
Development of a prognostic score for recommended transarterial chemoembolization candidates with spontaneous rupture of hepatocellular carcinoma
Introduction
One of the life-threatening complications of hepatocellular carcinoma (HCC) is intraperitoneal hemorrhage due to spontaneous rupture of the tumor. Several studies have confirmed emergency hepatectomy and transarterial chemoembolization (TACE) as the optimal treatment approaches for patients with spontaneous rupture of HCC (1-3). Hepatectomy is an effective treatment strategy for tumor rupture (4). However, severe cirrhosis or poor hepatic function usually reduces the tolerance of patients to surgical resection. Accordingly, the less invasive procedure, TACE, has been widely used to treat unresectable HCC.
Several studies have reported promising outcomes following TACE treatment in patients with spontaneous rupture of HCC (5-7). However, patients’ prognoses after TACE treatment can be affected by several factors, such as age, Barcelona Clinic Liver Cancer (BCLC) staging system, Child-Pugh score, maximum tumor size, and extrahepatic invasion (8). Considering these associated risk factors, identify significant prognostic factors regarding the outcome and timely operation are needed for HCC patients with spontaneous rupture. Despite this need, few studies have investigated the predictive factors associated with this life-threatening complication. Moreover, no guidelines exist that can aid in determining the appropriateness of TACE treatment for individual patients.
Individualized prediction of spontaneous rupture in HCC patients is necessary for the prediction of prognosis after TACE treatment. A predictive nomogram can indicate an alternative option for HCC patients with spontaneous rupture who might undergo TACE treatment. However, to our knowledge, no previous study has used a nomogram model to address this issue. Thus, the aim of the present study was to analyze the risk factors associated with the overall survival (OS) of HCC patients with spontaneous rupture after TACE treatment. Following this, we identified independent prognostic factors and developed a nomogram model for the risk prediction of patients with spontaneous rupture HCC based on widely available pretreatment clinical and laboratory data. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-531/rc).
Methods
Patients and study design
The medical records of 55 HCC patients with spontaneous tumor rupture were retrospectively reviewed. These patients underwent TACE between January 2015 and April 2019 at the Department of Hepatic Carcinoma of Fudan University Affiliated Zhongshan Hospital (Shanghai, China). We reviewed their clinical data, including age, sex, clinical presentation, and disease history. The results of the laboratory examinations at diagnosis were also reviewed. HCC tumor status, including the number of tumors, presence of portal vein thrombosis, tumor size, and distant metastasis were obtained by contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) scans.
The inclusion criteria for this study were as follows: (I) HCC was diagnosed either by imaging or histological evaluation according to the guidelines of the American Association for the Study of Liver Diseases (9). All the HCC tumors were staged according to the BCLC staging system (10). (II) The diagnosis of spontaneous HCC rupture was based on the disruption of the peritumoral liver capsule with surrounding fluid in the perihepatic region. This was detected by dynamic computed tomography and abdominal puncture, which could determine the presence of bloody fluid in the abdomen. (III) No HCC treatment was administered to the patients within 1 month prior to the diagnosis of HCC rupture. (IV) No other malignancies in addition to HCC. (V) Complete treatment or follow-up data.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study was approved by the Clinical Research Ethics Committee of Fudan University Affiliated Zhongshan Hospital (No. Y2017-163). Individual consent for this retrospective analysis was waived.
Follow up
Patients were followed-up 4 weeks after the operation and subsequently once every 6−12 weeks. The CT or MRI images and laboratory tests were collected. Follow-up period was terminated on June 1, 2020.
Treatment procedures
Under the guidance of digital subtraction angiography (DSA), TACE was performed through a femoral artery puncture with the Seldinger technique. Based on the number of tumors, their sizes, and the degree of hemorrhage, 5–20 mL of lipiodol mixed with epirubicin suspension and gelatin sponge fragments or granules were injected into the tumor-feeding arteries with a catheter under the guidance of DSA. Subsequently, 100–150 mg of oxaliplatin or 5-fluorouracil was slowly perfused into the tumor-feeding artery according to the body weight and liver function status of the patients. Finally, post-embolization angiography was performed to determine the extent of the embolization. More details of the treatment procedure can be found in our previously published article (8).
