
Colorectal cancer surgery in elderly patients 80 years and older: a comparison with younger age groups
Introduction
The number of people reaching old age is rapidly increasing worldwide. According to the World Population Prospects from the United Nations, people 65 years and older now comprise more than 20% of the world’s population. In Japan, that figure has climbed to nearly 30% over the last three decades, with life expectancy reaching 81 for males and 87 for females. This increase in life expectancy is accompanied by a higher incidence of malignancies (1), one of which is colorectal cancer (CRC). In Japan, CRC is the most common type of cancer and the second highest cause of cancer-related death after lung cancer (2). More than 150,000 new CRC cases are diagnosed annually in Japan, 80% of which are over 60 and 20% are over 80 years old, and one-third of these patients are lost each year (3). The incidence of CRC in the elderly is increasing across Japan (1) at a rate similar to other countries (4).
Curative resection with adjuvant chemotherapy is the standard treatment for CRC, but there has been some debate on whether surgery with curative intent should be performed on elderly patients. This age group tends to receive either less aggressive treatment or palliative surgery due to the higher incidence of complications and mortality (4-6). However, an evolving trend over the last two decades has revealed a significantly improved short-term survival rate after CRC surgery in patients over 65 years. The 30-day mortality rate has also dropped from 6 times as high (4) to 3 times as high (7) as younger age groups. For patients 75 years and older, Ketelaers et al. (8) recently reported that the rate fell from 5.8% [2006–2012] to 1.2% [2013–2017], a figure similar to younger age groups. It appears that age per se is not a risk factor that affects short-term outcome in elderly patients undergoing CRC surgery (9-11). Improvement of surgical procedures (laparoscopic over open surgery), preoperative assessments, and perioperative management have contributed to these trends, and CRC surgery will remain an important treatment modality as the elderly population continues to expand.
Physical and nutritional impairments have a decidedly negative impact on outcome after surgery (12,13). Frailty, sarcopenia, hypoalbuminemia, and other comorbidities are often seen among elderly patients undergoing both general surgery and CRC resection. Dolan et al. reported that sarcopenia, found in 19.6% of CRC elderly patients, correlated with more postoperative complications and higher mortality (14). Comorbidity was also identified as an independent risk factor for morbidity and mortality (15). Although these features were important in predicting outcome after CRC surgery in the elderly, most of these studies included patients over 65 years and limited postoperative follow-up to within 1 year. Although a few studies reported on long-term outcome after CRC resection, they lacked detailed analyses on the causes of patient death and the correlation with preoperative conditions.
In the present study, we aimed to determine the long-term outcome of elderly patients 80 years and older after CRC surgery in comparison with younger age groups, as well as to clarify the influence of oncological and physical parameters on their outcome. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-627/rc).
Methods
The present study was performed retrospectively on 346 patients (median age 70.5 years; range, 29–103 years) who underwent elective colorectal resection with curative intent (R0 and R1 resection) for primary colon cancer (n=283) or rectal cancer (n=63) between January 2013 and December 2017 at St. Mary’s Hospital. Patients with stage IV CRC (n=67) and those who underwent emergency surgery (n=23) were excluded from the study. Informed consent was obtained from individual patients and all data was collected retrospectively. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of St. Mary’s Hospital in Kurume, Japan (approval number 20-0701).
Patients were divided into three age groups: younger than 60 (n=47), between 60 and 79 (n=218), and 80 years and older (n=81), as younger age, elderly age, and very elderly age group, respectively, as previously reported (9,16-18). Preoperative data included gender, comorbidity (cardiovascular disease, hypertension, cerebral disease, renal disease, respiratory disease, diabetes mellitus, liver disease, cancer in other organs), American Society of Anesthesiologists Physical Status (ASA-PS) class, body mass index (BMI), and preoperative blood tests [hemoglobin, albumin, carcinoembryonic antigen (CEA), and prognostic nutrition index (PNI) calculated as 10 × serum albumin (g/dL) + 0.005 × total lymphocyte count (/mm3)] (19). The modified frailty index was determined by four comorbid conditions and one functional variable (13,20): a history of chronic obstructive pulmonary disease, congestive heart failure within 30 days of operation, diabetes mellitus requiring oral agents or insulin, hypertension requiring medication, and functional health status before operation (independent, partially independent, dependent). Each variable represents 1 point, for a total possible score of 5 points. A score of 2 or greater indicates frailty status (21). Intraoperative data included blood loss, operation time, and surgical method (open vs. laparoscopic). Tumor-related data included tumor location (right-sided/cecum to splenic flexure, left-sided/splenic flexure to sigmoid colon, or rectum), tumor margin status (R0/R1), tumor differentiation (well and moderate/poor and mucin), and tumor stage according to the 7th edition of the Union of International Cancer Control (UICC) (22). Immediate postoperative data included length of hospital stay, morbidity as denoted by a Clavien-Dindo class of III or greater (23), mortality within 30 days, and use of adjuvant chemotherapy. Parameters for postoperative outcome included mortality at 1 and 5 years in addition to cause of death, from which overall survival and disease-free survival rates were determined. Cause of death was categorized into CRC-related and “other”, which encompassed respiratory disease, cardiovascular disease, other primary cancer, sepsis, etc. The median follow-up period was 45.6 months (range, 0.2–92.1 months).
