
Anatomic patterns of recurrence in biliary tract cancers: does primary tumor site matter?
Introduction
Biliary tract cancers—including intrahepatic and extrahepatic cholangiocarcinoma (IHCCA and EHCCA, respectively) and primary gallbladder cancer (GBC) are relatively uncommon malignancies, both individually and as a group. Altogether, these account for approximately 18,000 diagnoses per year and place them well behind other more common malignancies in terms of incidence (1,2). As a result of their rarity, prospective study is difficult to perform and often includes a heterogeneous group of tumors when considering primary site. For example, the BILCAP trial establishing use of adjuvant capecitabine following resection included a relatively even mix of IHCCA, EHCCA, and GBC (3). Use of gemcitabine with cisplatin in the adjuvant setting is extrapolated from the ABC-02 trial, which also included a heterogeneous group of advanced biliary tract cancers (4). More recently, the PRODIGE 12–ACCORD 18 trial assessed use of adjuvant gemcitabine-oxaliplatin but also included a heterogeneous mix of primary tumors (5). The results of these studies are therefore applied to biliary tract cancers as a whole. However, there is growing evidence that these cancers are biologically distinct and it would therefore be logical to consider that they may recur differentially and thus warrant different treatment approaches (6).
Documented reports of anatomic patterns of recurrence of biliary tract cancers are uncommon (7-24), and even fewer have directly compared these patterns by site of the primary tumor (25,26). Moreover, amongst the studies that have documented recurrence patterns for biliary tract cancers, there are conflicting results, likely owing to overall rarity and subsequent small sample size, with some reporting local recurrence more common while others describing distant metastatic relapse (7,14,17,18,20,21,26). Understanding patterns of recurrence has critical implications as a predilection toward locoregional recurrence may benefit from aggressive locoregional therapies such as radical surgery and chemoradiotherapy, while conversely, distant disease recurrence may warrant early and aggressive systemic therapy.
In this context, we sought to examine our cohort of patients undergoing curative-intent resection of IHCCA, EHCCA, and GBC with the goal of directly comparing time to recurrence as well as anatomic patterns of first recurrence by primary tumor site. We sought to identify unique patterns of recurrence between the three entities, if they do indeed exist. Recognition of such differences would be valuable for informing timing and anatomic focus of surveillance imaging, as well as for rational development of prospective studies regarding novel neoadjuvant and/or adjuvant therapies. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-868/rc).
Methods
Study design and cohort
A retrospective observational cohort study was performed. Patients undergoing curative-intent resection of IHCCA, EHCCA, and GBC at our specialty cancer center were identified within our institutional database (2005–2020). Both perihilar and distal cholangiocarcinomas were included in the EHCCA group. Patients with incidentally discovered GBC who were referred for hepatic resection and portal lymphadenectomy were included for study. Patients were excluded if they had metastatic disease on presentation or less than 3 months of follow up time. Data was abstracted from October 2020 to December 2020.
Outcomes and covariates
The primary variable of interest was site of primary tumor (IHCCA, EHCCA, or GBC) which was determined by the final surgical pathology report. The primary outcomes of interest were recurrence free survival (RFS) and anatomic site of first recurrence. Recurrence was either biopsy-proven or determined radiographically with clinical impression. Anatomic site of recurrence could include multiple sites which were recorded and reported separately. If a patient had disease progression to additional sites after the initial recurrence, these sites were not included in the primary outcome. Overall survival (OS) was also considered as a secondary outcome of interest.
Clinical variables analyzed were age in years and sex (male versus female). Pathologic variables analyzed were tumor size (in centimeters), pathologic T stage, node status (N0, N+ or Nx), or resection type (R0, R1, or R2). As it is institutional practice to intraoperatively sample suspicious distant lymphadenopathy (such as aortocaval nodes) prior to proceeding with complete resection, node status refers to regional lymph nodes only. These data were obtained from the final surgical pathology report. Pathologic T stage was determined using the American Joint Commission on Cancer Staging Manual Eighth Edition according to primary tumor site (gallbladder, intrahepatic bile ducts, distal bile duct, perihilar bile duct). Receipt of systemic therapy was considered as a treatment variable and recorded as none, neoadjuvant, adjuvant, or perioperative but treated as a binary variable (none versus any) for analysis given category sizes. Receipt of radiotherapy was treated as a binary variable. Missing data was treated as unknown.