Statistical analyses
Categorical data were statistically analyzed using the chi-squared test or Fisher’s exact test and are expressed as frequencies and percentages; meanwhile, continuous variables were statistically analyzed using the independent Student’s t-test and are expressed as medians and ranges. OS rates were estimated using the Kaplan-Meier method. Factors which were found to be significant in the univariate analysis (P values less than 0.1) were subjected to multivariate analysis. Cox proportion hazards regression model were used to identify independent predictors (P values less than 0.05). Factors in the nomogram were identified with multivariate Cox regression analysis, and the concordance index was calculated. Statistical analyses were performed using SPSS software (version 21.0; IBM Corp.). Statistical analyses of the nomogram were performed using R software (version 3.2.0; R Foundation for Statistical Computing). Differences with P values of less than 0.05 were considered statistically significant.
Results
Baseline characteristics
The medical records of 55 patients with spontaneous ruptured HCC who underwent TACE between January 2015 and April 2019 were retrospectively reviewed. This study included 42 (76.4%) men and 13 (23.6%) women. The median age of the patients was 55 years (range 28–86 years; Table 1). Hepatitis B virus infection was the main etiological factor for HCC in patients (49/55, 89.0%). Only 1 patient had hepatitis C virus infection. A total of 23 (41.8%) patients presented with a single tumor, whereas 32 (58.2%) patients presented with >2 tumors. According to the Child-Pugh classification, 19 (34.5%) patients were class A, 28 (50.9%) were class B, and 8 (14.5%) were class C. According to the Barcelona Clinic Liver Cancer staging system, 28 (50.9%) patients were stage B, 27 (49.1%) were stage C, and no patients were stage A. Vascular invasion was observed in 21 (38.2%) patients, and distant metastasis was observed in 14 (25.5%) patients.
Table 1
| Variables | Cases (%) |
|---|---|
| Age over 60 years | 22 (40.0) |
| Hypertension history | 18 (32.7) |
| Child-Pugh: A/B/C | 19 (34.5)/28 (50.9)/8 (14.5) |
| AFP >400 ng/L | 33 (60.0) |
| ALT >120 U/L (3N) | 12 (21.8) |
| AST >120 U/L (3N) | 27 (49.1) |
| TBIL >30 μmol/L (1.5N) | 13 (23.6) |
| ALB ≤35 g/L | 24 (43.6) |
| PT >16 s | 7 (12.7) |
| Hb (g/L) >90/60–90/<60 | 21 (38.2)/30 (54.5)/4 (7.3) |
| Scr >1.5 N | 2 (3.6) |
| Positive HBsAg/HCV Ab status | 49 (89.0)/1 (1.8) |
| Maximum tumor size (cm): ≤5/>5 and ≤10/>10 | 11 (20.0)/26 (47.3)/18 (32.7) |
| Tumor number: single/multiple | 23 (41.8)/32 (58.2) |
| Capsule formation: yes/no | 25 (45.5)/30 (54.5) |
| Vascular thrombus: yes/no | 21 (38.2)/34 (61.8) |
| Extrahepatic invasion: yes/no | 14 (25.5)/41 (74.5) |
| BCLC stage: A/B/C | 0 (0.0)/28 (50.9)/27 (49.1) |
HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; N, normal level; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; ALB, albumin; PT, prothrombin time; Hb, hemoglobin; Scr, serum creatinine; HbsAg, hepatitis B surface antigen; HCV, hepatitis C virus; BCLC, Barcelona Clinic Liver Cancer.
Complications
The posttreatment adverse reactions were assessed. The majority of toxicity events were grade 2 or lower. All patients recovered and were discharged following symptomatic and supportive treatment, with the exception of 2 patients who had severe hemorrhagic shock and hepatic failure prior to TACE treatment. We reported these complications in a previously published article (8).
OS
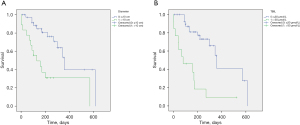
The median follow-up period was 23.0 months (range, 11.8–40.1 months). The median OS was 6.4 months, with 6-month and 1-year survival rates of 52.7% and 41.8%, respectively.
Univariate and multivariate analyses
Univariate analyses indicated the size of the largest tumor, total bilirubin (TBIL) levels, and aspartate aminotransferase (AST) levels to be associated with OS (P<0.1; Table 2). Multivariate Cox regression analysis indicated the diameter of the largest tumor (P=0.044) and TBIL level (P=0.036) to be independent prognostic factors for OS (Table 3).
Table 2
| Factors | P value |
|---|---|
| Hypertension history | 0.826 |
| Child-Pugh score | 0.164 |
| AFP >400 ng/L | 0.533 |
| ALT >3 N | 0.336 |
| AST >3 N | 0.045 |
| TBIL >1.5 N | 0.001 |
| Maximum tumor size | 0.002 |
| Vascular thrombus | 0.446 |
| Extrahepatic invasion | 0.231 |
| BCLC stage | 0.108 |
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; N, normal level; AST, aspartate aminotransferase; TBIL, total bilirubin; BCLC, Barcelona Clinic Liver Cancer.
