
Predictive effect of J waves on cardiac compression and clinical prognosis of esophageal tumors: a retrospective study
Introduction
Esophageal tumor is a major health problem associated with high mortality rate (1). The patients with esophageal tumors have poor prognosis with a 5-year survival rate of only 18.8% (2). The early detection and treatment may help in increasing the survival chance of the patients (3). At present, the endoscopy and pathological biopsy are the gold standards for the diagnosis of esophageal cancer (4). However, the appearance of the early stage of esophageal cancer has challenges because it can be located randomly throughout the esophagus tube (5). Additionally, the accurate detection requires experienced physicians and they are often overlooked during endoscopy surveillance (6). Besides, this process adds an additional invasive procedure (7). Thus, there is a pressing need to explore a more accurate and less invasive screening tool to allow the early detection of esophageal tumors.
J wave is common in electrocardiogram (ECG) as a distinctive deflection at the QRS-ST junction (STj), which can follow the QRS complex or be partly buried inside the QRS as notching or slurring (8). Traditionally, J waves were referred to as Osborne waves in hypothermic condition, and are associated with a host of conditions such as hypothermia (9), hypercalcemia (10), subarachnoid hemorrhage (11), myocardial ischemia (12,13), and especially ventricular arrhythmias (14,15). J wave syndromes (JWS) are disorders of ventricular repolarization that are characterized by prominent J waves on ECG, which may lead to lethal ventricular arrhythmias (16). It is considered that JWS consists of Brugada syndrome (BrS) and early repolarization syndrome (ERS) (17). The two syndromes have different characteristics of J waves, but both of them are closely related to transmural distribution of the transient outward current (Ito) (8).
BrS is closely related to genetic factors, yet it can also be caused by cardiac mass compression (18). Tarin et al. (19) reported a Brugada-like pattern ECG caused by compression of the right ventricular outflow tract by a giant mediastinal tumor, which disappeared after surgical resection. Similarly, Asteriou et al. (20) and Nakazato et al. (21) also demonstrated that mediastinal tumor was implicated with the appearance of Brugada-like ECG pattern which returned to normal after the tumor disappeared. Hayashi et al. (22) demonstrated that J waves were associated with the contact of lung tumors with the heart. In terms of esophageal tumors, due to the anatomical characteristics of esophagus close to heart and lungs, they may exert mechanical force to the heart. Interestingly, Sasaki et al. (23) reported a case in which Brugada-like ECG was detected after reconstructive surgery for an esophageal tumor. Taken together, we hypothesized that cardiac compression induced by esophageal tumors may be associated with the etiology of J waves.
Prognostic factors are essential in advancing patient tailored medicine. Several tumor and clinical characteristics may identify patients with a poor prognosis (24). Presently, there have been several prognostic factors for esophageal tumors, such as tumor size, histological grade, and lymph node involvement (24). Previous study has reported that patients with BrS are at high risk for sudden cardiac death (SCD) due to ventricular fibrillation (VF) (25). A recent study investigated the long-term prognosis of patients with JWS and revealed that the presence of global J waves was associated with a higher incidence of VF recurrence in patients with JWS (26). We speculated that J waves may also act as a prognostic factor for patients with esophageal tumors, although no lethal ventricular arrhythmia and other serious heart diseases were found in any of the above reports (19-23).
Therefore, our study aimed to investigate the relationship between J waves and cardiac compression by esophageal tumor and to compare the prediction of J waves on clinical prognosis with that of cardiac compression by esophageal tumor. Our results may provide theoretical basis for explanation the appearance of J waves, as well as predicting the prognosis in patients with esophageal tumors. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-371/rc).
Methods
We conducted this retrospective cohort study by reviewing previous cases, analyzing previous examinations and following up prognostic events. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Shanghai Chest Hospital (No. KS21004). All participants signed the informed consent.
Patient population
Data were retrospectively collected from all patients with esophageal tumors admitted to Shanghai Chest Hospital between August 2016 and November 2020. The inclusion criteria were as follows: (I) patients with complete ECG and CT imaging before treatment; (II) patients whose ECG recording and corresponding chest CT imaging were less than 4 weeks apart; (III) patients without any other chest tumor; and (IV) patients without ventricular block in ECG.
