
Efficacy and safety of radiotherapy combined with raltitrexed and irinotecan for treating unresectable recurrent colorectal cancer: a single-arm phase II trial
Introduction
Colorectal cancer is the third most common cancer in terms of incidence and mortality (1). After radical resection in colorectal cancer patients, approximately 10% will have local-regional failure accompanied by a series of symptoms including pelvic pain, bleeding, and urogenital disorders (2). While R0 resection (tumor resection with clear margins) for recurrent lesions significantly prolongs overall survival and is a potentially curative approach, only 20–30% of recurrent colorectal cancer patients are eligible for radical resection (3-5). Other therapeutic modalities include chemotherapy, radiotherapy and radiofrequency ablation. Radiofrequency ablation can relieve pelvic pain but it may cause considerable morbidity such as bleeding, abscess and fistula, so it is applied to symptomatic patients who are unable to benefit from other treatments. Radiotherapy is a vital approach for local control, which improves survival and quality of life (6) and even increases the viability of complete resection of recurrent tumors (7-9).
Most patients developed distant metastases when they had local recurrence. Therefore, systemic therapy is also crucial for tumor control. Current systemic therapeutic options for advanced or metastatic colorectal cancer include chemotherapeutic drugs [e.g., 5-fluorouracil (5-FU), irinotecan, oxaliplatin, and raltitrexed], targeted therapies (e.g., cetuximab, bevacizumab, and regorafenib), and immunotherapy (e.g., nivolumab and pembrolizumab). However, whether targeted therapies and immunotherapy provide good efficacy and safety when administered concomitantly with radiotherapy remains uncertain, so chemotherapeutic drugs were prioritized to be combined with radiotherapy. Since most recurrent patients have previously received fluoropyrimidine and oxaliplatin as adjuvant or first-line chemotherapy (10), we propose other chemotherapeutic agent combinations may be a better choice.
Irinotecan, a topoisomerase I inhibitor, is an effective chemotherapeutic agent for colorectal cancer, for which radiosensitizing properties were also identified (11). In our previous phase III trial, additional irinotecan significantly improved the pathologic complete response (pCR) rate (30% vs. 15%) when compared with conventional chemoradiotherapy for patients with locally advanced rectal cancer (12). Raltitrexed is a cytotoxic drug that selectively inhibits thymidylate synthase (TS), leading to DNA inhibition (13). Several studies have demonstrated that raltitrexed-based chemotherapy, including raltitrexed combined with irinotecan or oxaliplatin, shows efficacy similar to that of 5-FU-based doublets with favorable toxicity profile (14,15). Moreover, raltitrexed is recommended as second-line treatment after failure of standard first-line chemotherapy in metastatic colorectal cancer (16), and can be a viable option for patients refractory to or intolerant of 5-FU (patients with cardiac events or dihydropyrimidine dehydrogenase deficiency) (17). Irinotecan combined with raltitrexed has significant synergistic effect and acceptable toxicity.
Thus, we conducted a phase II trial to explore the effectiveness and safety of raltitrexed and irinotecan administered concurrently with radiotherapy in patients with recurrent colorectal cancer. We present the following article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-308/rc).
Methods
Patient eligibility
Patients were eligible if they had histologically confirmed primary colorectal adenocarcinoma and were diagnosed with locoregional recurrence histologically or clinically after primary surgical resection. Patients with distant metastases were also included. All patients had conditions refractory to chemotherapy with fluoropyrimidine and oxaliplatin, defined as progression of disease within 6 months of receiving 5-FU and oxaliplatin as neoadjuvant chemotherapy, adjuvant chemotherapy, or first-line treatment, or intolerance to adverse events such as cardiovascular events. The irradiated sites were assessed and were not eligible for surgery. Furthermore, eligible patients were aged 18–75 years, had a Karnofsky performance status score ≥70, and had adequate bone marrow function (hemoglobin ≥90 g/L, neutrophil count ≥1.5×109/L, and platelet count ≥1.5×109/L). Additionally, liver function [total bilirubin level ≤1.5× the upper limit of normal (ULN), albumin level ≥30 g/L, alanine transaminase (ALT) and aspartate aminotransferase (AST) ≤2.5× ULN], and kidney function (serum creatinine ≤ ULN) were prerequisites for inclusion. Conversely, the exclusion criteria were pregnancy or lactation or diagnosis with a serious illness, such as unstable angina or myocardial infarction, within the previous 12 months. All patients underwent uridine diphosphate glucuronosyltransferase 1A1 (UGT1A1) genotyping via polymerase chain reaction testing, and patients with UGT1A1 genotype *28*28 were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center (Shanghai, China) (approval number: 1603158-3) and written informed consent was obtained from all patients. The study was registered at ClinicalTrials.gov (No. NCT04499586) on July 31, 2020.