Table 3
| Factors | HR | 95% CI | P value |
|---|---|---|---|
| TBIL >30 μmol/L | 0.358 | 0.137–0.934 | 0.036 |
| Maximum tumor size | 1.012 | 1.000–1.025 | 0.044 |
| AST | 1.560 | 0.641–3.794 | 0.327 |
HR, hazard ratio; CI, confidence interval; TBIL, total bilirubin; AST, aspartate aminotransferase.
Development of the risk score model
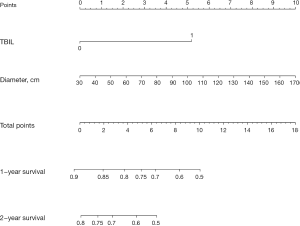
A nomogram for the stratification of individual patient’s risk was created based on 2 clinically detectable preoperative parameters: the diameter of the largest tumor and TBIL levels (Figure 1). The diameter of the largest tumor was treated as a continuous variable, while TBIL level (>30 and ≤30 µmol/L) was treated as a categorical factor.
The 1- and 2-year survival probabilities of individual patients could be predicted before the TACE procedure with the total points of size of the largest tumor and TBIL level (Figure 2). For example, the 1-year survival rate for patients having a tumor diameter less than 5 cm and a TBIL level of less than 30 µmol/L was approximately 82.5% according to the risk assessment model. However, the 1-year survival rate for patients having the same tumor diameter and a TBIL level >30 µmol/L was less than 50%. The concordance-index of the nomogram for the prediction of OS was 0.748 (95% CI: 0.691–0.805).
Discussion
Spontaneous rupture of HCC is one of the most common and lethal complications among liver emergencies (11). Previous studies have shown a very poor prognosis for those afflicted due to the various degrees of complications associated with HCC rupture, such as hypovolemic shock, acute hepatic or renal failure, and recurrent tumor rupture (12,13). Several studies have confirmed emergency hepatectomy and TACE to be the best treatment approaches for patients with HCC rupture (14-17). As HCC rupture is always accompanied by coagulopathy or poor liver function, only a limited number of patients can tolerate surgical hepatic resection (18,19). Compared with emergency hepatectomy for ruptured HCC, transarterial embolization (TAE) with staged hepatectomy can effectively decrease intraoperative blood loss and 30-day mortality (20).
Several studies have reported that TACE can be effective for patients with spontaneous rupture HCC and can achieve immediate hemostasis (7,21). Kim et al. reported that TACE effectively achieved hemodynamic stability and increased the 30-day survival in HCC patients with spontaneous rupture (6). Shin et al. reported a median OS time of 179.6 days in HCC patients with spontaneous rupture, with 3-month, 6-month, and 1-year survival rates of 54%, 48%, and 43%, respectively (5). Similarly, we found that the median OS of HCC patients with spontaneous rupture was 6.4 months (192 days), with 6-month and 1-year survival rates of 52.7% and 41.8%, respectively.
Several researchers have also investigated the possibly related predictive factors in order to improve our understanding of HCC ruptures. Shin et al. reported that higher requirements for blood transfusion, Child-Pugh class C, presence of portal vein thrombosis, and tumors involving both lobes were significant predictors of poor survival; however, the variable of TBIL levels was not capable of predicting OS (5). In contrast, our study found TBIL level to be an independent prognostic factor for OS. The reason for this difference can be attributed to the serum bilirubin levels reported by Shin et al. being <3.0 mg/dL in all patients (5). Lee et al. (22) also reported high bilirubin levels to be associated with early mortality following hemostasis by emergency TACE in HCC patients with spontaneous rupture. Kirikoshi et al. (23) reported a maximum tumor size >7 cm to be the only independent factor determining long-term survival in patients who underwent initial TACE after spontaneous HCC rupture. However, the multivariate analysis in our study indicated there to be no significant association between the diameter of the largest tumor (>7 cm) and survival rates. Furthermore, the endpoint of the largest tumor size >10 cm was determined to be an independent predictor of survival rate. This discrepancy may be due to the different follow-up times and baseline characteristics of the patients, but more studies are required to address these conflicting findings.
