
Perioperative lymphocyte-to-monocyte ratio changes plus CA199 in predicting the prognosis of patients with gastric cancer
Introduction
Gastric cancer (GC) is one of the most common malignant tumors of the digestive system, the fifth most common cancer worldwide, and the third leading cause of cancer-related death (1). China has a high incidence of GC and most patients have already developed advanced GC at the time of diagnosis. At present, radical gastrectomy is the gold standard treatment for GC globally, and postoperative adjuvant chemotherapy is also a traditional treatment model. With the improvement of diagnostic techniques and treatments, the incidence and mortality rates of GC have decreased over the last half-century; in particular, the discovery of widely available inexpensive biomarkers that assist in diagnosis, prognosis, determination of the need for adjuvant therapy, and efficacy monitoring. Yet, the prognosis of GC remains poor.
Thus, identifying appropriate prognostic factors is essential for stratifying patients with a high risk of mortality and facilitating future intensive adjuvant therapies and surveillance planning. In clinical practice, the tumor-node-metastasis (TNM) cancer staging system, developed by the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC), has been used to predict cancer prognosis. However, other important prognostic factors, such as age, gender, primary tumor location, depth of invasion, and cancer embolus, were not included. Moreover, patients with the same TNM stage of malignant tumors often exhibit different prognoses, which will have a significant impact on future treatment options (2). Therefore, it is necessary to identify other reliable biomarkers to predict the prognosis of patients with GC.
In the processes of tumorigenesis, development, and metastasis, the specific survival and progression of GC are affected by both the characteristics of the tumor itself and those of the host. Obtaining in vivo tumor data by non-invasive and simple methods to understand the characteristics of the tumor itself has always been a particular focus of research. In recent years, serum tumor markers (STMs) have gradually entered people’s field of vision, and have been widely used in clinical practice due to their convenience and ease of acceptance by patients. Elevated preoperative STM is often associated with tumor progression, leading to poor prognosis in patients with malignant tumors. Among them, carbohydrate antigen 199 (CA199) is an STM that has been used for malignant digestive tumors. It can be detected in the gastrointestinal, hepatobiliary and pancreatic systems, salivary glands and other normal epithelial tissues. Elevated CA199 has been widely used to screen for GC, pancreatic cancer, colorectal cancer and other digestive tract malignant tumors (3,4). During the progression of GC, tumor cells stay in a hypoxic environment for a longer time, resulting in elevated CA199 levels. Therefore, elevated levels of CA199 in gastric adenocarcinoma patients may indicate more aggressive tumor in this patient. Some studies have found that the positive rate of CA199 in GC before surgery increases with the increase of TNM stage, so serum CA199 can be used as a biomarker to predict the prognosis of GC patients (5,6). In the process of tumorigenesis, development and metastasis, its specific survival and progression are affected not only by the characteristics of the tumor itself, but also by the host. As described in the main text, lymphocyte-to-monocyte ratio (LMR) is associated with host immunity, but only indicates aspects of host defense in tumor development, from which the tumor’s own properties are not reflected. CA199 shows the hypoxic microenvironment during the development of GC, and is a non-invasive and simple method to understand the characteristics of the tumor itself. Using a single inflammatory marker as a measure of prognosis in cancer patients is a simplification of a complex system. Therefore, combining markers from these two aspects can develop better prognostic markers. However, since STM only indicates the characteristics of the tumor itself and does not consider the condition of the host, it usually has the problems of low specificity, low sensitivity, and a high false positive rate. Therefore, a comprehensive indicator that can better balance the relationship between tumor and host is needed. Coincidentally, the study by Ma et al. confirmed that STMs combined with inflammation-related indicators could be used in the prognostic analysis of GC patients (7).
The influence of the host on tumor survival and progression, mainly through systemic inflammatory response (SIR) and host immune response, is not only highly correlated with postoperative survival in patients with various cancers (8) but is also an important mediator of clinical benefit from surgery and chemotherapy. A study on SIR have shown that systemic inflammation markers (SIMs) are important prognostic factors in various tumors (7). These routine parameters from peripheral blood, including the ratios of neutrophils, lymphocytes, monocytes, platelets, and different blood cells, have been widely developed as prognostic biomarkers in patients with solid tumors. It is worth noting that lymphocytes and monocytes are the key immune cells in peripheral blood, and the changes in their counts can reflect the inflammatory status of the body and its response to malignant tumors. Previous research on the LMR also confirmed that the preoperative LMR can be used as an independent prognostic factor to guide treatment in many tumors, including GC, ovarian cancer, penile cancer, lung cancer, etc. (9-12). However, despite its potential as a prognostic factor for GC, preoperative LMR is susceptible to surgery and chemotherapy (13). Therefore, only longitudinal observation of the dynamic change value of LMR can more objectively reflect the influence of the host on the tumor.