Treatment
All patients were treated with intensity-modulated radiation therapy (IMRT) at a dose of 50–60 Gy in 25–30 fractions and with a photon beam of 6–10 MV and five- or seven-field treatment plan. Planning computed tomography (CT) was performed in the treatment position. Volume was defined in accordance with the International Commission on Radiation Units and Measurements (ICRU) 50 report and the gross tumor volume (GTV) was defined as the gross disease evaluated on clinical examination, magnetic resonance imaging (MRI), colonoscopy, ultrasound, and positron emission tomography (PET)-CT. The clinical target volume (CTV) consisted of the GTV and areas considered at significant risk of potential microscopic disease such as suspicious lateral lymph node metastases, and the planning target volume (PTV) was defined as CTV plus 5-mm margins in all directions.
Radiotherapy was delivered concurrently with two cycles of chemotherapy comprising an intravenous injection of irinotecan at a dose of 200 mg/m2 and an intravenous injection of raltitrexed at a dose of 3 mg/m2 every 3 weeks. After completion of chemoradiotherapy, the resectability of the lesion was reassessed by our multidisciplinary team. If the lesion was resectable, the patient underwent surgery, and if the lesion remained unresectable, the patients continued to receive more cycles of chemotherapy with the same regimen until disease progression, intolerant toxicity, or withdrawal of consent. Dose reductions or interruptions were permitted for grade 3 or 4 toxicities related to treatment.
Study objectives
The primary endpoint was the objective response rate (ORR) of the target tumor in the radiation field. We defined ORR as the proportion of patients with a confirmed complete response (CR) or partial response (PR), using the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Local progression was defined as progression within the radiation field, while regional progression was defined as locoregional progression outside the radiation field. The secondary endpoints included the disease control rate (DCR) [percentage of patients achieving CR, PR, or stable disease (SD)], progression-free survival (PFS) (time from enrollment to disease progression or death), local PFS (LPFS) (time from enrollment to local progression within the radiation field or death), and treatment safety.
Evaluation
Clinical and imaging examinations (MRI of the pelvis and abdomen, CT of the chest, or PET-CT) were performed at baseline, at the end of the radiotherapy and every 4 weeks during chemotherapy. Tumor response was assessed by the radiologist and investigator after completion of radiotherapy and was reconfirmed a month later according to RECIST version 1.1. Patients were followed up every 3 months during the first year after the treatment and every 6 months thereafter. Adverse events were recorded from the initiation of treatment to 6 months after the last round of chemotherapy and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
According to previous studies, ORR to second-line treatment for colorectal cancer after oxaliplatin failure was approximately 10% (18,19). The study was designed to test the null hypothesis of a 10% ORR and we hypothesized that the treatment in our study could achieve an ORR of 25%. The sample size was calculated using exact binomial test with a 10% one-sided significance level and a power of 80%. The estimated sample size was 25 patients, considering potential drop-out, we planned to enroll 30 patients. Descriptive statistics were used to summarize ORR, DCR and safety. PFS and LPFS were analyzed using the Kaplan-Meier method, the median follow-up time was calculated using the reverse Kaplan-Meier method. Antitumor activity and safety analyses were based on the intention to treat population that completed the radiotherapy and received at least one dose of raltitrexed and irinotecan. All statistical analyses were performed with R software (version 4.0).