Statistical analysis
Values were expressed as frequency and percentage. Differences in the categorical variables among the three age groups were compared using the Chi-square test or Fisher’s exact test as appropriate. Overall and disease-free survival rates were analyzed with the Kaplan-Meier method using the log-rank test. Overall survival was defined as the period from the date of surgery to the date of last follow-up or death. Disease-free survival was defined as the duration from surgery to the date of CRC recurrence. Statistically significant variables from the univariate analysis were subsequently tested by multivariate analysis using the Cox proportional hazards model to determine the association between the individual determinants and death from CRC and other causes, and the effect of each variable was assessed by the hazard ratio (HR) and 95% confidence interval (95% CI). A P value of less than 0.05 was considered to be statistically significant. All statistical analyses were performed using the JMP software package (version 13.0, SAS Institute, Cary, NC, USA).
Results
Preoperative characteristics of CRC patients
Differences in preoperative features among the three age groups are shown in Table 1. Of the 346 patients, 47 (13.6%) were younger than 60 years, 218 (63.0%) were between 60 and 79 years, and 81 (23.4%) were 80 years and older, respectively. Female patients prevailed in the oldest age group, while males dominated in the two younger groups. There was no significant difference in BMI among the three groups. Patients in the 80+ age group had a higher occurrence of comorbidity and both a higher ASA-PS class and modified frailty index than the other age groups. In addition, laboratory studies revealed that this age group had significantly lower levels of PNI, hemoglobin, and albumin along with significantly higher levels of CEA than those in the younger groups.
Table 1
| Characteristic | N | <60 years (n=47), n (%) | 60–79 years (n=218), n (%) | ≥80 years (n=81), n (%) | P value |
|---|---|---|---|---|---|
| Gender | <0.001 | ||||
| Male | 199 | 27 (57.4) | 141 (64.7) | 31 (38.3) | |
| Female | 147 | 20 (42.6) | 77 (35.3) | 50 (61.7) | |
| Comorbidities | <0.001 | ||||
| No | 87 | 31 (66.0) | 50 (22.9) | 6 (7.4) | |
| One or more | 259 | 16 (34.0) | 168 (77.1) | 75 (92.6) | |
| ASA-PS class | <0.001 | ||||
| 1 | 120 | 37 (78.7) | 70 (32.1) | 13 (16.0) | |
| ≥2 | 226 | 10 (21.3) | 148 (67.9) | 68 (84.0) | |
| Modified frailty index | <0.001 | ||||
| 0–1 | 208 | 44 (93.6) | 128 (59.0) | 36 (44.4) | |
| ≥2 | 138 | 3 (6.4) | 90 (41.0) | 45 (55.6) | |
| BMI (kg/m2) | 0.524 | ||||
| <25 | 270 | 36 (76.6) | 167 (76.6) | 67 (82.7) | |
| ≥25 | 76 | 11 (23.4) | 51 (23.4) | 14 (17.3) | |
| PNI | <0.001 | ||||
| <40 | 90 | 5 (10.6) | 46 (21.1) | 39 (48.1) | |
| ≥40 | 256 | 42 (89.4) | 172 (78.9) | 42 (51.9) | |
| Hb (g/dL) | <0.001 | ||||
| <11 | 136 | 12 (25.5) | 75 (34.6) | 49 (60.5) | |
| ≥11 | 210 | 35 (74.5) | 143 (65.4) | 32 (39.5) | |
| Alb (g/dL) | <0.001 | ||||
| <3.5 | 105 | 7 (14.9) | 57 (26.1) | 41 (50.6) | |
| ≥3.5 | 241 | 40 (85.1) | 161 (73.9) | 40 (49.4) | |
| CEA (ng/mL) | 0.038 | ||||
| <5 | 198 | 26 (55.3) | 135 (61.9) | 37 (45.7) | |
| ≥5 | 148 | 21 (44.7) | 83 (38.1) | 44 (54.3) |
ASA-PS, American Society of Anesthesiologists Physical Status; BMI, body mass index; PNI, prognostic nutrition index; Hb, hemoglobin; Alb, albumin; CEA, carcinoembryonic antigen.