12-lead ECG assessment
ECG images were collected using a digital electrocardiograph (aECG-12PWL, Nalong, China). The 12-lead ECG was recorded with patients in the supine position. It was recorded at rest for 12 s at a paper speed of 25 mm/s, with an amplification of 10 mm/mV. The J wave was defined as an elevation in the STj with an amplitude of ≥0.1 mV and a duration of ≥20 ms in at least two consecutive leads. The waveforms of the J waves were classified according to the standard proposed by Heng et al. (27). The prevalence and demographic characteristics of QRS end notching or slurring and of the associated STj amplitude were observed. Notching was defined as a positive deflection at the end of the QRS complex, and slurring was defined as a slower terminal waveform transitioning from the QRS complex to the ST segment. There were six different morphology types of the J waves in our study, including five conventional types and one mixed type. The different interpretations of morphology types are shown in Table 1. A mixed type was defined by a mixture of types 1 to 4, with STj not exceeding 0.1 mV. The classification of the J waves was also similar to that used by Heng et al. (27), except that V1 to V3 were included in our study (Table 1). The Brugada patterned ECG was defined as a J wave with an amplitude of ≥0.2 mV, and was considered malignant. The morphology of ST segments after J waves was observed and classified according to the method used by Tikkanen et al. (28): (I) type I, with a horizontal/descending ST segment; or (II) type II, with a concave/rapidly ascending ST segment.
Table 1
| J wave classification | J wave patterns according to leads | |||||||
|---|---|---|---|---|---|---|---|---|
| II, III, and aVF | I and aVL, | V4–V6 | I and aVL, V4–V6 | II, III, and aVF, V4–V6 | V1–V3 | Total | ||
| J wave morphology classification | ||||||||
| 1 (QRS end notching, STj ≥0.1 mV) | 8 | 1 | 0 | 0 | 2 | 3 | 14 | |
| 2 (QRS end notching, STj <0.1 mV) | 44 | 3 | 1 | 0 | 1 | 2 | 51 | |
| 3 (QRS end slurring, STj ≥0.1 mV) | 2 | 0 | 0 | 0 | 0 | 0 | 2 | |
| 4 (QRS end slurring, STj <0.1 mV) | 29 | 2 | 0 | 0 | 1 | 0 | 32 | |
| 5 (ST-segment upward sloping, STj ≥0.1 mV, no QRS end notching or slurring) | 14 | 1 | 7 | 0 | 4 | 1 | 27 | |
| Mixed | 9 | 0 | 0 | 2 | 4 | 0 | 15 | |
| Total | 106 | 7 | 8 | 2 | 12 | 6 | 141 | |
| ST-segment patterns | ||||||||
| Type I | 22 | 2 | 1 | 1 | 0 | 4 | 30 | |
| Type II | 84 | 5 | 7 | 1 | 12 | 2 | 111 | |
| J wave amplitude ≥0.2 mV | 26 | 1 | 0 | 0 | 3 | 0 | 30 | |
aVR, augmented voltage, right arm; aVL, augmented voltage, left arm; aVF, augmented voltage, left foot; QRS, QRS wave complex; STj, ST junction.
The presence of J waves in 12-lead ECGs was evaluated by three experienced cardiologists, and two of them independently evaluated all of the ECGs for J waves using the blind method. After the research organizer summarized the results, all ECGs with different conclusions were evaluated by the third cardiologist, who was blinded to the determination of her colleagues.
Chest computed tomography image assessment
A 128-slice spiral CT was applied in our study. CT scans were obtained with a 128-detector row scanner (Brilliance iCT, Philips, USA) at the end of inspiration during a single breath hold. Chest Enhanced Scan (mediastinal) three-dimensional reconstruction technology was applied and enhanced scanning was used. All of the chest CT scans were performed with a section thickness and interval of 5 mm and a pitch of 1.375. All patients were given 100 mL of non-ionic contrast agent intravenously. CT images were collected with the patient in the supine position. The esophageal segment where the esophageal tumor was located was recorded. The criteria for esophageal tumor compressing the heart were as follows: (I) the tumor contacted the pericardium directly; (II) the fat space between esophagus and pericardium had disappeared; and (III) the shape of the heart had changed under the external force of the tumor. Three experienced radiologists evaluated the CT images. Two of them independently analyzed all of the chest CT images and determined whether the esophageal tumor compressed the heart. After the organizer summarized the findings, the third radiologist (who was blinded to the determination of his colleagues) was invited to re-evaluate all of the CT images. The evaluation of CT images was carried out in a blinded manner to the ECG results.