Results
Patient characteristics
The 30 patients with recurrent colorectal cancer were enrolled between January 1, 2019 and July 14, 2020, and their baseline characteristics are reported in Table 1. The median age of the study population was 58 years (range, 23–74 years), and 56.7% were female. All patients enrolled in this study underwent curative surgery for primary cancer, with 12 (40.0%) having colon cancer and 18 (60.0%) having rectal cancer. The median interval between the initial surgery and local recurrence was 14 months (range, 4–109 months). Twelve patients (40.0%) with recurrent colorectal cancer developed distant metastases before enrollment (four patients developed liver metastases, five developed lung metastases, and three developed both), of whom seven patients with liver metastasis had undergone surgical resection or radiofrequency ablation and eight still presented with lung metastases. There were 39 local recurrent sites among all patients with 21 (70.0%) having a single site and nine (30.0%) having multiple sites. Eleven recurrence sites (28.2%) were in the presacral region, eight (20.5%) in the pelvic soft tissue, four (10.3%) in the anastomosis, 15 (38.5%) in the lymph node region, and one (2.6%) in the rectum (primary colon cancer implanted into the rectum). The median tumor size was 2.90 cm (range, 0.96–7.50 cm). All patients completed radiotherapy, and the median total dose was 54 Gy (range, 50–60 Gy), the median daily dose was 2.0 Gy (range, 1.8–2.2 Gy), and the main total dose was 50 Gy (43% patients) or 55 Gy (40% patients). The median number of overall chemotherapy cycles was five (range, 2–10).
Table 1
| Characteristics | No. of patients | Percentage |
|---|---|---|
| Age, median [range], years | 58 [23–74] | – |
| Sex | ||
| Male | 13 | 43.3 |
| Female | 17 | 56.7 |
| Primary site | ||
| Colon | 12 | 40.0 |
| Rectum | 18 | 60.0 |
| Anatomical sites of recurrent disease (n=39) | ||
| Presacral region | 11 | 28.2 |
| Pelvic soft tissue | 8 | 20.5 |
| Anastomosis | 4 | 10.3 |
| Lymph node | 15 | 38.5 |
| Rectum | 1 | 2.6 |
| Number of recurrent sites | ||
| 1 | 21 | 70.0 |
| ≥2 | 9 | 30.0 |
| Tumor size, median [range], cm | 2.90 [0.96–7.50] | – |
| Distant metastases | ||
| No | 18 | 60.0 |
| Yes | 12 | 40.0 |
| Carcinoembryonic antigen level | ||
| Normal (<5 ng/mL) | 11 | 36.7 |
| Increased (≥5 ng/mL) | 19 | 63.3 |
| Prior treatment | ||
| Surgery | 30 | 100.0 |
| Chemotherapy | 30 | 100.0 |
| Surgery-recurrence interval, median [range], months | 14 [4–109] | – |
Tumor response
All efficacy endpoints were assessed in the “intention to treat” analysis set, and all 30 patients were included. Tumor response was evaluated in all patients (Figure 1, Table 2), which showed the ORR and DCR were 40.0% (12/30) and 96.7% (29/30), respectively, with 6.7% (2/30) of patients achieving CR and 33.3% (10/30) achieving PR, while 17 patients had SD (56.7%) and one had disease progression after radiotherapy. As for the response of the non-irradiated sites (eight lung metastases), no patient had CR or PR, seven had confirmed SD (7/8; 87.5%), and one had progressive disease.
Table 2
| Response | Irradiated site (n=30), n (%) | Non-irradiated site (n=8), n (%) |
|---|---|---|
| Complete response | 2 (6.7) | 0 |
| Partial response | 10 (33.3) | 0 |
| Stable disease | 17 (56.7) | 7 (87.5) |
| Progressive disease | 1 (3.3) | 1 (12.5) |
| Objective response rate | 12 (40.0) | 0 |
| Disease control rate | 29 (96.7) | 7 (87.5) |
Survival
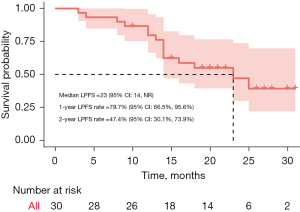
The median follow-up time was 22 months (range, 4–35 months). By the cut-off date, February 12, 2022, nine patients died, four were lost to follow-up, six patients had local failure in the irradiation field, four had regional progression outside the irradiation field, 13 had distant metastasis or metastatic progression. After completion of chemoradiation, two patients underwent surgery, one of whom remained progression-free, but the other experienced both local failure and distant metastasis. Two patients received radiofrequency ablation for pelvic lesions, of whom one had regional progression 2 months later and one achieved local control. Patients were not amenable to resection mainly because of extensive invasion of the pelvis (such as the sacrum and pelvic side wall). The median PFS was 13.5 months [95% confidence interval (CI): 10–16 months], the 1-year PFS rate was 56.7% (95% CI: 41.4–77.5%), and the 2-year PFS was 18.8% (95% CI: 8.7–40.3%) (Figure 2). The median LPFS was 23 months (95% CI: 14 months to not reached), the 1-year LPFS was 79.7% (95% CI: 66.5–95.6%), and the 2-year LPFS was 47.4% (95% CI: 30.1–73.9%) (Figure 3).