Surgical achievements were similar among the three groups (Table 2). There were no statistical differences in laparoscopic vs. open surgery, amount of blood loss, or duration of operation. Investigation for tumor-related factors revealed that while rectal cancer saw a higher incidence among younger patients, CRC in patients 80 years and older was more often right-sided (male: 15/49 and female: 34/49). Pathological examination of resected specimens according to UICC classification showed no differences in tumor stage and differentiation among groups (Table 2). Although all patients underwent surgery with curative intent, 26 specimens revealed residual tumor cells on the tumor margin (R1) across all groups: 2 (4.3%), 17 (7.8%), and 7 (8.6%) respectively. R0 resection rates followed at 95.7%, 92.2%, and 91.4% respectively. There was no significant difference in R0 resection among groups.
Table 2
| Characteristic | N | <60 years (n=47), n (%) | 60–79 years (n=218), n (%) | ≥80 years (n=81), n (%) | P value |
|---|---|---|---|---|---|
| Surgical procedure | 0.538 | ||||
| Laparoscopic surgery | 271 | 34 (72.3) | 174 (79.8) | 63 (77.8) | |
| Open surgery | 75 | 13 (27.7) | 44 (20.2) | 18 (22.2) | |
| Blood loss (mL) | 0.529 | ||||
| <150 | 239 | 32 (68.1) | 147 (67.4) | 60 (74.1) | |
| ≥150 | 107 | 15 (31.9) | 71 (32.6) | 21 (25.9) | |
| Operation time (min) | 0.189 | ||||
| <270 | 154 | 23 (48.9) | 89 (40.8) | 42 (51.9) | |
| ≥270 | 192 | 24 (51.1) | 129 (59.2) | 39 (48.1) | |
| Tumor location | 0.001 | ||||
| Right side (C-T) | 139 | 14 (29.8) | 76 (34.9) | 49 (60.5) | |
| Left side (D-Rs) | 144 | 21 (44.7) | 99 (45.4) | 24 (29.6) | |
| Rectal | 63 | 12 (25.5) | 43 (19.7) | 8 (9.9) | |
| UICC stage | 0.259 | ||||
| I/II | 219 | 28 (59.6) | 145 (66.5) | 46 (56.8) | |
| III | 127 | 19 (40.4) | 73 (33.5) | 35 (43.2) | |
| Tumor differentiation | 0.185 | ||||
| Well and moderate | 327 | 44 (93.6) | 209 (95.9) | 74 (91.4) | |
| Poor and mucin | 19 | 3 (6.4) | 9 (4.1) | 7 (8.6) | |
| Tumor margin status | 0.604 | ||||
| R0 | 320 | 45 (95.7) | 201 (92.2) | 74 (91.4) | |
| R1 | 26 | 2 (4.3) | 17 (7.8) | 7 (8.6) |
UICC, Union of International Cancer Control.
Short-term outcome
Table 3 illustrates differences in the immediate postoperative course among the three age groups. The duration of hospital stay after surgery (mean ± standard deviation) was 17.2±15.6 days in the younger than 60 group, 20.9±17.7 days in the 60–79 group, and 21.8±17.1 days in the 80 and older group, revealing that the oldest age group required significantly longer hospital stays after surgery. Immediate postoperative morbidity in patients with a Clavien-Dindo class of III or greater (16.0%), and 30-day mortality (2.5%) in patients 80 years and older were not statistically different from those of younger age groups; however, elderly patients faced more complications and higher mortality than younger age groups. Two patients over 80 years died within 30 days after surgery from heart failure and aspiration pneumonia. Adjuvant chemotherapy was administered postoperatively to patients across all age groups who were eligible for treatment: 26 (81.3%), 84 (58.3%), and 4 (6.5%).