Current research has confirmed the effect of the dynamic changes in SIM after surgery and chemotherapy on the survival of cancer patients (14-16). For example, a study has confirmed that the dynamic changes of perioperative LMR are closely related to the prognosis of patients with non-small cell lung cancer (NSCLC) (17). Moreover, Lin et al. showed that the re-measurement of the LMR after surgery helped to predict the long-term survival of GC patients (13). However, the prognostic significance of perioperative LMR dynamic changes in patients with GC has not yet been explored.
In the process of tumor development, tumor characteristics and host immune defense response are two key aspects. As described in the text, LMR is associated with host immunity, but only indicates the host defense aspect of tumor development and does not reflect the properties of the tumor itself. CA199 directly reflects the malignant potential of cancer, which complements this deficiency. And using a single inflammatory marker or tumor marker as a measure of prognosis in cancer patients is a simplification of a complex system. Therefore, better prognostic markers can be developed only by combining markers from these two aspects. We believe that the dynamic changes of perioperative LMR have limited predictive power for the prognosis of GC patients. Because only the inflammatory response brought by surgery to the patient and the immune defense response caused by the tumor are considered, but the invasiveness of the tumor itself is ignored, and CA199 makes up for this defect well. To our knowledge, this is the first time that the dynamic changes of LMR and tumor markers are combined to construct a novel prognostic model to predict the prognosis of GC patients.
Therefore, this study aimed to investigate the value of the dynamic change of perioperative LMR (ΔLMR; postoperative LMR minus preoperative LMR) in predicting the prognosis in patients with GC who underwent radical gastrectomy, and then to further explore whether the combination of ΔLMR with preoperative CA199 to construct a comprehensive index would further improve the prognostic accuracy. To our knowledge, this is the first tumor prognostic marker created by taking into account the characteristics of the tumor itself as well as the influence of the host on the tumor. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-411/rc).
Methods
Ethics
This retrospective study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Research Ethics Committees of Nantong Tumor Hospital and the Affiliated Hospital of Nantong University (No. 2022-A 04 and No. 2022-K053-01). Since this was a retrospective study, informed consent from the patients was not required.
Patients
The information of patients who underwent radical gastrectomy between 2010 and 2016 was collected from the databases of two institutional medical centers. The inclusion criteria were as follows: (I) patients with pathologically confirmed primary gastric adenocarcinoma; (II) patients that underwent total or partial radical gastrectomy; (III) those with pathologic TNM (pTNM) stage I to III according to pathological diagnosis after surgery; and (IV) patients that did not receive any treatment before surgery, such as neoadjuvant chemoradiotherapy.
A total of 1,894 patients were collected, and those who were lost to follow-up (n=118), with non-primary GC (n=96), with serious missing data (n=801), with benign status (n=134), with non-adenocarcinoma (n=59), and with TNM staging IV (n=12), and those who received neoadjuvant or palliative chemotherapy only (n=8) were excluded. Finally, 666 patients were included, of which 456 from the Affiliated Hospital of Nantong University were classified as the training set, and 210 from the Nantong Tumor Hospital were included as the validation set. The last follow-up date was August 13, 2020. Overall survival (OS) was the outcome in this study, which was defined as the period from the date of radical gastrectomy to the date of death or last follow-up. The OS outcome was collected blinded to clinical information.
Data collection
We collected and summarized the clinicopathological characteristics of the patients, including age, sex, tumor location, ulceration, cancer embolus, perineural invasion, depth of infiltration (T stage), lymph node status (N stage), and TNM stage. TNM stage was redefined according to the 8th Edition AJCC guidelines. The patients’ peripheral complete blood counts, including lymphocytes, monocytes, and tumor marker CA199 level, were checked regularly in all patients 1 week before and after radical gastrectomy by two technicians who were blinded to their clinical characteristics. Preoperative serum levels of lymphocytes and monocytes were measured within 7 days before gastrectomy, while postoperative measurement of lymphocytes and monocytes levels were usually measured at the 1 week after surgery and before administration of adjuvant chemotherapy. The LMR was calculated by dividing the lymphocyte count by the monocyte count in the peripheral blood. ΔLMR could be obtained by subtracting the preoperative LMR from the postoperative LMR, as shown in the formula:
Continuous variables were transformed into categorical ones to make the statistical analysis more convenient and objective. The serum CA199 level was determined through a latex immunoassay (Mitsubishi Chemical Ltd., Japan), and its normal value was 37 ng/mL. This cutoff value has been widely used clinically, and clinicians can make judgments more quickly. The ΔLMR is the LMR, with no recognized normal value. The receiver operating characteristic (ROC) curve was used to calculate the Youden index to determine the maximum value, which was used as the optimal critical value of ΔLMR, as is shown in Figure S1. The cutoff value of ΔLMR was −2.73.