Toxicity
The incidence of toxicity of all 30 patients is presented in Table 3. The incidence of grade 3 or 4 adverse events was 26.7%. One patient experienced grade 4 neutropenia and five experienced grade 3 hematological toxicities including neutropenia (three patients, 10.0%), thrombocytopenia (one patient, 3.3%) and anemia (one patient, 3.3%). Grade 3 non-hematological toxicities included ALT elevation in two patients (6.7%), AST elevation in one patient (3.3%), nausea or vomiting in one patient (3.3%), and diarrhea in one patient (3.3%). Two patients required interruption of chemotherapy because of abnormal liver transaminase levels, which recovered after hepatoprotective treatment.
Table 3
| Toxicity | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|
| Hematological | ||||
| Neutropenia | 3 (10.0) | 11 (36.7) | 3 (10.0) | 1 (3.3) |
| Thrombocytopenia | 11 (36.7) | 2 (6.7) | 1 (3.3) | 0 |
| Anemia | 2 (6.7) | 8 (26.7) | 1 (3.3) | 0 |
| Non-hematological | ||||
| ALT elevation | 7 (23.3) | 5 (16.7) | 2 (6.7) | 0 |
| AST elevation | 11 (36.7) | 2 (6.7) | 1 (3.3) | 0 |
| Nausea/vomiting | 2 (6.7) | 4 (13.3) | 1 (3.3) | 0 |
| Diarrhea | 3 (10.0) | 2 (6.7) | 1 (3.3) | 0 |
ALT, alanine transaminase; AST, aspartate aminotransferase.
Discussion
We evaluated the clinical outcomes of administration of raltitrexed and irinotecan concurrent with radiotherapy for treating patients with unresectable recurrent colorectal cancer and found IMRT with chemotherapy of raltitrexed and irinotecan resulted in good ORR, PFS, and LPFS, and the toxicity rate was within an acceptable range.
While surgical resection remains the mainstay treatment for patients with local recurrent colorectal cancer, it is often not feasible or adequate because of sidewall or high sacral fixation and may cause a significant increase in late complications such as leakage and abscess (3,20). While non-surgical treatments such as radiotherapy are essential for local treatment, the optimal chemotherapeutic agents to be used concurrently with radiation remain uncertain. We administered raltitrexed and irinotecan concurrently with radiation, considering that most recurrent patients had received fluoropyrimidine and oxaliplatin as adjuvant chemotherapy. Irinotecan is a key chemotherapeutic drug for colorectal cancer as its active metabolite SN-38 can induce single strand DNA breakage and form a stabilized SN-38-topoisomerase I-DNA cleavable complex, which leads to irreversible double-strand DNA breaks and cell death (11). Irinotecan has shown radiosensitization properties in preclinical models and early clinical trials, and a recent phase III trial demonstrated the addition of irinotecan to capecitabine-based neoadjuvant chemoradiation improved the pCR rate for locally advanced rectal cancer (12), which further confirmed its synergistic effect with radiotherapy. Raltitrexed can also enhance radiation sensitivity and has a different cytotoxicity mechanism with 5-FU (21). Hence, the combination of radiation and raltitrexed-irinotecan was analyzed in our study.
Cai et al. (22) investigated the efficacy of capecitabine and irinotecan administered with IMRT with a total radiation dose of 55–61 Gy in 71 patients with recurrent rectal cancer and reported a 46.5% ORR and a 1-year LPFS rate of 74.2%. In addition, another study indicated dose-escalated radiotherapy (≥70 Gy) for patients with recurrent colorectal cancer without distant metastases improved PFS (23), although only 11 patients (50.0%) received chemotherapy, including 5-FU, capecitabine, tegafur, oxaliplatin, and doxifluridine, which was determined by their physicians. A higher dose may help achieve a better local response but could also increase adverse events which cause further intolerance to chemotherapy. A retrospective study including 18 local recurrent patients without distant metastases and who received chemoradiotherapy reported a 39% overall response and 17% CR rate (24), although their concurrent regimen was 5-FU with oxaliplatin, which was not suitable for our patients. Additionally, that study only included patients with local recurrent rectal cancer without distant metastases.