Statistical analysis
Descriptive statistics are presented as frequency for categorical variables and median with interquartile range (IQR) for continuous variables; univariate analyses were performed using Chi-square, Fisher’s exact, or Kruskal-Wallis tests, as appropriate. RFS, the primary outcome, was estimated using the Kaplan-Meier method with date of surgery as the starting point (or date of re-resection for incidental GBCs). Date of recurrence was defined as failure and patients were censored if they had no recurrence at time of last follow up. Median follow up time was assessed using the reverse Kaplan-Meier method (27). Multivariable analysis was performed using Cox proportional hazard modeling. A backward, stepwise approach was taken with all covariates initially included in the multivariable model with sequential removal if P>0.05. Anatomic patterns of initial site of recurrence were described and compared using Chi-square or Fisher’s Exact test. All statistical analyses were performed using Stata version 14 (28). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board at Moffitt Cancer Center (Protocol #16646) and individual consent for this retrospective analysis was waived.
Results
Characteristics of study cohort
From 2005 to 2020, there were 142 patients who underwent curative-intent resection of IHCCA, EHCCA, and GBC and met study criteria, after excluding 20 patients with less than 3 months follow up and 5 with metastatic disease identified at surgery (Figure S1). Clinical and pathologic characteristics of the cohort are described in Table 1. There were 50 IHCCAs (35%), 32 EHCCAs (23%), and 60 GBCs (42%). Of the GBCs, 42 (70%) were diagnosed incidentally following cholecystectomy and subsequently underwent hepatic resection and portal lymphadenectomy; in these cases, common bile duct resection was uncommon (n=4). The median age of the study cohort was 67 years (IQR 61–74) and the majority were female (n=92, 65%). There was no significant difference in age by tumor site (P=0.61). Patients with GBC were more commonly female (77% versus 64% for IHCCA and 44% for EHCCA, P=0.007). IHCCAs were more commonly pT1 lesions and more commonly did not have lymph nodes sampled at time of resection (Nx). Notably, there were no R2 resections and the majority were R0 (n=127, 89%). Ninety-one patients (64%) received systemic chemotherapy in the neoadjuvant (n=6, 4%), adjuvant (n=72, 51%), or perioperative setting (n=13, 9%). Known chemotherapy regimens included gemcitabine-cisplatin (n=22), gemcitabine only (n=29), 5FU/capecitabine (n=10), FOLFOX/XELOX (n=14), and FOLFOXIRI (n=1). Chemoradiotherapy was more commonly administered for EHCCA and GBC than for IHCCA (34% and 33% versus 14%, respectively, P=0.035).
Table 1
| Variable | Overall cohort, n=142 | IHCCA, n=50 | EHCCA*, n=32 | GBC, n=60 | P |
|---|---|---|---|---|---|
| Age | 67 [61–74] | 68 [61–75] | 67 [62–76] | 66 [61–72] | 0.61 |
| Female sex | 92 [65] | 32 [64] | 14 [44] | 46 [77] | 0.007 |
| Race | |||||
| White | 121 [85] | 47 [94] | 31 [97] | 43 [72] | 0.01 |
| Black | 8 [6] | 2 [4] | 0 [0] | 6 [10] | |
| Hispanic | 11 [8] | 1 [2] | 1 [3] | 9 [15] | |
| Asian | 2 [1.4] | 0 [0] | 0 [0] | 2 [3] | |
| Tumor size (cm) | 3.0 [1.6–5.0] | 5.0 [3.5–7.0] | 1.7 [1.2–2.2] | 2.5 [1.3–4.0] | 0.001 |
| Pathologic T stage | <0.001 | ||||
| pT0** | 2 [1.4] | 1 [2] | 1 [3] | 0 | |