ΔLMR and CA199 were combined to establish the ΔLMR-CA199 score, which was defined as follows: a score of 0 denoted both low ΔLMR (≤−2.73) and low CA199 (≤37); a score of 1 represented low ΔLMR (≤−2.73) and high CA199 (>37) or high ΔLMR (>−2.73) and low CA199 (≤37); and a score of 2 signified both high ΔLMR (>−2.73) and high CA199 (>37).
Statistical analysis
SPSS version 25 and Windows statistical software (IBM Corp., NY, USA) were used for all statistical analyses. Continuous variables were expressed as ranges and medians, and categorical variables were represented as percentages and frequencies. The comparison of the differences in categorical variables between the groups was conducted using the chi-square test. The log-rank test and Kaplan-Meier approach were used to compare results, including OS.
The Cox risk regression model was employed to distinguish the risk factors related to prognosis, and the 95% confidence intervals (CIs) and hazard ratios (HRs) were calculated. In univariate analyses, the variables with P<0.05 were included in the multivariate analyses. All statistical analyses were two-tailed, with P<0.05 indicating a statistically significant difference. Finally, nomograms with several significant prognostic factors identified using the Cox hazard models was established using R software (version 4.0.3; https://www.r-project.org/). We further assessed the performance of the nomogram using Harrell’s concordance index (C-index), where a larger value indicates higher prognostic value. Calibration curves for our nomogram were depicted to explain the concordance between the predicted survival and the observed survival. The statistical analysis was performed using R software (version 4.0.3).
Results
Patient characteristics
The data of 1,894 patients between 2010 and 2016 from two institutional medical centers were collected, excluding patients who were lost to follow-up (n=118), with non-primary GC (n=96), with serious missing data (n=801), with benign status (n=134), with non-adenocarcinoma (n=59), and with TNM staging IV (n=12), and who underwent neoadjuvant or palliative chemotherapy only (n=8). Finally, 666 patients with primary GC who underwent total or partial radical gastrectomy were included, of which 456 were from Affiliated Hospital of Nantong University (training set) and 210 were from the Nantong Tumor Hospital (validation set) according to the same enrollment criteria. The entire patient screening process is shown in Figure 1.
The training set comprised 456 patients, including 314 men (68.86%) and 142 women (31.14%). The median age of the patients was 64 (range, 22–88) years. These patients were classified into TNM I, II, and III stages, with 141 (31.33%), 110 (24.44%), and 199 (44.22%) patients, respectively. The median OS time was 99.93 (range, 0.63–127.07) months. The validation set comprised 210 patients, including 141 men (67.14%) and 69 women (32.86%), with a median age of 64 (range, 22–95) years. In total, 37 (17.87%), 42 (20.29%), and 128 (61.84%) patients were in the TNM I, II, and III stages, respectively. The median OS time for patients in the validation set was 60.97 (range, 2.37–104.53) months. The clinicopathological characteristics and hematological indexes of all included patients are summarized in Table 1.
Table 1
| Characteristics | Training set | Validation set | |||||
|---|---|---|---|---|---|---|---|
| Patients (%) | OS (months)m | P value | Patients (%) | OS (months)m | P value | ||
| Age (years) | 0.263 | 0.052 | |||||
| ≤60 | 38.38 | 99.93 | 35.71 | 61.03 | |||
| >60 | 61.62 | 99.95 | 64.29 | 60.82 | |||
| Sex | 0.958 | 0.314 | |||||
| Male | 68.86 | 99.82 | 67.14 | 61.10 | |||
| Female | 31.14 | 100.37 | 32.86 | 60.67 | |||
| Tumor location | 0.411 | 0.147 | |||||
| Upper | 21.60 | 99.67 | 11.06 | 63.50 | |||
| Middle | 22.72 | 99.12 | 39.42 | 61.10 | |||
| Lower | 55.68 | 100.43 | 49.52 | 59.30 | |||
| Ulceration | 0.173 | 0.560 | |||||
| No | 35.29 | 99.93 | 55.39 | 60.67 | |||
| Yes | 64.71 | 100.12 | 44.61 | 61.44 | |||
| Adjuvant chemotherapy | 0.165 | 0.985 | |||||
| Yes | 34.88 | 99.62 | 53.33 | 60.85 | |||
| No | 65.12 | 100.67 | 46.67 | 60.97 | |||
| T stage | <0.001 | 0.003 | |||||