Clinical event assessment
Severe cardiac events were defined as follows: SCD, VF, sustained ventricular tachycardia (VT), or acute coronary syndrome (ACS). We tracked the survival status and severe cardiac events of the patients since the initial admission. Follow-up for clinical events was mainly performed by review of the inpatient or outpatient medical records, Holter recording, and telemetry data. Telephone tracking of events was used as a supplement for patients with incomplete medical records.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software (IBM Co., Armonk, NY, US). Normally distributed continuous variables were expressed as mean ± standard deviation. Qualitative data were expressed in the form of n (%). Group comparisons were conducted using the chi -square test. The odds ratio (OR) and corresponding 95% confidence interval (CI) were calculated to investigate significant differences in the distribution of each characteristic between groups. Univariate and multivariate logistic regression analysis were used to explore whether there was an independent significant association between two clinical variables. The variables with P<0.1 in univariate logistic regression analysis were included in multivariate analysis, and the “enter” method was used to fit the model. The variables with P<0.05 in multivariate analysis were considered to be independently correlated. Differences in the median survival time between groups were compared by Kaplan-Meier cumulative survival curves and by using the log-rank test. Differences in the risk of death between groups were calculated by a Cox proportional hazards model. A two-sided P<0.05 was considered statistically significant.
Results
Baseline characteristics
Between August 2016 and November 2020, a total of 385 patients who suffered from esophageal tumors were included in the initial study group. The final analysis included 273 patients (Figure 1), including 228 males and 45 females, with a mean age of 63.8±7.5 years (range, 36–91 years). Of these, 165 patients underwent surgical resection of esophageal tumor, 211 patients received radiotherapy, and 192 patients received chemotherapy.
J waves occurred in 141 (51.6%) patients. Table 1 shows the characteristics of the J waves, which were far more prevalent in the inferior leads (II, III, and aVF) than other leads. Type II was the most frequent morphology pattern. The presence of J waves with an amplitude ≥0.2 mV was observed in 30 patients (11.0%). No typical Brugada-patterned ECG was observed.
No multifocal esophageal tumors were found in the CT images. Esophageal tumors were located in the cervical segment of the esophagus in seven patients, the upper-thoracic segment in 26 patients, the middle-thoracic segment in 71 patients, and the lower-thoracic segment in 40 patients. Esophageal tumors crossed the upper and middle thoracic segments in 28 patients, and the middle and lower thoracic segments in 101 patients. Cardiac compression by esophageal tumor was observed in 114 (41.8%) patients.
Relationship between J waves and cardiac compression by esophageal tumor
Table 2 shows the relationship between J waves and cardiac compression by esophageal tumor. The sensitivity and specificity of cardiac compression by tumor to the presence of J waves were 78.1% and 67.3%, respectively. The OR of cardiac compression by tumor to J waves was 7.33 (95% CI: 4.21–12.74; P<0.001). The consistency test result of J waves and cardiac compression by tumor showed that kappa coefficient was 0.44 ± 0.05, suggesting that the consistency intensity was medium. The univariate and multivariate logistic regression analysis of the significant factors of J waves were shown in Table S1. In the univariate model, cardiac compression and severe cardiac events were the factors with P<0.1. They were included in multivariate regression analysis. The results showed that cardiac compression was an independent correlation factor of J waves (P<0.001). The correlation between the classification of J waves and prediction of cardiac compression by tumor was analyzed in Table 3 (n=141). Type 2 occurred more frequently in the compression group (36/89, 40.4%) than in non-compression group (15/52, 28.8%).
Table 2
| Group | Cardiac compression by tumor | χ2 | P value | OR (95% CI) | Kappa value | |
|---|---|---|---|---|---|---|
| + (n=114) | − (n=159) | |||||
| J waves (+) (n=141) | 89 (78.1) | 52 (32.7) | 54.72 | <0.001 | 7.33 (4.21–12.74) | 0.44±0.05 |
| J waves (−) (n=132) | 25 (21.9) | 107 (67.3) | ||||
Values are expressed as n (%) or mean ± SD. OR, odds ratio; CI, confidence interval.