Table 3
| Variables | N | <60 years (n=47), n (%) | 60–79 years (n=218), n (%) | ≥80 years (n=81), n (%) | P value |
|---|---|---|---|---|---|
| Short-term outcome | |||||
| Postoperative mortality within 30 days | 2 | 0 (0) | 0 (0) | 2 (2.5) | 0.072 |
| Postoperative morbidity within 30 days (Clavien-Dindo ≥ III) | 33 | 2 (4.3) | 18 (8.3) | 13 (16.0) | 0.068 |
| Postoperative hospital stay | 0.028 | ||||
| <21 days | 254 | 41 (87.2) | 159 (72.9) | 54 (66.7) | |
| ≥21 days | 92 | 6 (12.8) | 59 (27.1) | 27 (33.3) | |
| Survival at 1 year post-surgery | 0.001 | ||||
| Alive | 327 | 46 | 212 | 69 | |
| Dead | 19 | 1 | 6 | 12 | |
| Long-term outcome | |||||
| 5-year survival | 92 | 90.8% | 86.6% | 52.1% | <0.001 |
| 5-year disease-free survival | 77 | 78.4% | 81.9% | 81.7% | 0.875 |
| Recurrence at 5 years | 59 | 9 | 38 | 12 | 0.797 |
| Number of deaths at 5 years | 60 | 4 | 26 | 30 | |
| Cause of death | <0.001 | ||||
| Colorectal cancer | 30 | 3 (75.0) | 18 (69.2) | 9 (30.0) | |
| Other | 30 | 1 (25.0) | 8 (30.8) | 21 (70.0) | |
| Cause of other deaths | |||||
| Respiratory disease | 10 | 3 | 7 | ||
| Cardiovascular disease | 8 | 3 | 5 | ||
| Other primary cancer | 3 | 1 | 2 | ||
| Sepsis | 3 | 3 | |||
| Other | 6 | 1 | 1 | 4 |
Long-term outcome
The median follow-up period was 45.6 months, with a range of 0.2–92.1 months. The breakdown by group is as follows: 46.7 months for the younger than 60 group, 48.3 months for the 60–79 group, and 33.3 months for the 80 years and older group. During follow-up, 59 patients (17.1%) developed local or distant metastases: 19.1% in the younger than 60 group, 17.4% in those between 60 and 79, and 14.8% in the 80 and older group. Of the 346 patients, a total of 60 patients died: 8.5% of those younger than 60, 11.9% of those between 60 and 79, and 37.0% of those 80 and older. Of the 30 deaths in the oldest group, 9 were directly from CRC and the remaining 21 were from other causes. These causes included respiratory disease (30.0%), cardiovascular disease (23.8%), other primary cancer (9.5%), sepsis (14.3%), and others such as senility, renal failure or suicide (19.0%). Seventy percent of deaths encountered in patients 80 years and older during the follow-up period were primarily from respiratory failure and cardiovascular disease, not cancer. As shown in Table 3 and Figure 1, there was no significant difference among age groups in disease-free survival, while overall survival was significantly lower in patients 80 years and older (Table 3, Figure 2).
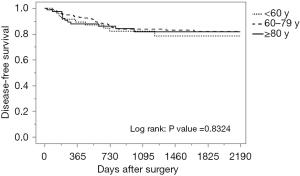
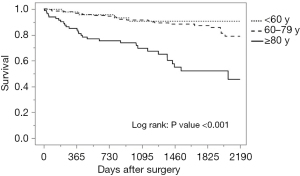
Factors influencing cause of death
A univariate analysis of CRC death revealed that tumor margin status, UICC stage, CEA, tumor differentiation, postoperative hospital stay, morbidity, BMI, and operative blood loss were significant factors influencing cause of death. Multivariate analysis showed that margin status, UICC stage, CEA, tumor differentiation, and postoperative hospital stay were found to be important risk factors (Table 4). Age was not a significant factor for CRC death. A univariate analysis of other causes of death revealed that age, modified frailty index, PNI, surgical procedure, operation time, morbidity, and postoperative hospital stay were significant factors. Age, modified frailty index, PNI, and surgical procedure were found to be significant risk factors for mortality from causes other than CRC through the multivariate analysis.