| T1 | 23.56 | 103.70 | 14.98 | 64.97 | |||
| T2 | 15.78 | 101.03 | 14.49 | 62.10 | |||
| T3 | 40.44 | 90.50 | 21.74 | 48.77 | |||
| T4 | 20.22 | 99.60 | 48.79 | 59.30 | |||
| Lymph node metastasis | <0.001 | <0.001 | |||||
| No | 41.69 | 103.47 | 26.09 | 63.97 | |||
| Yes | 58.31 | 95.40 | 73.91 | 59.10 | |||
| Cancer embolus | 0.004 | <0.001 | |||||
| No | 88.47 | 100.43 | 55.91 | 62.15 | |||
| Yes | 11.53 | 38.13 | 44.09 | 48.50 | |||
| Perineural invasion | <0.001 | 0.223 | |||||
| No | 83.81 | 100.77 | 50.00 | 62.07 | |||
| Yes | 16.19 | 46.00 | 50.00 | 60.03 | |||
| TNM stage | <0.001 | 0.001 | |||||
| I | 31.33 | 103.75 | 17.87 | 64.13 | |||
| II | 24.44 | 101.52 | 20.29 | 64.38 | |||
| III | 44.22 | 53.10 | 61.84 | 48.42 | |||
| CA199 (U/mL) | 0.001 | <0.001 | |||||
| ≤37 | 82.31 | 100.77 | 83.98 | 62.13 | |||
| >37 | 17.69 | 52.62 | 16.02 | 34.83 | |||
| ΔLMR | 0.001 | <0.001 | |||||
| ≤−2.73 | 67.36 | 100.43 | 55.32 | 62.17 | |||
| >−2.73 | 32.64 | 95.07 | 44.68 | 45.65 | |||
SIR, systemic inflammatory response; GC, gastric cancer; OS (months)m, median overall survival; TNM, tumor-node-metastasis; CA199, carbohydrate antigen 199; ΔLMR, postoperative lymphocyte-monocyte ratio minus preoperative lymphocyte-monocyte ratio.
Univariate and multivariate analyses of the prognostic factors associated with OS
As shown in Table 2, the results of the univariate analysis in the training set indicated that OS was related to lymph node metastasis (P<0.001), T stage (P<0.001), perineural invasion (P<0.001), cancer embolus (P<0.001), CA199 (P<0.001), TNM stage (P<0.001), and ΔLMR (P<0.001). Subsequently, the multivariate analysis of these variables was performed, which showed that T (P=0.017), TNM stage (P<0.001), CA199 (P=0.038), and ΔLMR (P=0.009; Table 2) are the independent prognostic factors for GC.
Table 2
| Characteristics | Training set | Validation set | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age (years) | 1.164 (0.849–1.594) | 0.345 | 1.639 (0.991–2.709) | 0.054 | |||||||
| ≤60 | |||||||||||
| >60 | |||||||||||
| Sex | 0.971 (0.702–1.343) | 0.857 | 1.154 (0.703–1.895) | 0.571 | |||||||
| Male | |||||||||||
| Female | |||||||||||
| Tumor location | 0.890 (0.743–1.065) | 0.203 | 1.369 (0.951–1.97) | 0.091 | |||||||
| Upper | |||||||||||
| Middle | |||||||||||
| Lower | |||||||||||
| Ulceration | 1.232 (0.890–1.706) | 0.208 | 1.102 (0.696–1.746) | 0.679 | |||||||
| No | |||||||||||
| Yes | |||||||||||
| Adjuvant chemotherapy | 0.719 (0.516–1.001) | 0.051 | 0.974 (0.620–1.530) | 0.910 | |||||||
| Yes | |||||||||||
| No | |||||||||||
| T | 1.544 (1.329–1.794) | <0.001 | 0.017 | 1.390 (1.103–1.751) | 0.005 | 0.420 | |||||
| T1 | Reference | Reference | |||||||||
| T2 | 1.887 (0.815–4.372) | 0.138 | 0.941 | ||||||||
| T3 | 1.815 (0.630–5.227) | 0.269 | 0.590 | ||||||||
| T4 | 0.953 (0.308–2.946) | 0.934 | 0.678 | ||||||||
| Lymph node metastasis | 3.528 (2.446–5.088) | <0.001 | 0.120 | 3.526 (1.692–7.347) | 0.001 | 0.238 | |||||
| No | |||||||||||
| Yes | |||||||||||
| Cancer embolus | 2.009 (1.405–3.134) | <0.001 | 0.209 | 3.051 (1.843–5.049) | <0.001 | 2.772 (1.536–5.004) | 0.001 | ||||
| No | |||||||||||
| Yes | |||||||||||
| Perineural invasion | 2.176 (1.528–3.098) | <0.001 | 0.190 | 1.323 (0.82–2.136) | 0.252 | ||||||
| No | |||||||||||
| Yes | |||||||||||
| TNM stage | 2.345 (1.896–2.902) | <0.001 | <0.001 | 2.135 (1.439–3.168) | <0.001 | 0.333 | |||||
| I | Reference | Reference | |||||||||
| II | 1.033 (0.424–2.518) | 0.943 | 0.343 | ||||||||
| III | 3.127 (1.220–8.013) | 0.018 | 0.157 | ||||||||
| CA199 (U/mL) | 2.067 (1.419–3.010) | <0.001 | 1.567 (1.026–2.394) | 0.038 | 3.419 (1.958–5.969) | <0.001 | 2.879 (1.436–5.771) | 0.003 | |||
| ≤37 | |||||||||||
| >37 | |||||||||||
| ΔLMR | 1.836 (1.320–2.554) | <0.001 | 1.623 (1.127–2.338) | 0.009 | 2.196 (1.29–3.738) | 0.004 | 2.289 (1.189–4.405) | 0.013 | |||
| ≤−2.73 | |||||||||||
| >−2.73 | |||||||||||
SIR, systemic inflammatory response; OS, overall survival; HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis; CA199, carbohydrate antigen 199; ΔLMR, postoperative lymphocyte-monocyte ratio minus preoperative lymphocyte-monocyte ratio.