It should be noted that 12 patients (40.0%) in our study already had metastases before treatment. Thus, we opted for intensive chemotherapy with moderate-intensity radiation to achieve good local control and distant control at the same time. The addition of agents targeting vascular endothelial growth factor (VEGF) or epidermal growth factor receptor (EGFR) to cytotoxic chemotherapeutic combinations has shown efficacy in treating metastatic colorectal cancer (25-27). A randomized control trial comparing radiation concurrent with raltitrexed and irinotecan with or without bevacizumab administration in patients with recurrent colorectal cancer will be conducted in the future.
Neoadjuvant chemoradiotherapy is the standard treatment for patients with locally advanced rectal cancer. However, in our study, none of the patients received neoadjuvant chemoradiotherapy or postoperative radiotherapy. For patients who underwent pelvic irradiation for the primary tumor, several studies have shown that further irradiation using hyperfractionation could also provide good local response at a dose of 30–40 Gy (28-31).
In our study, toxicities were acceptable and manageable. Neutropenia was the most common, with grades 3–4 recorded in 13.3% of patients, and although irinotecan was administered, the incidence of diarrhea was infrequent with grades 1–3 diarrhea observed in 20% of patients. This may be attributed to the 3-week schedule with a relatively low dose of 200 mg/m2, which was used in two phase III trials (AXEPT and CinClare), and because patients with UGT1A1 *28*28 genotype were excluded (12,32). Special attention should be given to hepatic function, as raltitrexed can cause elevation of transaminase levels.
A few limitations of this study exist. First, the follow-up period was relatively short (median: 22 months), and long-term survival may be elucidated upon further follow-up. Secondly, patients with distant metastasis were included in this study, so the tumor burden and physical condition were of heterogeneity. However, this reflected the reality that most patients develop distant metastasis when diagnosed with local recurrence. Moreover, our study included relatively few patients (n=30), and a large-scale study should be undertaken to evaluate the effects of this chemoradiotherapy regimen in the future.
Conclusions
IMRT with concurrent raltitrexed and irinotecan is a feasible treatment for unresectable recurrent colorectal cancer, which allows good tumor response and local control with acceptable toxicity profile.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-308/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-308/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-308/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study protocol was approved by the Institutional Review Board of the Fudan University Shanghai Cancer Center (Shanghai, China) (approval number: 1603158-3) and written informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Camilleri-Brennan J, Steele RJ. The impact of recurrent rectal cancer on quality of life. Eur J Surg Oncol 2001;27:349-53. [Crossref] [PubMed]
- Kodeda K, Derwinger K, Gustavsson B, et al. Local recurrence of rectal cancer: a population-based cohort study of diagnosis, treatment and outcome. Colorectal Dis 2012;14:e230-7. [Crossref] [PubMed]
- Nielsen MB, Laurberg S, Holm T. Current management of locally recurrent rectal cancer. Colorectal Dis 2011;13:732-42. [Crossref] [PubMed]
- Rahbari NN, Ulrich AB, Bruckner T, et al. Surgery for locally recurrent rectal cancer in the era of total mesorectal excision: is there still a chance for cure? Ann Surg 2011;253:522-33. [Crossref] [PubMed]
- Bouchard P, Efron J. Management of recurrent rectal cancer. Ann Surg Oncol 2010;17:1343-56. [Crossref] [PubMed]
- Mohiuddin M, Marks GM, Lingareddy V, et al. Curative surgical resection following reirradiation for recurrent rectal cancer. Int J Radiat Oncol Biol Phys 1997;39:643-9. [Crossref] [PubMed]
- Garcia-Aguilar J, Cromwell JW, Marra C, et al. Treatment of locally recurrent rectal cancer. Dis Colon Rectum 2001;44:1743-8. [Crossref] [PubMed]
