies have found multiple combined tests can be an effective predictor of survival in GC patients (38). For example, Hirahara et al. reported that combining NLR and PLR could predict the chemotherapy response and prognosis in patients with advanced GC (39), and Arigami et al. found that combining fibrinogen and NLR could be used as a prognostic marker (40). However, a combination method in KT requires more in-depth research in the future. More studies should also be conducted to address the potential prognostic value of NLR, PLR, and SII in KT.
We also explored the impact of ovarian metastasis and its corresponding treatment on the survival of patients, and the results showed that in patients with metachronous ovarian metastasis, the survival impact of metastasis occurred at intervals of more than 1 year. The prognosis was good for patients with GC resection, ovarian metastasis resection, and chemotherapy, especially for those who underwent a two-drug regimen chemotherapy. Our findings are the same as most reports (41,42), such as Yan et al., who reported that metastasectomy combined with chemotherapy in patients with synchronous ovarian metastases of GC could have a survival benefit (43). Cho et al. found that metastasectomy, signet-ring cell pathology, and peritoneal carcinomatosis were prognostic factors for survival (44).
Interestingly, we found that contrary to our conventional understanding, patients with ovarian metastases larger than 5 cm had a better prognosis, which may be related to the higher rate of oophorectomy in patients with large metastatic lesions. Ovarian metastasis can lead to local pelvic compression symptoms, such as dysuria or difficulties with defecation, abdominal/pelvic effusion, and pain, which can seriously affect the quality of life of patients. Therefore, active ovarian metastasis resection is beneficial to prolong survival and improve the quality of life of these patients. In particular, we found a two-drug chemotherapy regimen was superior to three-drug therapy in our study, which may be related to the high burden of ovarian metastases and poor patient status, and is consistent with some previous studies (45,46). Current National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO) guidelines also recommend dual drugs as the first choice for patients with KT-GC, and three-drug for patients with better physical condition. However, the sample size of this study was limited, and the results need to be validated by large-scale clinical trials.
Conclusions
Fibrinogen, size of ovarian metastasis tumor, chemotherapy after ovarian metastasis, peritoneal metastasis, and oophorectomy are independent prognostic factors for KT-GC patients. Attention should be paid to the coagulation function, inflammatory system, and nutritional status of KT patients to correct hypercoagulable states and hypoalbuminemia as soon as possible, as well as to whether the patient has peritoneal metastasis. Once diagnosed, chemotherapy should be actively administered, and the two-drug regimen may be better. Prospective randomized trials are required to determine the optimal treatment strategy for KT, and in-depth mechanistic research should be performed.
Acknowledgments
Funding: This research was funded by the Shanghai Municipal Committee of Science and Technology (No. 21Y11913200).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-464/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-464/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-464/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Ren Ji Hospital (No. KY2021-183-B). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zhong J, Chen Y, Wang LJ. Emerging molecular basis of hematogenous metastasis in gastric cancer. World J Gastroenterol 2016;22:2434-40. [Crossref] [PubMed]
- Tan P, Yeoh KG. Genetics and Molecular Pathogenesis of Gastric Adenocarcinoma. Gastroenterology 2015;149:1153-62.e3. [Crossref] [PubMed]
- McCracken KW, Catá EM, Crawford CM, et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014;516:400-4. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Krukenberg F. Ueber das Fibrosarcoma ovarii mucocellulare (carcinomatodes). Arch Gynakol 1896;50:287-321. [Crossref]
- Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol 2006;30:277-99. [Crossref] [PubMed]
- Wang B, Tang Q, Xu L, et al. A comparative study of RTK gene status between primary tumors, lymph-node metastases, and Krukenberg tumors. Mod Pathol 2021;34:42-50. [Crossref] [PubMed]
- Wang J, Shi YK, Wu LY, et al. Prognostic factors for ovarian metastases from primary gastric cancer. Int J Gynecol Cancer 2008;18:825-32. [Crossref] [PubMed]
- Kubeček O, Laco J, Špaček J, et al. The pathogenesis, diagnosis, and management of metastatic tumors to the ovary: a comprehensive review. Clin Exp Metastasis 2017;34:295-307. [Crossref] [PubMed]
- Alrajban WA, Khubrani RA, Almalki MS, et al. Extensive Paneth cell metaplasia in an ovarian Krukenberg tumor: report of an unusual case and literature review. J Surg Case Rep 2018;2018:rjy323. [Crossref] [PubMed]
- Okamoto T, Suzuki H, Fukuda K. Gastric linitis plastica due to signet-ring cell carcinoma with Krukenberg tumors diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Clin J Gastroenterol 2021;14:994-1003. [Crossref] [PubMed]