Similarly, the results of the univariate analysis in the validation cohort suggested that OS was obviously associated with the following factors: T (P=0.005), lymph node metastasis (P<0.001), cancer embolus (P<0.001), TNM stage (P<0.001), CA199 (P<0.001), and ΔLMR (P=0.004). The multivariate analysis suggested that cancer embolus (P=0.001), CA199 (P=0.003), and ΔLMR (P=0.013) were correlated with OS, as shown in Table 2.
Combining ΔLMR and CA199 to create the ΔLMR-CA199 score
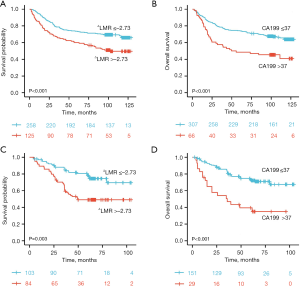
In both the validation and training set, Kaplan-Meier survival analysis indicated that patients with high CA199 (>37 U/mL) and high ΔLMR (>−2.73) had a worse prognosis compared to those with low CA199 (≤37 U/mL) and low ΔLMR (≤−2.73) (Figure 2: all P<0.05). Thus, ΔLMR and CA199 were combined to establish the ΔLMR-CA199 score. According to the aforementioned scoring criteria, all of the patients could be classified into low-risk (scored 0), medium-risk (scored 1), and high-risk (scored 2) groups. The survival outcomes were remarkably different between the three groups (P<0.001), as illustrated in Figure 3A. This obvious difference was also found in the validation set (Figure 3B; P<0.001), suggesting that the higher the ΔLMR-CA199 risk score, the worse the prognosis.
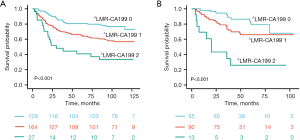
Prognostic role of ΔLMR-CA199 in advanced GC
According to the outcomes of the univariate analysis, the TNM stage was found to be a prognostic factor related to GC, regardless of whether it was in the validation or training set (Table 2). As shown in Figure S2A,S2B, significant survival differences were observed in patients with TNM stages I–III and in the training set between the three risk-stratified groups. However, in the validation set, significant survival differences were only found in patients with TNM stages II–III between the groups (Figure S2C,S2D). We believe that ΔLMR-CA199 could be used to more accurately identify advanced GC patients with poor prognosis.
Prognostic role of ΔLMR-CA199 in postoperative adjuvant chemotherapy
Moreover, 218 (45.61%) patients with advanced GC received additional postoperative adjuvant chemotherapy after curative gastrectomy. As shown in Figure S3A, we found that the ΔLMR-CA199 score was significantly different in patients with stage II–III GC who received postoperative adjuvant chemotherapy between the three risk-stratified groups. We further explored the response of these three groups of patients to postoperative adjuvant chemotherapy based on the ΔLMR-CA199 score. As shown in Figure S3B-S3D, significant survival differences were observed in patients with a ΔLMR-CA199 risk score of 1, but not in those with ΔLMR-CA199 risk scores of 0 or 2. In other words, adjuvant chemotherapy after surgery was more beneficial for patients with a ΔLMR-CA199 score of 1.