| pT1 | 41 [29] | 27 [54] | 6 [19] | 8 [13] | |
| pT2 | 61 [43] | 16 [32] | 10 [31] | 35 [58] | |
| pT3 | 37 [26] | 5 [10] | 15 [47] | 17 [28] | |
| pT4 | 1 [0.7] | 1 [2] | 0 | 0 | |
| Node status | 0.003 | ||||
| N0 | 94 [66] | 32 [64] | 20 [63] | 42 [70] | |
| N+ | 38 [27] | 9 [18] | 11 [34] | 18 [30] | |
| Nx | 10 [7] | 9 [18] | 1 [3] | 0 [0] | |
| Resection type | |||||
| R0 | 127 [89] | 43 [86] | 26 [81] | 58 [97] | 0.034 |
| R1 | 15 [11] | 7 [14] | 6 [19] | 2 [3] | |
| R2 | 0 | – | – | – | |
| GBC tumor site | |||||
| Fundus | – | – | – | 20 [37] | |
| Body | – | – | – | 9 [17] | |
| Neck | – | – | – | 3 [6] | |
| Unknown | – | – | – | 28 [47] | |
| GBC surface location | |||||
| Liver | – | – | – | 16 [34] | |
| Peritoneal | – | – | – | 14 [30] | |
| Unknown | – | – | – | 30 [30] | |
| Systemic therapy | 0.83 | ||||
| None | 51 [36] | 21 [42] | 11 [35] | 19 [32] | |
| Neoadjuvant | 6 [4] | 2 [4] | 1 [3] | 3 [5] | |
| Adjuvant | 72 [51] | 21 [42] | 18 [56] | 33 [55] | |
| Perioperative | 13 [9] | 6 [12] | 2 [6] | 5 [8] | |
| Chemoradiation | 0.035 | ||||
| None | 104 [73] | 43 [86] | 21 [66] | 40 [67] | |
| Any | 38 [27] | 7 [14] | 11 [34] | 20 [33] | |
| Recurrence | |||||
| None | 79 [56] | 28 [56] | 13 [41] | 37 [62] | |
| Any | 63 [44] | 22 [44] | 19 [59] | 23 [38] |
Data are presented as n [%] or median [range]. *, EHCCA includes both perihilar and distal cholangiocarcinoma; **, Two patients had complete pathologic response following neoadjuvant therapy. IHCCA, intrahepatic cholangiocarcinoma; EHCCA, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer.
Recurrence and recurrence-free survival
During the course of follow up, there were 64 recurrences and 78 deaths. Median follow up time in this study was 42 months (range 3.3–133 months). Median RFS was 30.8 months, which was not significantly different between the three primary tumor sites (Figure 1A, P=0.44). Estimated 3-year RFS was 48.2% overall (95% CI: 38.5–57.1%), 48.5% for IHCCA (95% CI: 32.5–62.8%), 40.8% for EHCCA (95% CI: 22.9–58.0%), and 52.7% for GBC (95% CI: 36.8–66.4%). Over 80% of documented recurrences occurred within 24 months regardless of primary tumor site.
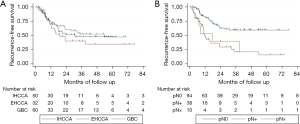
Multivariable Cox regression was performed to assess for underlying differences in RFS by primary tumor site masked by differences in clinical and pathologic characteristics (Table 2). There remained no significant difference in RFS by tumor site after adjustment for age, sex, pathologic T stage, nodal status, margin status, receipt of systemic chemotherapy, or receipt of chemoradiotherapy. Tumor size was not included in this analysis due to inherent differences in tumor growth patterns by anatomic site (e.g., mass-forming versus growth along biliary tracts). In the reduced multivariable model, nodal positivity was found to be independently associated with poor RFS after adjustment for other covariates (HR 3.92; 95% CI: 2.18–7.02, P<0.001 referent to N0 pathology). There was no significant difference in the strength of association between nodal positivity and RFS by primary tumor site (P=0.29 for the interaction term). Receipt of chemoradiotherapy appeared to be associated with prolonged RFS but did not reach statistical significance (HR 0.54; 95% CI: 0.29–1.01, P=0.052). Estimated 3-year RFS was 23.0% (95% CI: 10.1–38.9%) for N+ patients compared to 59.8% (95% CI: 47.5–70.2%) for N0 (Figure 1B).