Table 4
| Variables | Deaths from colorectal cancer | Deaths from causes other than colorectal cancer | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||||
| Age | 0.165 | <0.001 | <0.001 | ||||||||
| <60 years | Reference | – | Reference | – | – | ||||||
| 60–79 years | 1.38 (0.46–5.95) | 0.593 | 2.14 (0.39–39.61) | 0.431 | 0.75 (0.11–14.77) | 0.802 | |||||
| ≥80 years | 2.85 (0.85–12.83) | 0.929 | 16.82 (3.51–301.60) | <0.001 | 4.93 (0.84–93.78) | 0.081 | |||||
| Gender, male | 0.88 (0.42–1.89) | 0.746 | 1.56 (0.75–3.48) | 0.239 | |||||||
| Modified frailty index ≥2 | 1.94 (0.92–4.12) | 0.081 | 5.77 (2.60–14.55) | <0.001 | 3.44 (1.50–9.13) | 0.003 | |||||
| PNI <40 | 1.60 (0.66–3.51) | 0.278 | 4.26 (2.07–8.88) | <0.001 | 2.48 (1.11–5.61) | 0.026 | |||||
| Hb <11 g/dL | 1.11 (0.51–2.35) | 0.783 | 1.67 (0.81–3.44) | 0.165 | |||||||
| BMI <25 kg/m2 | 4.30 (1.29–26.69) | 0.014 | 1.02 (0.25–6.99) | 0.976 | 1.35 (0.59–3.64) | 0.503 | |||||
| CEA ≥5 ng/mL | 4.47 (1.87–12.34) | 0.001 | 5.88 (2.05–20.06) | <0.001 | 1.32 (0.56–3.06) | 0.521 | |||||
| Tumor margin R1 | 13.13 (6.06–27.72) | <0.001 | 10.59 (3.97–29.20) | <0.001 | 3.43 (0.82–9.73) | 0.084 | |||||
| Open procedure | 2.12 (0.93–4.55) | 0.072 | 2.97 (1.42–6.15) | 0.005 | 2.44 (1.02–5.91) | 0.045 | |||||
| Tumor location | 0.533 | 0.080 | |||||||||
| Right side | Reference | – | Reference | – | |||||||
| Left side | 0.75 (0.30–1.80) | 0.512 | 0.68 (0.31–1.42) | 0.301 | |||||||
| Rectal | 1.28 (0.50–3.18) | 0.593 | 0.24 (0.04–0.86) | 0.026 | |||||||
| UICC stage III | 5.62 (2.56–13.62) | <0.001 | 6.00 (2.32–17.14) | <0.001 | 1.87 (0.89–3.86) | 0.098 | |||||
| Tumor differentiation poor and mucin | 4.53 (1.52–11.00) | 0.010 | 7.01 (1.85–25.43) | 0.005 | 0.83 (0.05–3.86) | 0.847 | |||||
| Postoperative hospital stay ≥21 days | 3.63 (1.72–7.76) | 0.001 | 5.36 (1.70–17.11) | 0.005 | 2.70 (1.30–5.55) | 0.009 | 1.91 (0.76–4.71) | 0.167 | |||
| Clavien-Dindo class ≥ III | 3.16 (1.24–7.10) | 0.018 | 1.05 (0.32–3.55) | 0.939 | 2.71 (1.00–6.22) | 0.049 | 2.45 (0.74–7.70) | 0.139 | |||
| Operation time ≥270 min | 1.29 (0.61–2.72) | 0.450 | 3.36 (1.59–7.73) | 0.001 | 2.37 (0.99–6.04) | 0.054 | |||||
| Operative blood loss ≥150 mL | 3.58 (1.70–7.89) | 0.001 | 1.01 (0.38–2.69) | 0.989 | 1.07 (0.47–2.27) | 0.866 | |||||
HR, hazard ratio; CI, confidence interval; PNI, prognostic nutrition index; BMI, body mass index; Hb, hemoglobin; CEA, carcinoembryonic antigen; UICC, Union of International Cancer Control.
Discussion
This study aimed to determine the long-term outcome, cause of death, and risk factors in elderly patients 80 years and older undergoing CRC surgery. The results showed that there were no significant differences in short-term morbidity, mortality, or 5-year disease-free survival between elderly patients 80 years and older and patients in younger age groups. R1 resection, advanced cancer stage, presence of pathological undifferentiated tumor, elevated CEA, and longer postoperative hospital stay were identified as risk factors for CRC deaths. In contrast, long-term overall survival was significantly reduced in elderly patients 80 years and older compared to younger age groups. Seventy percent of deaths encountered in patients 80 years and older were primarily from respiratory failure and cardiovascular diseases. Advanced age, frailty, low PNI, and open procedures were important risk factors for deaths from causes other than CRC.