A prognostic score system can facilitate clinical counseling, guide treatment, and optimize follow-up plans. In recent years, an increasing number of nomograms have been applied in the management of cancer and other diseases (24-26). Nomograms are easy, convenient, and highly accurate, and can help in making more tailored clinical decisions. Wang et al. (27) developed a “six-and-twelve” score for HCC that can predict individual outcomes with a prognostic model for recommended or ideal TACE candidates. Although TACE has been widely used for spontaneous rupture HCC, no existing model exists for predicting survival. Recently, Qiu et al. (28) and Cheng et al. (29) constructed an artificial neural network-based model and a model for end-stage liver disease score, respectively, to predict the periprocedural prognostic factors of patients with spontaneous rupture HCC. However, patients from these models were treated with TAE. Meanwhile, TACE is recommended in the 2021 edition of the Chinese guidelines for interventional therapy and is more commonly used in China compared to other countries. Therefore, predicting the prognosis of the patients with spontaneous rupture HCC and treated with TACE is even more meaningful in the Chinese context.
In the present study, we developed a nomogram to predict the risk of HCC spontaneous rupture after TACE treatment. To our knowledge, our study is the first to investigate the application of this nomogram to HCC rupture following TACE treatment and its associated risks. Moreover, the concordance index of this nomogram for OS prediction was 0.748 (95% CI: 0.691–0.805), while the concordance index in the interval validation indicated that this nomogram could be widely and accurately used. Our proposed nomogram provides an easy-to-use tool for predicting the OS of patients with ruptured HCC. With an estimate of the individual risk for each patient, clinicians can make more suitable and informed decisions regarding the treatment of HCC rupture. However, this nomogram requires external validation, and a larger sample size is needed to determine its suitability for predicting TACE in patients with HCC rupture. To these ends, multicenter studies should be conducted in the future.
In summary, we found the diameter of the largest tumor and TBIL level to be independent prognostic factors for predicting the OS of patients with ruptured HCC. The subsequently developed nomogram demonstrated relatively good accuracy for estimating the prediction of survival in patients with ruptured HCC upon undergoing TACE treatment. According to the risk assessment model, we can evaluate the approximate 1- and 2-year survival rates based on the patient’s tumor diameter and TBIL level after TACE treatment for ruptured HCC. This study may help improve perioperative strategies and maximize favorable treatment outcomes.
Acknowledgments
Funding: This work was supported by the Young Scientists Fund of the National Natural Science Foundation of China (grant Nos. 81702310 and 81502007) and the Outstanding Youth Science Foundation of Fudan University Affiliated Zhongshan Hospital (No. 2019ZSYXQN22).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-531/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-531/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-531/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Clinical Research Ethics Committee of Fudan University Affiliated Zhongshan Hospital (No. Y2017-163). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu Q, Qiao GL, Xu C, et al. Partial hepatectomy for spontaneous tumor rupture in patients with hepatocellular carcinoma: a retrospective cohort study. Cancer Manag Res 2017;9:525-37. [Crossref] [PubMed]
- Wu JJ, Zhu P, Zhang ZG, et al. Spontaneous rupture of hepatocellular carcinoma: Optimal timing of partial hepatectomy. Eur J Surg Oncol 2019;45:1887-94. [Crossref] [PubMed]
- Jin YJ, Lee JW, Park SW, et al. Survival outcome of patients with spontaneously ruptured hepatocellular carcinoma treated surgically or by transarterial embolization. World J Gastroenterol 2013;19:4537-44. [Crossref] [PubMed]
- Zhang XF, Wei T, Liu XM, et al. Spontaneous tumor rupture and surgical prognosis of patients with hepatocellular carcinoma. Scand J Gastroenterol 2012;47:968-74. [Crossref] [PubMed]
- Shin BS, Park MH, Jeon GS. Outcome and prognostic factors of spontaneous ruptured hepatocellular carcinoma treated with transarterial embolization. Acta Radiol 2011;52:331-5. [Crossref] [PubMed]