Table 2
| Variable | Model including all variables | Reduced model | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P | Hazard ratio (95% CI) | P | ||
| Primary Tumor Site | |||||
| IHCCA | Ref | – | Ref | – | |
| EHCCA | 1.55 (0.76–3.16) | 0.22 | 1.49 (0.78–2.87) | 0.23 | |
| GBC | 1.13 (0.55–2.29) | 0.74 | 1.11 (0.59–2.11) | 0.74 | |
| Age | 0.99 (0.97–1.02) | 0.59 | |||
| Female sex | 1.25 (0.73–2.14) | 0.42 | |||
| Pathologic T stage | 0.91 (0.64–1.29) | 0.60 | |||
| T0/T1 | Ref | – | |||
| T2 | 0.92 (0.46–1.85) | 0.82 | |||
| T3/T4 | 1.18 (0.54–2.58) | 0.68 | |||
| Nodal status | |||||
| N0 | Ref | – | Ref | – | |
| N+ | 3.24 (1.72–6.08) | <0.001 | 3.92 (2.18–7.02) | <0.001 | |
| Nx | 3.62 (1.38–9.48) | 0.009 | 3.09 (1.21–7.90) | 0.018 | |
| Margin status | |||||
| R0 | Ref | – | |||
| R1 | 1.17 (0.53–2.60) | 0.70 | |||
| Receipt of systemic therapy | |||||
| None | Ref | – | |||
| Any | 1.66 (0.83–3.29) | 0.15 | |||
| Receipt of radiotherapy | |||||
| None | Ref | – | Ref | ||
| Any | 0.42 (0.22–0.84) | 0.014 | 0.54 (0.29–1.01) | 0.052 | |
All variables were initially included in the model. Backward, stepwise removal was performed to arrive at the final reduced model. Primary tumor site was not significantly associated with RFS. IHCCA, intrahepatic cholangiocarcinoma; EHCCA, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer.
No significant difference in OS was found between the three primary tumor sites (P=0.17, Figure 2A). Median OS was 52 months for IHCCA, 32 months for EHCCA, and 50 months for GBC. Nodal positivity was significantly associated with poor OS with median OS of 56 months for pN0 patients compared to 33 months for pN+ patients (P=0.0003, Figure 2B). This association remained statistically significant in multivariable Cox regression (HR 2.08, P=0.012). Receipt of chemotherapy (HR 2.12, P=0.015) and chemoradiotherapy (HR=0.52, P=0.24) were also significantly associated with OS.

Most common anatomic sites of first recurrence
Among 64 patients who recurred, the most common anatomic site of recurrence was within the liver (n=49, 77%), in isolation (n=32) or synchronous with other site of recurrence (n=17). Other sites of recurrences included lung (n=11, 17%), periportal region (n=8, 13%), peritoneum (n=9, 14%), retroperitoneal nodes (n=5, 8%), and bone (n=1, 2%). There was no significant difference in RFS between hepatic and extrahepatic recurrence (P=0.10) or between isolated liver recurrences and synchronous liver-extrahepatic recurrences (P=0.77).
Patterns of anatomic sites of recurrence by primary tumor site
Exploratory analysis was performed to assess for differential anatomic patterns of recurrence between the three biliary tract cancers. Significant variations were observed overall (Table 3, P=0.049, Fisher exact). This was driven by the fact that recurrence of GBC was uniquely associated with disease in the periportal region (n=7/23, 30%) when compared to IHCC (n=0/22, 0%) or EHCCA (n=1/19, 5%). Of note, these regional recurrences were commonly in the setting of simultaneous hepatic recurrence (n=5). In contrast, pulmonary metastases were more commonly observed at time of first recurrence in patients with IHCCA (n=6/22, 27%) than for EHCCA (n=3/19, 16%) or GBC (n=2/23, 9%). In particular, the pattern of simultaneous liver-lung metastases was more commonly observed with IHCCA (n=5/22, 23%) than for EHCCA (n=2/19, 11%) or GBC (n=1/23, 4%). First recurrence of EHCCA was characterized by disease in the peritoneum (n=5/19, 26%) compared to either IHCCA or GBC (n=2/22, 9% and n=2/23, 9% respectively).