Table 3
| J wave classification | Cardiac compression by tumor | χ2 | P value | OR (95% CI) | |
|---|---|---|---|---|---|
| + (n=89) | − (n=52) | ||||
| J wave morphology type | 2.26 | 0.81 | 0.89 (0.72–1.10) | ||
| 1 | 9 (10.1) | 5 (9.6) | |||
| 2 | 36 (40.4) | 15 (28.8) | |||
| 3 | 1 (1.1) | 1 (1.9) | |||
| 4 | 18 (20.2) | 14 (26.9) | |||
| 5 | 16 (18.0) | 11 (21.2) | |||
| Mixed | 9 (10.1) | 6 (11.5) | |||
| ST-segment patterns | 0.21 | 0.65 | 1.22 (0.52–2.85) | ||
| Type I | 69 (77.5) | 42 (80.8) | |||
| Type II | 20 (22.5) | 10 (19.2) | |||
| J wave amplitude ≥0.2 mV | 2.82 | 0.09 | 0.50 (0.22–1.13) | ||
| No | 74 (83.1) | 37 (71.2) | |||
| Yes | 15 (16.9) | 15 (28.8) | |||
Values are expressed as n (%). OR, odds ratio; CI, confidence interval.
No significant difference was observed in the distribution of morphology patterns (P=0.81), ST-segment patterns (P=0.65), and J wave amplitude patterns (P=0.09) between the two groups.
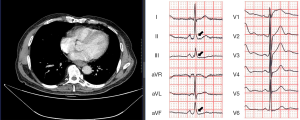
Figure 2 shows a case who presented J waves with type 4 morphology in ECG on admission, and CT image demonstrated that the esophageal tumor compressed the left atrium.
Correlation between the locations of tumors and the leads of J waves
All of the esophageal tumors compressed the heart from the left atrium. The relationship between the locations of the esophageal tumors and the leads presenting J waves was analyzed (Table 4). Patients with J waves in ECGs whose esophageal tumors compressed the heart were included in the analysis (n=89). No tumor was located in the cervical segment of the esophagus in the analysis, and there was no significant correlation between the locations of tumors and the leads presenting J waves (P=0.52).
Table 4
| Leads presenting J waves | Segment of the esophagus where the tumor was located | ||||
|---|---|---|---|---|---|
| Upper-thoracic | Middle- thoracic |
Lower- thoracic |
Upper-middle thoracic | Middle-lower thoracic | |
| I, aVL | 0 (0) | 4 (12.9) | 1 (8.3) | 0 (0) | 2 (5.1) |
| I, aVF, V4–V6 | 0 (0) | 0 (0) | 1 (8.3) | 1 (16.7) | 0 (0) |
| II, III, aVF | 0 (0) | 22 (71.0) | 10 (83.3) | 4 (66.7) | 27 (69.2) |
| II, III, avF, V4–V6 | 0 (0) | 2 (6.5) | 0 (0) | 1 (16.7) | 3 (7.7) |
| V1–V3 | 0 (0) | 3 (9.7) | 0 (0) | 0 (0) | 3 (7.7) |
| V4–V6 | 1 (100.0) | 0 (0) | 0 (0) | 0 (0) | 4 (10.3) |
χ2=0.42, P=0.52. Values are expressed as n (%). Upper-middle thoracic means that the tumor crossed the upper and middle thoracic segments; middle-lower thoracic means that the tumor crossed the middle and lower thoracic segments. aVR, augmented voltage, right arm; aVL, augmented voltage, left arm; aVF, augmented voltage, left foot.
Follow-up
All 273 subjects were tracked for clinical events. Severe cardiac events occurred in 30 (10.9%) patients (14, ACS; 16, sustained VT) during a mean follow-up period of 858.1±460.5 days. Also, 61 patients (22.3%) died during follow-up, among which no SCD occurred. Further analysis showed that the presence of J waves was significantly associated with severe cardiac events (OR =2.84; 95% CI: 1.22–6.63, P=0.01) (Table 5). There was no significant correlation between cardiac compression by tumor and severe cardiac events (P=0.08), though the proportion of patients with compression was higher in the cardiac events group than that in the non-event group (56.7% vs. 39.9%).
Table 5
| Group | Severe cardiac events | χ2 | P value | OR (95% CI) | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Grouped according to J waves | 6.24 | 0.01 | 2.84 (1.22–6.63) | ||
| J waves (+) (n=141) | 22 (73.3) | 119 (49.0) | |||
| J waves (−) (n=132) | 8 (26.7) | 124 (51.0) | |||
| Grouped according to cardiac compression by tumor | 3.08 | 0.08 | 1.97 (0.92–4.24) | ||
| Compression (+) (n=114) | 17 (56.7) | 97 (39.9) | |||
| Compression (−) (n=159) | 13 (43.3) | 146 (60.1) | |||
Values are expressed as n (%). OR, odds ratio; CI, confidence interval.