There is an evolving trend demonstrating significant improvement in short-term survival rates after CRC surgery over the last 2 decades. In a systematic review of 34,194 patients conducted in 2000, the Colorectal Cancer Collaborative Group (4) reported that 30-day mortality of patients over 85 years old was 6.2 times higher than that of patients under 65. Eleven years later, an analysis of 19,375 patients by Al-Refaie et al. (7) using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) reported that 30-day mortality of patients over 80 years was only three times higher compared to those in the 40–55 age range. Most recently, Ketalaers et al. (8) described improvement in 30-day mortality of patients over 75 years from 5.8% [2006–2012] to 1.2% [2013–2017]. Surgical procedure (laparoscopic vs. open surgery), anesthesia, and postoperative management have contributed to this decrease, but CRC surgery for the elderly remains a significant concern as the demographic continues to grow.
Our data showed that patients 80 years and older frequently presented with comorbidity, higher ASA-PS class, advanced modified frailty index, and lower levels of PNI, albumin and hemoglobin. Those between 60–79 years exhibited a similar trend to those under 60 years. Of interest was the female patient population presenting with more right-sided colon cancer and less rectal cancer—a finding similar to previous reports (24). In addition, a higher CEA level in patients 80 years and older might be caused by more right-sided cancer with malignant potential (25,26). Since these characteristics often distinguish elderly patients from their younger counterparts, careful preoperative geriatric assessments including comorbidity, physical activity, and nutritional impairment are important for elderly patients undergoing CRC surgery.
There was no significant difference among age groups in 30-day morbidity. Postoperative morbidity in patients with a Clavien-Dindo class of III or greater, and mortality in patients 80 years and older within 30 days after surgery were 16.0% and 2.5%, respectively—findings that appear relatively low when compared to the 11–31.7% morbidity rate (27-29) and 2–7% mortality rate (28,30). As described by Ketelaers et al. (8), these results suggest that short-term outcome after CRC surgery has nearly equalized for older and younger CRC patients, although prolonged length of postoperative stay is required. Despite the absence of significant difference in the frequency of morbidity among age groups, the incidence of anastomotic leakage, paralytic ileus, and pneumonia tended to be higher in patients 80 years and older (data not shown). The frequency of dysphagia, decline of ADLs, and delirium after surgery were also encountered more frequently in the oldest patient group.
There were no statistically significant differences in disease-free survival and recurrence rate among the three age groups. Our study showed that risk factors for CRC death were R1 resection, advanced tumor stage, a CEA level of >5 ng/mL, pathological undifferentiated tumor and longer postoperative hospital stay, all of which were findings similar to previous reports (27,31-33). These results demonstrate that elderly patients undergoing CRC surgery receive oncological benefit. As Papamichael et al. previously described (34), radical curative surgery for elderly patients with CRC should not be withheld solely due to advanced age. Virk et al. reported the 10-year survival rate for patients undergoing surgery for CRC was 25.45% in the 80–89 age group, while the rate for those who did not receive treatment or surgery was a dismal 0.96% (16).