Table 3
| Sites | IHCCA (n=22) | EHCCA (n=19) | GBC (n=23) | P |
|---|---|---|---|---|
| Liver | 17 [77] | 14 [74] | 18 [78] | 0.94 |
| Lung | 6 [27] | 3 [16] | 2 [9] | 0.25 |
| Periportal region | 0 | 1 [5] | 7 [30] | 0.003 |
| Peritoneum | 2 [9] | 5 [26] | 2 [9] | 0.23 |
| Retroperitoneal nodes | 3 [14] | 1 [5] | 1 [4] | 0.51 |
| Bone | 0 | 1 [5] | 0 | 0.30 |
Data are presented as n [%]. Overall table, P=0.049 (Fisher Exact). Columns total to larger than column total as patients frequently recurred in multiple sites simultaneously. IHCCA, intrahepatic cholangiocarcinoma; EHCCA, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer.
Discussion
Carcinomas of the biliary tract are relatively uncommon malignancies making prospective study challenging, often with a heterogeneous pool of IHCCA, EHCCA, and GBC when such study is completed. As a result, recommendations regarding adjuvant therapies and surveillance are relatively uniform across primary tumor site. However, these are clearly distinct biologic entities and may have distinct patterns of recurrence. In this study, we sought to examine whether anatomic patterns of first recurrence for IHCCA, EHCCA, and GBC differ by primary tumor site.
In the current study, there was no difference in time to recurrence by primary tumor site, but unique patterns of recurrence were observed when stratified by anatomic site. Median RFS overall was 32.6 months, which did not significantly differ between IHCCA, EHCCA, or GBC. This remained the case after adjustment for clinical, pathologic, and treatment covariates. Nodal positivity was the strongest predictor of poor RFS and was a negative prognostic indicator in all three sites of biliary tumor origin (HR 3.92). With regard to anatomic site of first recurrence, the most common site was within the liver (77% overall), in isolation or synchronous with other sites of recurrence. This did not differ between the three cancer types. The variance in anatomic patterns of recurrence was primarily driven by the fact that regional recurrence within the porta hepatis was uniquely seen in patients with GBC (30% compared to 0% in IHCCA and 5% in EHCCA). The pattern of simultaneous pulmonary-hepatic recurrence was more commonly seen in IHCCA than EHCCA or GBC (23% versus 11% and 4%, respectively). A trend toward more common peritoneal recurrence was seen for EHCCA (26% compared to 9% for both IHCCA and GBC).
The finding that RFS was not significantly different between the three biliary tract cancers is informative and supports current recommendations by the NCCN for timing of surveillance imaging. Current recommendations for surveillance imaging are essentially the same for IHCCA, EHCCA, and GBC, which is to consider imaging of the chest, abdomen and pelvis every 3–6 months for two years, then every 6–12 months for up to five years (29). Indeed, in the current study, over 80% of all recurrences were documented with 24 months, regardless of primary site. This study also confirms that these are unfortunately aggressive malignancies and supports efforts to identify risk factors for early and very early recurrence as undertaken by the US Extrahepatic Biliary Malignancy Consortium and others (10,30-32). Those with nodal positivity—regardless of primary tumor site—warrant close monitoring given very high risk of recurrence. The finding that nodal positivity is the most important prognostic factor for disease recurrence is consistent with previous studies across tumor sites (7,9,12,13,15,17-19).
The current finding of unique anatomic patterns of recurrence by primary tumor site may help to guide both surveillance and future study of neoadjuvant and adjuvant strategies. The finding that GBCs frequently recur with disease in the regional nodal basin may be counterintuitive given these patients routinely undergo regional lymphadenectomy. However, this is consistent with findings from other published series, including from Jung, Choi, and Kim et al. (8,15,16). It should be noted, however, that these recurrences were often seen in the setting of hepatic recurrence, consistent with the findings of the US Extrahepatic Biliary Malignancy Consortium (20). These findings should reinforce to clinicians that suspicious findings in the porta hepatis should not be overlooked with the assumption that the regional basin has been cleared as recurrence in this area. Recurrent disease in this area is likely a result of residual microscopic disease despite lymphadenectomy. These findings may support a role for a regional adjuvant therapy such as chemoradiation in addition to systemic adjuvant therapy. In this series, resection of the common bile duct was infrequently required.