- Lee JH, Kim DY, Kim SY, et al. Clinical outcomes of chemoradiotherapy for locally recurrent rectal cancer. Radiat Oncol 2011;6:51. [Crossref] [PubMed]
- Hong YS, Kim SY, Lee JS, et al. Oxaliplatin-Based Adjuvant Chemotherapy for Rectal Cancer After Preoperative Chemoradiotherapy (ADORE): Long-Term Results of a Randomized Controlled Trial. J Clin Oncol 2019;37:3111-23. [Crossref] [PubMed]
- Illum H. Irinotecan and radiosensitization in rectal cancer. Anticancer Drugs 2011;22:324-9. [Crossref] [PubMed]
- Zhu J, Liu A, Sun X, et al. Multicenter, Randomized, Phase III Trial of Neoadjuvant Chemoradiation With Capecitabine and Irinotecan Guided by UGT1A1 Status in Patients With Locally Advanced Rectal Cancer. J Clin Oncol 2020;38:4231-9. [Crossref] [PubMed]
- Avallone A, Di Gennaro E, Silvestro L, et al. Targeting thymidylate synthase in colorectal cancer: critical re-evaluation and emerging therapeutic role of raltitrexed. Expert Opin Drug Saf 2014;13:113-29. [Crossref] [PubMed]
- Barni S, Ghidini A, Coinu A, et al. A systematic review of raltitrexed-based first-line chemotherapy in advanced colorectal cancer. Anticancer Drugs 2014;25:1122-8. [Crossref] [PubMed]
- Liu Y, Wu W, Hong W, et al. Raltitrexed-based chemotherapy for advanced colorectal cancer. Clin Res Hepatol Gastroenterol 2014;38:219-25. [Crossref] [PubMed]
- Scheithauer W, Kornek GV, Schuell B, et al. Second-line treatment with oxaliplatin + raltitrexed in patients with advanced colorectal cancer failing fluoropyrimidine/leucovorin-based chemotherapy. Ann Oncol 2001;12:709-14. [Crossref] [PubMed]
- Batra A, Rigo R, Hannouf MB, et al. Real-world Safety and Efficacy of Raltitrexed in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer 2021;20:e75-81. [Crossref] [PubMed]
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311-9. [Crossref] [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [Crossref] [PubMed]
- Sorrentino L, Belli F, Guaglio M, et al. Prediction of R0/R+ surgery by different classifications for locally recurrent rectal cancer. Updates Surg 2021;73:539-45. [Crossref] [PubMed]
- Aparicio J, Vicent JM, Maestu I, et al. Multicenter phase II trial evaluating a three-weekly schedule of irinotecan plus raltitrexed in patients with 5-fluorouracil-refractory advanced colorectal cancer. Ann Oncol 2003;14:1121-5. [Crossref] [PubMed]
- Cai G, Zhu J, Palmer JD, et al. CAPIRI-IMRT: a phase II study of concurrent capecitabine and irinotecan with intensity-modulated radiation therapy for the treatment of recurrent rectal cancer. Radiat Oncol 2015;10:57. [Crossref] [PubMed]
- Jo S, Choi Y, Park SK, et al. Efficacy of Dose-Escalated Radiotherapy for Recurrent Colorectal Cancer. Ann Coloproctol 2016;32:66-72. [Crossref] [PubMed]
- Watanabe J, Shoji H, Hamaguchi T, et al. Chemoradiotherapy for Local Recurrence of Rectal Cancer: A Single Center Study of 18 Patients. In Vivo 2019;33:1363-8. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [Crossref] [PubMed]
- Qin S, Li J, Wang L, et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J Clin Oncol 2018;36:3031-9. [Crossref] [PubMed]
- Valentini V, Morganti AG, Gambacorta MA, et al. Preoperative hyperfractionated chemoradiation for locally recurrent rectal cancer in patients previously irradiated to the pelvis: A multicentric phase II study. Int J Radiat Oncol Biol Phys 2006;64:1129-39. [Crossref] [PubMed]
- Cai G, Zhu J, Hu W, et al. Accelerated hyperfractionated intensity-modulated radiotherapy for recurrent/unresectable rectal cancer in patients with previous pelvic irradiation: results of a phase II study. Radiat Oncol 2014;9:278. [Crossref] [PubMed]
- Mohiuddin M, Marks G, Marks J. Long-term results of reirradiation for patients with recurrent rectal carcinoma. Cancer 2002;95:1144-50. [Crossref] [PubMed]
- Dijkstra EA, Mul VEM, Hemmer PHJ, et al. Re-Irradiation in Patients with Recurrent Rectal Cancer is Safe and Feasible. Ann Surg Oncol 2021;28:5194-204. [Crossref] [PubMed]
- Xu RH, Muro K, Morita S, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): a multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol 2018;19:660-71. [Crossref] [PubMed]
(English Language Editor: B. Draper)