- Ikeshita C, Komatsu S, Shibata R, et al. Conversion Surgery for Advanced Gastric Cancer with Ovarian Metastasis-A Case Report with Review of the Literatures. Gan To Kagaku Ryoho 2021;48:1709-11. [PubMed]
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559-64. [Crossref] [PubMed]
- Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76:182-8. [Crossref] [PubMed]
- Wu HW, Qin CY, Huang JL, et al. Correlations of β-catenin, Ki67 and Her-2/neu with gastric cancer. Asian Pac J Trop Med 2014;7:257-61. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Smyth EC, Nilsson M, Grabsch HI, et al. Gastric cancer. Lancet 2020;396:635-48. [Crossref] [PubMed]
- Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68:284-96. [Crossref] [PubMed]
- Webb PM, Jordan SJ. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2017;41:3-14. [Crossref] [PubMed]
- Zulfiqar M, Koen J, Nougaret S, et al. Krukenberg Tumors: Update on Imaging and Clinical Features. AJR Am J Roentgenol 2020;215:1020-9. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Lionetti R, De Luca M, Travaglino A, et al. Prognostic factors in Krukenberg tumor. Arch Gynecol Obstet 2019;300:1155-65. [Crossref] [PubMed]
- Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med 2006;130:1725-30. [Crossref] [PubMed]
- Zhang C, Hou W, Huang J, et al. Effects of metastasectomy and other factors on survival of patients with ovarian metastases from gastric cancer: a systematic review and meta-analysis. J Cell Biochem 2019;120:14486-98. [Crossref] [PubMed]
- Baracos VE. Cancer-associated malnutrition. Eur J Clin Nutr 2018;72:1255-9. [Crossref] [PubMed]
- Sachlova M, Majek O, Tucek S. Prognostic value of scores based on malnutrition or systemic inflammatory response in patients with metastatic or recurrent gastric cancer. Nutr Cancer 2014;66:1362-70. [Crossref] [PubMed]
- Young A, Chapman O, Connor C, et al. Thrombosis and cancer. Nat Rev Clin Oncol 2012;9:437-49. [Crossref] [PubMed]
- Falanga A, Marchetti M, Russo L. Hemostatic Biomarkers and Cancer Prognosis: Where Do We Stand? Semin Thromb Hemost 2021;47:962-71. [Crossref] [PubMed]
- Nøst TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol 2021;36:841-8. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Liu C, Li X. Stage-Dependent Changes in Albumin, NLR, PLR, and AFR are Correlated with Shorter Survival in Patients with Gastric Cancer. Clin Lab 2019; [Crossref] [PubMed]
- Shi H, Jiang Y, Cao H, et al. Nomogram Based on Systemic Immune-Inflammation Index to Predict Overall Survival in Gastric Cancer Patients. Dis Markers 2018;2018:1787424. [Crossref] [PubMed]
- Gu L, Wang M, Cui X, et al. Clinical significance of peripheral blood-derived inflammation markers in advanced gastric cancer after radical resection. BMC Surg 2020;20:219. [Crossref] [PubMed]
- Zhang Y, Lu JJ, Du YP, et al. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine (Baltimore) 2018;97:e0144. [Crossref] [PubMed]
- Wu M, Pan Y, Jia Z, et al. Preoperative Plasma Fibrinogen and Serum Albumin Score Is an Independent Prognostic Factor for Resectable Stage II-III Gastric Cancer. Dis Markers 2019;2019:9060845. [Crossref] [PubMed]
- Staton CA, Brown NJ, Lewis CE. The role of fibrinogen and related fragments in tumour angiogenesis and metastasis. Expert Opin Biol Ther 2003;3:1105-20. [Crossref] [PubMed]
- Guo J, Chen S, Chen Y, et al. Combination of CRP and NLR: a better predictor of postoperative survival in patients with gastric cancer. Cancer Manag Res 2018;10:315-21. [Crossref] [PubMed]
- Hirahara T, Arigami T, Yanagita S, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer 2019;19:672. [Crossref] [PubMed]
- Arigami T, Uenosono Y, Matsushita D, et al. Combined fibrinogen concentration and neutrophil-lymphocyte ratio as a prognostic marker of gastric cancer. Oncol Lett 2016;11:1537-44. [Crossref] [PubMed]
- Feng Q, Pei W, Zheng ZX, et al. Clinicopathologic characteristics and prognostic factors of 63 gastric cancer patients with metachronous ovarian metastasis. Cancer Biol Med 2013;10:86-91. [PubMed]
- Ma F, Li Y, Li W, et al. Metastasectomy Improves the Survival of Gastric Cancer Patients with Krukenberg Tumors: A Retrospective Analysis of 182 patients. Cancer Manag Res 2019;11:10573-80. [Crossref] [PubMed]
- Yan D, Du Y, Dai G, et al. Management Of Synchronous Krukenberg Tumors From Gastric Cancer: a Single-center Experience. J Cancer 2018;9:4197-203. [Crossref] [PubMed]
- Cho JH, Lim JY, Choi AR, et al. Comparison of Surgery Plus Chemotherapy and Palliative Chemotherapy Alone for Advanced Gastric Cancer with Krukenberg Tumor. Cancer Res Treat 2015;47:697-705. [Crossref] [PubMed]
- Acikgoz Y, Aktürk Esen S, Ucar G, et al. The Comparison of mDCF and mFOLFOX-6 as First-Line Treatment in Metastatic Gastric Cancer. Cureus 2021;13:e14882. [Crossref] [PubMed]
- Mizrak Kaya D, Nogueras González GM, Harada K, et al. Efficacy of Three-Drug Induction Chemotherapy Followed by Preoperative Chemoradiation in Patients with Localized Gastric Adenocarcinoma. Oncology 2020;98:542-8. [Crossref] [PubMed]
(English Language Editor: B. Draper)