The finding of simultaneous hepatic-pulmonary initial recurrence is consistent with the multi-institutional report from Hu and colleagues which found a high rate of extrahepatic-intrahepatic recurrence in cases of resected IHCCA (11). This is in contrast to the MSKCC experience which illustrates a picture of hepatic-only initial recurrence in the majority of patients, simultaneous intrahepatic-extrahepatic in a minority, and extrahepatic-only relapse presenting in a later timeframe (33). In addition to high likelihood of recurrent disease in the liver, we observed a high frequency of peritoneal disease at time of initial recurrence for patients with resected EHCCA. This appears to be consistent with previous reports documenting an incidence of 11-26% for peritoneal recurrence (9,21,25,34). Future study for neoadjuvant and adjuvant systemic regimens are warranted for both IHCCA and EHCCA given the high likelihood for hepatic and extrahepatic recurrence.
Limitations to this study are notably its small sample size as a result of the rarity of biliary tract cancers as previously discussed. Statistical analysis is limited and observations should be considered in light of the sample size and in the context with the few other previously published series. It is likely that our series contains an element of patient selection bias as our institution is a regional referral center. There are likely patients in the general population that are not referred to our center due to progressive disease at time of diagnosis. Similarly, loss to follow up is a concern for a referral center and some recurrences may not be captured. Median follow up time in this study was 42 months. Results from the current series may be less generalizable to populations in other geographic regions, an important consideration given global variations in biliary tract cancer incidence. For example, in the current study the majority of IHCCA cases were in women, while in Asian countries IHCCA has a higher incidence in males. Additionally, the majority of patients were white so racial and/or ethnic influences on tumor biology cannot be well studied in this cohort.
Conclusions
Recurrence following resection of biliary tract cancers is common and frequently occurs within two years. In the current study, the timing of recurrence does not appear to differ by primary tumor site. Hepatic involvement is common at time of first recurrence regardless of primary tumor site. However, disease recurrence within the peri-portal region may be more common following resection of GBC despite routine portal lymphadenectomy. Pulmonary disease may be seen more commonly with recurrence of IHCCA while peritoneal recurrence may occur in the setting of resected EHCCA. These subtleties in recurrence patterns may help to inform neoadjuvant/adjuvant therapy clinical trial design and/or creation of novel treatment strategies for biliary tract cancers.
Acknowledgments
The major findings of this study were presented as an On Demand Abstract Presentation at the AHPBA Annual Meeting 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-868/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-868/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-868/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-868/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Review Board at Moffitt Cancer Center (Protocol #16646) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017;24:1073274817729245. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol 2019;20:663-73. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J Clin Oncol 2019;37:658-67. [Crossref] [PubMed]
- Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet 2015;47:1003-10. [Crossref] [PubMed]
- Barreto SG, Pawar S, Shah S, et al. Patterns of failure and determinants of outcomes following radical re-resection for incidental gallbladder cancer. World J Surg 2014;38:484-9. [Crossref] [PubMed]
- Choi SB, Han HJ, Kim CY, et al. Surgical outcomes and prognostic factors for T2 gallbladder cancer following surgical resection. J Gastrointest Surg 2010;14:668-78. [Crossref] [PubMed]