Construction and verification of a nomogram
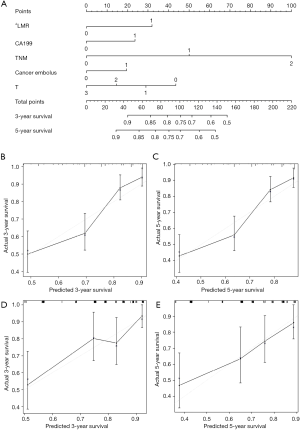
Combining the comprehensive multivariate Cox analysis results, five factors were used to construct a nomogram to predict the 3- and 5-year OS of patients with GC in the training set. The nomogram is shown in Figure 4A. First, the C-index was used to evaluate the reliability of this nomogram. The C-index for the validation and training sets was 0.693 and 0.710, respectively, indicating that the nomogram had good predictive power. The calibration curves were then employed to verify the predictive value of the nomogram. As shown in Figure 4B,4C, the calibration curves of the 3- and 5-year survival in the training set were very similar to those predicted by the nomogram and were also matched in the validation set (Figure 4D,4E).
Discussion
Although radical gastrectomy is the gold standard treatment for GC, a considerable number of patients still have a short survival time after radical resection (18). The most common causes of death are local recurrence and distant metastasis. Cancer-specific survival and disease progression are affected on the one hand by the characteristics of the tumor itself, and on the other by the host SIR. STM and SIM are meaningful indicators of these two aspects, respectively. In recent years, a previous study has shown that the combination of the two is likely to become a novel biomarker for predicting long-term prognosis and guiding treatment in cancer patients (18). Both CA199 and LMR have been reported to be important prognostic factors in GC. However, surgery and chemotherapy are important factors that affect the LMR levels in patients with GC (14,15). In the past, most studies on GC were mainly limited to detecting the preoperative LMR level for prognostic evaluation. Few studies paid attention to the dynamic changes of perioperative LMR in GC patients. In this study, we aimed to investigate the effect of the longitudinal change in LMR before and after surgery (ΔLMR) in GC patients on long-term prognosis and to further explore whether the combination of CA199 and ΔLMR could improve the prognostic accuracy. The effectiveness of LMR and CA199 in predicting prognosis and monitoring efficacy in GC patients has been explored since their discovery. To date, guidelines still do not recommend CA199 as an effective marker for GC, and the behavior and usefulness of the combination of ΔLMR and CA199 has not been sufficiently studied to make any guideline-oriented recommendations.
Increasing evidence suggests that SIR is highly associated with the occurrence, development, metastasis, and prognosis of various cancers (19-21). Associated immune inflammatory cells play a major role in SIR. In our study, we found that ΔLMR was an independent prognostic factor for GC in both cohorts. Possible reasons for this are as follows. Firstly, lymphocytes make up one-third of peripheral blood leukocytes, and play a leading role in the cell-mediated immune system, possibly helping to eliminate tumor cells (22,23). Alternatively, monocytes can promote tumor progression by inducing angiogenesis and immunosuppression in the tumor microenvironment (23). Therefore, it is not surprising that the LMR can be employed to predict the prognosis of GC patients, as it may reflect the SIR that inhibits both tumor development as well as the metastatic process. Secondly, this perioperative change in the LMR could be due to curative gastrectomy, which removes the tumor burden from the host. That is, the stress associated with surgery and postoperative inflammation might exacerbate the change in LMR during the perioperative period. In contrast, static LMR has limitations in predicting whether a tumor would return to a new equilibrium because inflammation is a dynamic process. Hence, the persistence of higher ΔLMR compared to preoperative LMR might indicate the presence of long-term chronic inflammatory responses, which could predict a poor prognosis in cancer patients. Lin et al. studied the influence of changes in SIM before and after surgery on the prognosis of GC. Their results indicated that ΔLMR was related to the prognosis of GC (13). Consistent with their results, the present study found that the elevated ΔLMR in patients with GC tended to indicate a poor prognosis. These results all suggest that ΔLMR has a better prognostic value than static LMR.
In our study, we found that CA199 was an independent prognostic factor for GC in both cohorts. Tumor markers are important indicators of the existence of tumors in the body. Increased levels of tumor markers may reveal the existence of relatively highly malignant tumors and aggressive metastasis (24). A previous study has suggested that tumor markers should be included in the AJCC TNM staging system to improve the prognostic prediction for colorectal cancer (25). On the contrary, the guidelines of the Japanese Gastric Cancer Association (JGCA), the National Comprehensive Cancer Network (NCCN), and the European Society for Medical Oncology (ESMO) did not recommend the incorporation of preoperative tumor markers, such as CA199, into the prognosis of GC (26-28). As one of the members of the Lewis family, CA199 is also called sialic acid A and has been confirmed to have unique diagnostic and prognostic values in malignant digestive tract tumors. Many previous reports have explored the significance of CA199 in predicting the prognosis of GC (3,4,29). The same results were also found in this study. Kannagi et al. showed that elevated CA199 levels were significantly correlated with the depth of gastric tumor infiltration. They explained that during the progression of GC, tumor cells spend a longer time in the hypoxic environment, resulting in an increased level of CA199 (30). It is worth mentioning that there is no clear correlation between postoperative CA199 levels and the prognosis of cancer patients. Given that CA199 loses its predictive significance after the solid tumor disappears from the body owing to surgical resection, only the preoperative CA199 level was discussed in this study. In our study, based on multivariate Cox analysis, the CA199 level was found to independently predict the prognosis of GC patients.