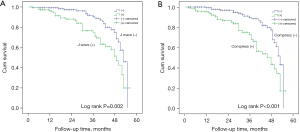
The median survival time of the non-J wave group was longer than that of the J wave group (53.0 vs. 49 months, P=0.002, Figure 3A). The Cox proportional hazards model showed that the presence of J waves was significantly associated with death [hazard ratio (HR) =2.28; 95% CI: 1.35–3.84; P=0.002]. Also, the median survival time of the non-compression group was significantly longer than that of the compression group (48.0 vs. 53.0 months, P<0.001, Figure 3B). The Cox proportional hazards model demonstrated that cardiac compression by esophageal tumor was significantly associated with death (HR =2.51; 95% CI: 1.51–4.17; P<0.001).
A total of 157 patients had ECG and CT image follow-up records at intervals of >6 months, and these subjects were included in the comparative statistics. The amplitude of J waves changed in 44 (28.0%) patients, J waves disappeared in 27 (17.2%) patients, and the morphology pattern of the J waves changed in 10 patients (6.4%). According to CT imaging, cardiac compression was relieved in 61 patients (38.9%). In patients undergoing surgery, J waves disappeared in 15 patients (10.2%), with CT imaging indicating disappearance of cardiac compression.
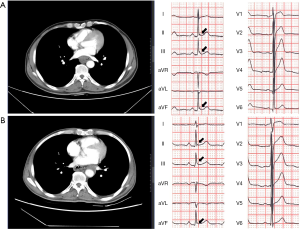
Figure 4 shows a representative case with an esophageal tumor presenting with dynamic J wave changes on 12-lead ECG during the postoperative period. The J wave amplitude decreased to negative on ECG recorded 1 month after resection of the tumor.
Among the 35 (22.3%) patients with decreased J wave amplitude, 19 patients had corresponding CT imaging indicating a change from compression to non-compression. There was a significant correlation between the decrease of J wave amplitude and the corresponding CT imaging changes (P=0.03, Table 6). No corresponding CT imaging changes were observed in nine (5.7%) patients with increased J wave amplitude.
Table 6
| Decrease of J wave amplitude | CT imaging changes, n (%) | χ2 | P value | |
|---|---|---|---|---|
| No change | From compression (+) to compression (−) | |||
| Decrease | 16 (16.7) | 19 (31.1) | 4.52 | 0.03 |
| No decrease | 80 (83.3) | 42 (68.9) | ||
Discussion
- In this study, we report the following findings:
- There was a significant correlation between the presence of J waves and cardiac compression by esophageal tumor. Furthermore, decreased J wave amplitude was significantly correlated with the change from compression to non-compression on CT images.
- There was a significant correlation between severe cardiac events and the presence of J waves, but not with cardiac compression by tumor.
- The presence of J waves and cardiac compression by tumor could both evaluate the survival time of patients with esophageal tumors.
Our study demonstrated that cardiac compression by esophageal tumor could be one cause of J waves. This conclusion may also be applicable when the heart is compressed by other intrathoracic tumors. However, there were some differences between our study and previous case reports. In the case reported by Sasaki et al., the patient underwent resection of the esophagus followed by retro-sternum placement of the stomach. The anterior right ventricle was compressed by that stomach, and transient Brugada-like ECG appeared (23). Similarly, the case reported by Asteriou et al. exhibited a Brugada-like ECG due to the compression of the right ventricular outflow tract caused by a giant mediastinal lipoma (20). The presence of Brugada-like ECG in these two cases corresponded to the location of tumorous compression on the heart.
In our study, all the tumors compressed the heart from the left atrium. The majority of the J waves caused by compression appeared in inferior leads (63/89, 70.8%). The J waves presenting in anterior precordial leads (V1–V3) were observed in only three patients, without any Brugada-like morphology characteristics. It is worth noting that the distribution of J waves caused by tumors in our study was similar to the clinical study of Hayashi et al., where most of the J waves caused by lung tumors also presented in inferior leads (31/40, 77.5%) (22). There was no relationship between the leads of the J waves and the position of tumor contact on the heart both in our study and in the study conducted by Hayashi et al. (22). These phenomena indicate that the mechanical compression of the right ventricle by a large organ or mass may cause Brugada-like ECG, but the compression of the atrium by an intrathoracic tumor may cause J waves. In a study with a certain number of samples, the possibility of the atrium being compressed by mass is much larger than that of the ventricle, making J waves easier to detect. Hayashi et al. (22) speculated that the heart being compressed by lung cancer moves downward, and therefore, the inferior wall attaching to the diaphragm might exert a contact force, resulting in J waves. In the absence of sufficient electrophysiological evidence, we think that this scenario is the most reasonable at present.