- Kim JY, Lee JS, Oh DH, et al. Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol 2012;24:640-5. [Crossref] [PubMed]
- Maoz D, Sharon E, Chen Y, et al. Spontaneous hepatic rupture: 13-year experience of a single center. Eur J Gastroenterol Hepatol 2010;22:997-1000. [Crossref] [PubMed]
- Zou J, Li C, Chen Y, et al. Retrospective analysis of transcatheter arterial chemoembolization treatment for spontaneously ruptured hepatocellular carcinoma. Oncol Lett 2019;18:6423-30. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Gómez-Rodríguez R, Romero-Gutiérrez M, Artaza-Varasa T, et al. The value of the Barcelona Clinic Liver Cancer and alpha-fetoprotein in the prognosis of hepatocellular carcinoma. Rev Esp Enferm Dig 2012;104:298-304. [Crossref] [PubMed]
- Rathor M, Lal A, Dhiman RK. Spontaneous rupture of hepatocellular carcinoma. J Clin Exp Hepatol 2014;4:188-9. [Crossref] [PubMed]
- Hsin IF, Hsu CY, Huang HC, et al. Liver failure after transarterial chemoembolization for patients with hepatocellular carcinoma and ascites: incidence, risk factors, and prognostic prediction. J Clin Gastroenterol 2011;45:556-62. [Crossref] [PubMed]
- Zhu Q, Li J, Yan JJ, et al. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol 2012;18:7302-7. [Crossref] [PubMed]
- Chen ZY, Qi QH, Dong ZL. Etiology and management of hemmorrhage in spontaneous liver rupture: a report of 70 cases. World J Gastroenterol 2002;8:1063-6. [Crossref] [PubMed]
- Han XJ, Su HY, Shao HB, et al. Prognostic factors of spontaneously ruptured hepatocellular carcinoma. World J Gastroenterol 2015;21:7488-94. [Crossref] [PubMed]
- Li J, Huang L, Liu CF, et al. Risk factors and surgical outcomes for spontaneous rupture of BCLC stages A and B hepatocellular carcinoma: a case-control study. World J Gastroenterol 2014;20:9121-7. [PubMed]
- Lee HS, Choi GH, Choi JS, et al. Staged partial hepatectomy versus transarterial chemoembolization for the treatment of spontaneous hepatocellular carcinoma rupture: a multicenter analysis in Korea. Ann Surg Treat Res 2019;96:275-82. [Crossref] [PubMed]
- Dewar GA, Griffin SM, Ku KW, et al. Management of bleeding liver tumours in Hong Kong. Br J Surg 1991;78:463-6. [Crossref] [PubMed]
- Lai EC, Wu KM, Choi TK, et al. Spontaneous ruptured hepatocellular carcinoma. An appraisal of surgical treatment. Ann Surg 1989;210:24-8. [Crossref] [PubMed]
- Zhang FQ, Li L, Huang PC, et al. Transarterial embolization with hepatectomy for ruptured hepatocellular carcinoma: a meta-analysis. Minim Invasive Ther Allied Technol 2022;31:676-83. [Crossref] [PubMed]
- Singh Bhinder N, Zangan SM. Hepatocellular carcinoma rupture following transarterial chemoembolization. Semin Intervent Radiol 2015;32:49-53. [Crossref] [PubMed]
- Lee KH, Tse MD, Law M, et al. Development and validation of an imaging and clinical scoring system to predict early mortality in spontaneous ruptured hepatocellular carcinoma treated with transarterial embolization. Abdom Radiol (NY) 2019;44:903-11. [Crossref] [PubMed]
- Kirikoshi H, Saito S, Yoneda M, et al. Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol 2009;9:29. [Crossref] [PubMed]
- Tang X, Zhou X, Li Y, et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Patients with Initially Diagnosed Metastatic Esophageal Cancer: A SEER-Based Study. Ann Surg Oncol 2019;26:321-8. [Crossref] [PubMed]
- Ding Y, Mao Z, Ruan J, et al. Nomogram-Based New Recurrence Predicting System in Early-Stage Papillary Thyroid Cancer. Int J Endocrinol 2019;2019:1029092. [Crossref] [PubMed]
- Chou WC, Chang KP, Lu CH, et al. Complementary role of the Memorial Sloan Kettering Cancer Center nomogram to the American Joint Committee on Cancer system for the prediction of relapse of major salivary gland carcinoma after surgery. Head Neck 2017;39:860-7. [Crossref] [PubMed]
- Wang Q, Xia D, Bai W, et al. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol 2019;70:893-903. [Crossref] [PubMed]
- Qiu Y, Wang T, Yang X, et al. Development and Validation of Artificial Neural Networks for Survival Prediction Model for Patients with Spontaneous Hepatocellular Carcinoma Rupture After Transcatheter Arterial Embolization. Cancer Manag Res 2021;13:7463-77. [Crossref] [PubMed]
- Cheng YT, Teng W, Lui KW, et al. MELD score is the better predictor for 30-day mortality in patients with ruptured hepatocellular carcinoma treated by trans-arterial embolization. Am J Cancer Res 2021;11:3726-34. [PubMed]
(English Language Editor: J. Gray)