Since STM only indicates the characteristics of the tumor itself, and SIM only indicates the influence of the host on the tumor, using either of the two indicators alone cannot accurately predict the prognosis of cancer patients. Therefore, a comprehensive indicator that can better balance the relationship between tumor and host is needed. Surprisingly, Ma et al.’s view concurs with ours, and their study showed that STM combined with other inflammatory markers can better determine the prognosis of GC patients (7). Therefore, based on the results of the multivariate analysis performed in this study, the ΔLMR and CA199 were combined to explore their prognostic capability. It was found that compared to a single marker, ΔLMR-CA199 as a comprehensive marker could better predict the prognosis of GC patients in both the training and validation sets. In addition, considering that most patients with advanced GC receive postoperative adjuvant therapy, our further stratified analysis showed that patients with a ΔLMR-CA199 score of 1 were more likely to benefit from postoperative adjuvant chemotherapy than those with a score of 0 or 2. Vyas et al. suggested that chemotherapy-enhanced inflammation may lead to treatment failure and metastasis (31), which could explain these results. The reported preoperative positivity rate for tumor markers is approximately 30%. Although CA199 may reflect the prognosis after radical gastrectomy, its specificity is still low. And ΔLMR-CA199 can detect poor prognosis more sensitively than the two alone. In addition, changes in ΔLMR-CA199 even after initiation of chemotherapy made it possible to assess the treatment response to chemotherapy and thus to change the regimen appropriately. The known reasons accounting for the association between higher ΔLMR-CA199 risk score and worse oncological outcomes of GC are summarized in Figure 5.
However, this study had some limitations that should be noted. Firstly, although this was a multi-center study, selection bias was almost inevitable because of the retrospective nature, and further prospective studies are needed to determine whether our findings are generally applicable. Secondly, only OS outcomes were considered for GC prognosis, and data on disease-free survival and progression-free survival were lacking. Thirdly, since the two included hospitals did not regularly test patients for other tumor markers that may be related to GC, such as carcinoembryonic antigen (CEA), CA72-4, etc., this study could not compare CA199 with other tumor markers. We hope that future research will rectify this deficiency. Fourthly, this study focused on gastric adenocarcinoma, and other pathological types were not included. Finally, dichotomous categorization using cutoff values, despite being a commonly applied method in previous clinical research, can lead to several problems. Therefore, decreased statistical power and incomplete correction for confounding factors could be unavoidable.
In conclusion, this study confirmed the significance of ΔLMR in the prediction of GC prognosis through large-scale multi-center data analysis and identified for the first time that ΔLMR-CA199 can more accurately predict the prognosis of GC patients. In addition, we also found that postoperative adjuvant chemotherapy may be more beneficial for patients with a ΔLMR-CA199 score of 1. These findings add to our understanding of the changes of postoperative tumor-host inflammatory status and tumor markers, as well as the prognostic value of inflammatory markers in GC patients. Firstly, ΔLMR may be a better predictor of tumor response and survival time than static LMR, which may be related to changes in lymphocytes during treatment. Secondly, the combination of STM and SIM may better predict the prognosis of cancer patients, facilitating better stratification of cancer patients and individualized postoperative treatment, and also providing new directions for future tumor biomarker research.
Acknowledgments
The authors appreciate Affiliated Hospital of Nantong University and Nantong Tumor Hospital for their valuable support in data collection and follow-up.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-411/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-411/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-411/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Research Ethics Committees of Nantong Tumor Hospital and the Affiliated Hospital of Nantong University (No. 2022-A 04 and No. 2022-K053-01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zhu MH, Zhang KC, Yang ZL, et al. Comparing prognostic values of the 7th and 8th editions of the American Joint Committee on Cancer TNM staging system for gastric cancer. Int J Biol Markers 2020;35:26-32.