The cellular and ionic basis of the Ito-mediated J wave was reported in previous studies. Factors that affect the gating properties of Ito or ventricular stimulation may alter the appearance of J waves (8). Mechano-gated ion channels might be affected by mechanical forces on the myocardium (29), altering Ito availability and contributing to the presence of J waves. Furthermore, some gene mutations have been found in people with JWS (30,31). For example, inherited Brs is related to mutations in several genes. SCN5A mutations are linked to both ischemia JWS as well as BrS (8). The detection of these gene mutations is very helpful in exploring the mechanism of J wave formation due to cardiac compression by tumor. This is expected to be carried out with corresponding conditions.
Unlike the study conducted by Hayashi et al. (22), there was no subject whose J waves appeared with development of esophageal tumor in our study. However, the disappearance of J waves in 15 subjects whose tumors were surgically resected was detected during the follow-up. Obviously, the J waves in these patients were directly related to cardiac compression by tumor. This phenomenon showed that normal ECG could be restored some time after the removal of mechanical compression caused by tumor, but this dynamic change only appeared in a portion of the cases in our study. The sensitivity and specificity of the change from compression to non-compression for decreased J wave amplitude was 31.1% and 83.3%, respectively. These results suggest that most of the change from compression to non-compression may not be predicted by the decrease of J wave amplitude, although the appearance of this dynamic change of J waves cannot be ignored, as it may predict the change from compression to non-compression.
At present, there is no unified scheme for J wave morphological classification. The method used by Heng et al. (27) is a comprehensive, standard, and elaborate one, and was applied in our study. It is worth emphasizing that STj elevation without notching or slurring was also included in the statistics. There was no significant difference in the distribution of morphological classification between the compression and non-compression groups, except the proportion of type 2 J waves between two groups. We cannot compare the results with the data from Heng et al.’s study (27), which was conducted in a White healthy population. The next stage of our research will involve a sufficient number of healthy Yellow subjects as the control group. Tikkanen et al. speculated ST-segment morphology and found that subjects with horizontal/descending ST segments had an increased HR of arrhythmic death (28). In our study, we did not observe an effect of ST segment morphological differences on the predictive value of J waves for compression, nor did J waves with an amplitude >0.2 mV, which were considered to increase the cardiac risk. The distribution of J waves in the compression and non-compression groups was approximately uniform.
Previous studies have demonstrated that the presence of J waves could predict lethal cardiac events. Haïssaguerre et al. reported that an increased prevalence of J waves in the inferior or lateral leads was found in the subjects with a history of idiopathic VF (32). Naruse et al. demonstrated that the presence of J waves was an independent predictor of VF within 48 h after acute myocardial infarction (AMI) (33). Tsuda et al. performed a clinical study that showed that the presence of J waves was significantly associated with cardiac events (SCD, VF, sustained VT, etc.) in hypertrophic cardiomyopathy (34). In our study, there was a significant correlation between J waves and severe cardiac events. However, the presence of J waves was not an independent predictor of severe cardiac events in multivariate regression analysis, possibly due to limited sample size of the present study. Further studies with larger sample sizes are warranted.
According to the eighth edition Staging of Esophageal and Esophagogastric Epithelial Cancers (35), pericardial invasion is defined as stage T4a, which suggests a relatively poor prognosis. Cardiac invasion of esophageal tumors is rare but lethal (36,37). In our study, we found that the cardiac compression by esophageal tumor was negatively correlated with the survival time of patients, although cardiac compression was not statistically associated with severe cardiac events. The presence of cardiac compression by tumor can be seen as an important predictor of esophageal tumor progression. However, we can only observe this phenomenon through imaging, which is not conducive for us to find it in time. The presence of J waves also predicted a poor prognosis in our study, which provides us with a practical and simple way to evaluate the survival time of patients with esophageal tumors. Obviously, the examination method of ECG is easier to be accepted by patients. It should be emphasized that the appearance of J waves can indicate the prognosis of patients with esophageal tumors, but it is not a factor affecting the prognosis.