- Groot Koerkamp B, Wiggers JK, Allen PJ, et al. Recurrence Rate and Pattern of Perihilar Cholangiocarcinoma after Curative Intent Resection. J Am Coll Surg 2015;221:1041-9. [Crossref] [PubMed]
- Hu HJ, Jin YW, Shrestha A, et al. Predictive factors of early recurrence after R0 resection of hilar cholangiocarcinoma: A single institution experience in China. Cancer Med 2019;8:1567-75. [Crossref] [PubMed]
- Hu LS, Zhang XF, Weiss M, et al. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2019;26:2549-57. [Crossref] [PubMed]
- Hughes MA, Frassica DA, Yeo CJ, et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J Radiat Oncol Biol Phys 2007;68:178-82. [Crossref] [PubMed]
- Hyder O, Hatzaras I, Sotiropoulos GC, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery 2013;153:811-8. [Crossref] [PubMed]
- Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001;234:507-17; discussion 517-9. [Crossref] [PubMed]
- Jung W, Jang JY, Kang MJ, et al. Effects of Surgical Methods and Tumor Location on Survival and Recurrence Patterns after Curative Resection in Patients with T2 Gallbladder Cancer. Gut Liver 2016;10:140-6. [Crossref] [PubMed]
- Kim TG. Patterns of initial failure after resection for gallbladder cancer: implications for adjuvant radiotherapy. Radiat Oncol J 2017;35:359-67. [Crossref] [PubMed]
- Kim WS, Choi DW, You DD, et al. Risk factors influencing recurrence, patterns of recurrence, and the efficacy of adjuvant therapy after radical resection for gallbladder carcinoma. J Gastrointest Surg 2010;14:679-87. [Crossref] [PubMed]
- Kobayashi A, Miwa S, Nakata T, et al. Disease recurrence patterns after R0 resection of hilar cholangiocarcinoma. Br J Surg 2010;97:56-64. [Crossref] [PubMed]
- Komaya K, Ebata T, Shirai K, et al. Recurrence after resection with curative intent for distal cholangiocarcinoma. Br J Surg 2017;104:426-33. [Crossref] [PubMed]
- Margonis GA, Gani F, Buettner S, et al. Rates and patterns of recurrence after curative intent resection for gallbladder cancer: a multi-institution analysis from the US Extra-hepatic Biliary Malignancy Consortium. HPB (Oxford) 2016;18:872-8. [Crossref] [PubMed]
- Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg 2004;139:514-23; discussion 523-5. [Crossref] [PubMed]
- Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg 2001;193:384-91. [Crossref] [PubMed]
- Wiggers JK, Groot Koerkamp B, Ovadia Z, et al. Patterns of recurrence after resection of gallbladder cancer without routine extrahepatic bile duct resection. HPB (Oxford) 2014;16:635-40. [Crossref] [PubMed]
- Zhou W, Qian L, Rong Y, et al. Prognostic factors and patterns of recurrence after curative resection for patients with distal cholangiocarcinoma. Radiother Oncol 2020;147:111-7. [Crossref] [PubMed]
- Jarnagin WR, Ruo L, Little SA, et al. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer 2003;98:1689-700. [Crossref] [PubMed]
- Jung SJ, Woo SM, Park HK, et al. Patterns of initial disease recurrence after resection of biliary tract cancer. Oncology 2012;83:83-90. [Crossref] [PubMed]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015.
- Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:541-65. [Crossref] [PubMed]
- Sahara K, Tsilimigras DI, Kikuchi Y, et al. Defining and Predicting Early Recurrence after Resection for Gallbladder Cancer. Ann Surg Oncol 2021;28:417-25. [Crossref] [PubMed]
- Tsilimigras DI, Sahara K, Wu L, et al. Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg 2020;155:823-31. [Crossref] [PubMed]
- Zhang XF, Beal EW, Chakedis J, et al. Defining Early Recurrence of Hilar Cholangiocarcinoma After Curative-intent Surgery: A Multi-institutional Study from the US Extrahepatic Biliary Malignancy Consortium. World J Surg 2018;42:2919-29. [Crossref] [PubMed]
- Doussot A, Gonen M, Wiggers JK, et al. Recurrence Patterns and Disease-Free Survival after Resection of Intrahepatic Cholangiocarcinoma: Preoperative and Postoperative Prognostic Models. J Am Coll Surg 2016;223:493-505.e2. [Crossref] [PubMed]
- Takao S, Shinchi H, Uchikura K, et al. Liver metastases after curative resection in patients with distal bile duct cancer. Br J Surg 1999;86:327-31. [Crossref] [PubMed]