- Feng F, Tian Y, Xu G, et al. Diagnostic and prognostic value of CEA, CA19-9, AFP and CA125 for early gastric cancer. BMC Cancer 2017;17:737. [Crossref] [PubMed]
- Shimada H, Noie T, Ohashi M, et al. Clinical significance of serum tumor markers for gastric cancer: a systematic review of literature by the Task Force of the Japanese Gastric Cancer Association. Gastric Cancer 2014;17:26-33. [Crossref] [PubMed]
- Huang ZB, Zhou X, Xu J, et al. Prognostic value of preoperative serum tumor markers in gastric cancer. World J Clin Oncol 2014;5:170-6. [Crossref] [PubMed]
- Uda H, Kanda M, Tanaka C, et al. Perioperative Serum Carcinoembryonic Antigen Levels Predict Recurrence and Survival of Patients with Pathological T2-4 Gastric Cancer Treated with Curative Gastrectomy. Dig Surg 2018;35:55-63. [Crossref] [PubMed]
- Ma Y, Lin J, Lin J, et al. A novel prognosis marker based on combined preoperative carcinoembryonic antigen and systemic inflammatory response for resectable gastric cancer. J Cancer 2021;12:927-35. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Xu Z, Xu W, Cheng H, et al. The Prognostic Role of the Platelet-Lymphocytes Ratio in Gastric Cancer: A Meta-Analysis. PLoS One 2016;11:e0163719. [Crossref] [PubMed]
- Urabe M, Yamashita H, Seto Y. Pretreatment Neutrophil to Lymphocyte Ratio Independently Predicts Disease-specific Survival in Patients With Resectable Gastroesophageal Junction and Gastric Cancer. Ann Surg 2017;266:e76-7. [Crossref] [PubMed]
- Lin JP, Lin JX, Cao LL, et al. Preoperative lymphocyte-to-monocyte ratio as a strong predictor of survival and recurrence for gastric cancer after radical-intent surgery. Oncotarget 2017;8:79234-47. [Crossref] [PubMed]
- Wang K, Diao F, Ye Z, et al. Prognostic value of systemic immune-inflammation index in patients with gastric cancer. Chin J Cancer 2017;36:75. [Crossref] [PubMed]
- Lin JX, Wang ZK, Huang YQ, et al. Dynamic Changes in Pre- and Postoperative Levels of Inflammatory Markers and Their Effects on the Prognosis of Patients with Gastric Cancer. J Gastrointest Surg 2021;25:387-96. [Crossref] [PubMed]
- Yalon M, Toren A, Jabarin D, et al. Elevated NLR May Be a Feature of Pediatric Brain Cancer Patients. Front Oncol 2019;9:327. [Crossref] [PubMed]
- Chan JCY, Diakos CI, Chan DLH, et al. A Longitudinal Investigation of Inflammatory Markers in Colorectal Cancer Patients Perioperatively Demonstrates Benefit in Serial Remeasurement. Ann Surg 2018;267:1119-25. [Crossref] [PubMed]
- Templeton AJ, Knox JJ, Lin X, et al. Change in Neutrophil-to-lymphocyte Ratio in Response to Targeted Therapy for Metastatic Renal Cell Carcinoma as a Prognosticator and Biomarker of Efficacy. Eur Urol 2016;70:358-64. [Crossref] [PubMed]
- Song YJ, Wang LX, Hong YQ, et al. Lymphocyte to monocyte ratio is associated with response to first-line platinum-based chemotherapy and prognosis of early-stage non-small cell lung cancer patients. Tumour Biol 2016;37:5285-93. [Crossref] [PubMed]
- Park SH, Lim DH, Sohn TS, et al. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial Ann Oncol 2021;32:368-74. [Crossref] [PubMed]
- Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Klump KE, McGinnis JF. The role of reactive oxygen species in ocular malignancy. Adv Exp Med Biol 2014;801:655-9. [Crossref] [PubMed]
- Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol 1998;10:588-94. [Crossref] [PubMed]
- Hung K, Hayashi R, Lafond-Walker A, et al. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 1998;188:2357-68. [Crossref] [PubMed]
- Kanakis G, Kaltsas G. Biochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP-NETs). Best Pract Res Clin Gastroenterol 2012;26:791-802. [Crossref] [PubMed]
- Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 2000;88:1739-57. [Crossref] [PubMed]
- Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-v49. [Crossref] [PubMed]
- Wang W, Chen XL, Zhao SY, et al. Prognostic significance of preoperative serum CA125, CA19-9 and CEA in gastric carcinoma. Oncotarget 2016;7:35423-36. [Crossref] [PubMed]
- Kannagi R. Carbohydrate antigen sialyl Lewis a--its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J 2007;30:189-209. [PubMed]
- Vyas D, Laput G, Vyas AK. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther 2014;7:1015-23. [Crossref] [PubMed]
(English Language Editor: A. Kassem)