Limitations
Firstly, echocardiography examination was not included in our study. Echocardiography examination would help to better evaluate the morphological changes of the heart under the influence of mechanical force caused by tumors. Secondly, subjects who had bundle branch block in ECG were excluded, which could have affected our results. Thirdly, healthy Yellow subjects were not included as the control group. Comparing these subjects with the subjects in our study could help to better evaluate the prediction of the distribution characteristics of J wave morphology patterns.
Conclusions
The current study demonstrates that the presence of J waves can be caused by cardiac compression by esophageal tumor and may disappear after surgical resection of the tumor. Cardiac compression by esophageal tumor is significantly related to severe cardiac events and can be used as a predictor of survival time in patients with esophageal tumors.
Acknowledgments
Funding: This work was supported by Science and Technology Commission of Shanghai Municipality (No. 19140905302).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-371/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-371/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-371/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Okadome K, Baba Y, Yagi T, et al. Prognostic Nutritional Index, Tumor-infiltrating Lymphocytes, and Prognosis in Patients with Esophageal Cancer. Ann Surg 2020;271:693-700. [Crossref] [PubMed]
- Ghatwary N, Zolgharni M, Ye X. Early esophageal adenocarcinoma detection using deep learning methods. Int J Comput Assist Radiol Surg 2019;14:611-21. [Crossref] [PubMed]
- Jiang Y, Gong Y, Rubenstein JH, et al. Toward real-time quantification of fluorescence molecular probes using target/background ratio for guiding biopsy and endoscopic therapy of esophageal neoplasia. J Med Imaging (Bellingham) 2017;4:024502. [Crossref] [PubMed]
- Trovato C, Sonzogni A, Ravizza D, et al. Confocal laser endomicroscopy for in vivo diagnosis of Barrett's oesophagus and associated neoplasia: a pilot study conducted in a single Italian centre. Dig Liver Dis 2013;45:396-402. [Crossref] [PubMed]
- Armato SG, Petrick NA, Ghatwary N, et al. Automatic grade classification of Barretts Esophagus through feature enhancement[C]//Medical Imaging: Computer-aided Diagnosis. International Society for Optics and Photonics, 2017.
- Van D, Zinger S, Schoon EJ, et al. Supportive automatic annotation of early esophageal cancer using local gabor and color features. Neurocomputing 2014;144:92-106. [Crossref]
- Thomas L, Lapa C, Bundschuh RA, et al. Tumour delineation in oesophageal cancer–A prospective study of delineation in PET and CT with and without endoscopically placed clip markers. Radiother Oncol 2015;116:269-75. [Crossref] [PubMed]
- Li GL, Yang L, Cui CC, et al. J wave syndromes: a decade of progress. Chin Med J (Engl) 2015;128:969-75. [Crossref] [PubMed]
- Mililis P, Bazoukis G, Bakalakos A, et al. The J-waves of hypothermia. J Thorac Dis 2018;10:529-30. [Crossref] [PubMed]
- Netelenbos JC, Verheugt FW, de Rijk SH. Hypercalcemia and J waves. Am J Cardiol 1987;59:724. [Crossref] [PubMed]
- Carrillo-Esper R, Limón-Camacho L, Vallejo-Mora HL, et al. Non-hypothermic J wave in subarachnoid hemorrhage. Cir Cir 2004;72:125-9. [PubMed]
- Azarov JE, Ovechkin AO, Vaykshnorayte MA, et al. Prolongation of The Activation Time in Ischemic Myocardium is Associated with J-wave Generation in ECG and Ventricular Fibrillation. Sci Rep 2019;9:12202. [Crossref] [PubMed]
- Sato A, Tanabe Y, Chinushi M, et al. Analysis of J waves during myocardial ischaemia. Europace 2012;14:715-23. [Crossref] [PubMed]
- Seong CS, Gwag HB, Hwang JK, et al. Clinical significance of fragmented QRS complexes or J waves in patients with idiopathic ventricular arrhythmias. PLoS One 2018;13:e0194363. [Crossref] [PubMed]
- Di Diego JM, Antzelevitch C. J wave syndromes as a cause of malignant cardiac arrhythmias. Pacing Clin Electrophysiol 2018;41:684-99. [Crossref] [PubMed]
- Badri M, Patel A, Yan GX. Cellular and ionic basis of J-wave syndromes. Trends Cardiovasc Med 2015;25:12-21. [Crossref] [PubMed]
- Di Diego JM, Patocskai B, Barajas-Martinez H, et al. Acacetin suppresses the electrocardiographic and arrhythmic manifestations of the J wave syndromes. PLoS One 2020;15:e0242747. [Crossref] [PubMed]
- Coppola G, Corrado E, Curnis A, et al. Update on Brugada Syndrome 2019. Curr Probl Cardiol 2021;46:100454. [Crossref] [PubMed]
- Tarin N, Farre J, Rubio JM, et al. Brugada-like electrocardiographic pattern in a patient with a mediastinal tumor. Pacing Clin Electrophysiol 1999;22:1264-6. [Crossref] [PubMed]
- Asteriou C, Lazopoulos A, Giannoulis N, et al. Brugada-like ECG pattern due to giant mediastinal lipoma. Hippokratia 2013;17:368-9. [PubMed]
- Nakazato Y, Ohmura T, Shimada I, et al. Brugada-like Precordial ST Elevation on ECG by Anterior Mediastinal Infective Mass Lesion. Indian Pacing Electrophysiol J 2003;3:184. [PubMed]
- Hayashi H, Wu Q, Horie M. The relationship between J waves and contact of lung cancer with the heart. Ann Noninvasive Electrocardiol 2017;22:e12433. [Crossref] [PubMed]
- Sasaki A, Nakazato Y. Brugada-like electrocardiogram detected after reconstructive operation for oesophageal cancer. Europace 2010;12:1542. [Crossref] [PubMed]
- Ende VD, Veer T. Prognostic and Predictive Factors for the Curative Treatment of Esophageal and Gastric Cancer in Randomized Controlled Trials: A Systematic Review and Meta-Analysis. Cancers 2019;11:530. [Crossref] [PubMed]
- Hiraoka M, Takagi M, Yokoyama Y, et al. Prognosis and risk stratification of young adults with Brugada syndrome. J Electrocardiol 2013;46:279-283. [Crossref] [PubMed]
- Kamakura T, Shinohara T, Yodogawa K, et al. Long-term prognosis of patients with J-wave syndrome. Heart 2020;106:299-306. [Crossref] [PubMed]
- Heng SJ, Clark EN, Macfarlane PW. End QRS notching or slurring in the electrocardiogram: influence on the definition of "early repolarization". J Am Coll Cardiol 2012;60:947-8. [Crossref] [PubMed]
- Tikkanen JT, Junttila MJ, Anttonen O, et al. Early repolarization: electrocardiographic phenotypes associated with favorable long-term outcome. Circulation 2011;123:2666-73. [Crossref] [PubMed]
- Peyronnet R, Nerbonne JM, Kohl P. Cardiac Mechano-Gated Ion Channels and Arrhythmias. Circ Res 2016;118:311-29. [Crossref] [PubMed]
- Hou C, Sun X, Jiang X, et al. SNTA1 altered reactive oxygen species production is associated with J wave syndromes. Gene Reports 2021;24:101250. [Crossref]
- Behr ER. J-Wave Syndromes, SCN5A, and Cardiac Conduction Reserve: Two Sides of the Same Coin? J Am Coll Cardiol 2021;78:1618-20. [Crossref] [PubMed]
- Haïssaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358:2016-23. [Crossref] [PubMed]
- Naruse Y, Nogami A, Harimura Y, et al. Difference in the Clinical Characteristics of Ventricular Fibrillation Occurrence in the Early Phase of an Acute Myocardial Infarction Between Patients With and Without J Waves. J Cardiovasc Electrophysiol 2015;26:872-8. [Crossref] [PubMed]
- Tsuda T, Hayashi K, Konno T, et al. J Waves for Predicting Cardiac Events in Hypertrophic Cardiomyopathy. JACC Clin Electrophysiol 2017;3:1136-42. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Seca L, Barra S, Matos H. Cardiac invasion of esophageal tumor. Rev Esp Cardiol (Engl Ed) 2013;66:664. [Crossref] [PubMed]
- Yagawa Y, Narumiya K, Kudo K, et al. Cardiac metastasis after esophagogastrectomy for esophageal adenocarcinoma with an antemortem diagnosis. Clin J Gastroenterol 2022;15:77-84. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