In our study, the 5-year overall survival rate was 52.1% in patients 80 years and older, which was similar to the 40–58% survival rates reported by others (17,27,33). Based on these results, we believe that advanced age is not a contraindication for surgery in patients with CRC. However, our study showed that seventy percent of deaths encountered in patients over 80 years died of causes not related to CRC during the follow-up period. Major reasons for other causes of death were respiratory failure, especially by aspiration pneumonia, followed by cardiovascular disease and sepsis. A large-scale analysis (4) found a that the incidence of respiratory failure in patients over 85 years was 3 times higher than those under 65 years. Previous studies indicated that aspiration pneumonia is caused by impairments of the swallowing and cough reflexes after experiencing a decrease of muscle strength in swallowing and respiration (18,29). Poor oral health in frail older patients increased the incidence of aspiration pneumonia (35). Furthermore, dysphagia is associated with oral, physical, cognitive and psychological frailty in elderly patients (36,37). In this study, 55.6% of patients 80 years and older who met the criteria for modified frailty experienced dysphagia at a rate of 2.5% (2/81) before surgery and 18.5% (15/81) after surgery (data not shown). Ten (83.3%) of the 12 patients 80 and older who died within 1 year after surgery had experienced difficulty with mobility. Postoperative dysphagia was found in 6 patients (50.0%), modified frailty in 9 patients (75.0%), and low PNI in 7 patients (58.3%) (data not shown). Preoperative frailty, postoperative dysphagia, and deficits in nutritional status are all factors likely establishing aspiration pneumonia as the first leading cause of non-cancer death (38). Emphasis on oral health care and swallowing training might be useful for prevention of aspiration pneumonia in elderly frail patients. A population-based study reported that patients with CRC are associated with an increased risk of cardiovascular death, especially during the first year after diagnosis (39). In this study, 5 of the 8 deaths from cardiovascular disease (62.5%) occurred within 1 year. CRC patients should be screened early after diagnosis for cardiovascular disease.
We found that advanced age, frailty, low PNI, and open procedure were independent risk factors for other causes of death after CRC surgery; however, there are only a few reports that analyze these risk factors. Next to age, modified frailty index was found to be the second most important risk factor for mortality from non-CRC causes. Frailty is a state of vulnerability characterized by an age-associated decline in physiological and functional reserve across multiple organ systems and is associated with a greater risk of adverse postoperative outcomes such as severe complications, decreased long-term survival, higher readmission rates, and longer hospital stays (19), all of which are similar to outcomes in patients with sarcopenia (40,41). Preoperative evaluation of frailty could be a promising risk stratification tool for older patients undergoing general surgery (20). A low preoperative PNI level was found to be the third risk factor for survival and is significantly associated with the incidence of postoperative complications and poor prognosis in patients with CRC after laparoscopic surgery (15). Preoperative assessment of modified frailty index and PNI may help reliably predict both non-CRC related deaths after surgery as well as postoperative complications, which could prove useful when selecting elderly patients undergoing CRC surgery. Those with a higher risk may benefit from prehabilitation therapy before surgery (42). Our data also indicated that a laparoscopic approach to CRC surgery was beneficial for elderly patients, even those with comorbidities and decreased physical activity. This finding was corroborated in a previous report (43,44).
The present study has several limitations. It is a retrospective study that lacks assessment of postoperative quality of life. Patients with stage IV CRC and those who underwent emergency surgery were excluded, which further reduced the relatively small sample size. This limitation may have contributed to the lack of statistical difference in morbidity and mortality among the three age groups. Strengths of this study include the availability of detailed perioperative information on the population undergoing CRC surgery, as well as the promising long-term outcomes identified. In addition, the study highlighted the potential for preoperative evaluation to become a powerful tool in understanding which elderly patients might benefit the most from surgery.
Many elderly patients 80 years and older who undergo CRC surgery experience oncological benefits similar to younger patients, and this holds true even for those near the age of life expectancy. Appropriate preoperative assessment of certain indices such as modified frailty index and PNI can be an important step in selection of patients for CRC surgery and improvement of outcome during recovery. Higher-risk patients may find benefit in prehabilitation therapy, a mode of ancillary treatment currently utilized at our institute.
Acknowledgments
We would like to thank two staff members from St. Mary’s Hospital: Mrs. Takiko Miyakawa, cancer registrar, for her efforts in data collection, and Professor Chiyo Tsutsumi in medical biostatistics for her assistance with statistical methods and assessment.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-627/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-627/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-627/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from individual patients and all data was collected retrospectively. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the institutional review board of St. Mary’s Hospital in Kurume, Japan (approval number 20-0701).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hori M, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2009: a study of 32 populationbased cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2015;45:884-91. [Crossref] [PubMed]
- National cancer center Japan. Latest cancer statistics. Available online: https://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed on July 18, 2021.
- National cancer center Japan. Cancer statistics prediction. Available online: https://ganjoho.jp/reg_stat/statistics/stat/short_pred.html. Accessed on July 18, 2021.
- Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968-74. [Crossref] [PubMed]
- Bojer AS, Roikjær O. Elderly patients with colorectal cancer are oncologically undertreated. Eur J Surg Oncol 2015;41:421-5. [Crossref] [PubMed]
- Smith FM, Rao C, Oliva Perez R, et al. Avoiding radical surgery improves early survival in elderly patients with rectal cancer, demonstrating complete clinical response after neoadjuvant therapy: results of a decision-analytic model. Dis Colon Rectum 2015;58:159-71. [Crossref] [PubMed]
- Al-Refaie WB, Parsons HM, Habermann EB, et al. Operative outcomes beyond 30-day mortality: colorectal cancer surgery in oldest old. Ann Surg 2011;253:947-52. [Crossref] [PubMed]
- Ketelaers SHJ, Orsini RG, Burger JWA, et al. Significant improvement in postoperative and 1-year mortality after colorectal cancer surgery in recent years. Eur J Surg Oncol 2019;45:2052-8. [Crossref] [PubMed]
- Bircan HY, Koc B, Ozcelik U, et al. Are there any differences between age groups regarding colorectal surgery in elderly patients? BMC Surg 2014;14:44. [Crossref] [PubMed]
- Marusch F, Koch A, Schmidt U, et al. The impact of the risk factor "age" on the early postoperative results of surgery for colorectal carcinoma and its significance for perioperative management. World J Surg 2005;29:1013-21; discussion 1021-2. [Crossref] [PubMed]
- Lim SW, Kim YJ, Kim HR. Laparoscopic surgery for colorectal cancer in patients over 80 years of age: the morbidity outcomes. Ann Surg Treat Res 2017;92:423-8. [Crossref] [PubMed]
- Weimann A, Braga M, Carli F, et al. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr 2017;36:623-50. [Crossref] [PubMed]
- Fagard K, Leonard S, Deschodt M, et al. The impact of frailty on postoperative outcomes in individuals aged 65 and over undergoing elective surgery for colorectal cancer: A systematic review. J Geriatr Oncol 2016;7:479-91. [Crossref] [PubMed]
- Dolan DR, Knight KA, Maguire S, et al. The relationship between sarcopenia and survival at 1 year in patients having elective colorectal cancer surgery. Tech Coloproctol 2019;23:877-85. [Crossref] [PubMed]
- Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg 2004;8:1061-7. [Crossref] [PubMed]
- Virk GS, Jafri M, Mehdi S, et al. Staging and survival of colorectal cancer (CRC) in octogenarians: Nationwide Study of US Veterans. J Gastrointest Oncol 2019;10:12-8. [Crossref] [PubMed]
- Mothes H, Bauschke A, Schuele S, et al. Surgery for colorectal cancer in elderly patients: how can we improve outcome? J Cancer Res Clin Oncol 2017;143:1879-89. [Crossref] [PubMed]
- Aquina CT, Mohile SG, Tejani MA, et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. Br J Cancer 2017;116:389-97. [Crossref] [PubMed]
- Tominaga T, Nagasaki T, Akiyoshi T, et al. Prognostic nutritional index and postoperative outcomes in patients with colon cancer after laparoscopic surgery. Surg Today 2020;50:1633-43. [Crossref] [PubMed]
- Ko FC. Preoperative Frailty Evaluation: A Promising Risk-stratification Tool in Older Adults Undergoing General Surgery. Clin Ther 2019;41:387-99. [Crossref] [PubMed]
- Chimukangara M, Helm MC, Frelich MJ, et al. A 5-item frailty index based on NSQIP data correlates with outcomes following paraesophageal hernia repair. Surg Endosc 2017;31:2509-19. [Crossref] [PubMed]
- Song YX, Gao P, Wang ZN, et al. Can the tumor deposits be counted as metastatic lymph nodes in the UICC TNM staging system for colorectal cancer? PLoS One 2012;7:e34087. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The ClavienDindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Roque-Castellano C, Fariña-Castro R. Colorectal cancer surgery in selected nonagenarians is relatively safe and it is associated with a good long-term survival: an observational study. World J Surg Oncol 2020;18:120. [Crossref] [PubMed]
- Weiss JM, Pfau PR, O'Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol 2011;29:4401-9. [Crossref] [PubMed]
- Don XX, Mai M, Ogino T. A clinicopathological study on colonic carcinomas arising from right side colon. Gan No Rinsho 1989;35:1421-8. [PubMed]
- Weerink LBM, Gant CM, van Leeuwen BL, et al. LongTerm Survival in Octogenarians After Surgical Treatment for Colorectal Cancer: Prevention of Postoperative Complications is Key. Ann Surg Oncol 2018;25:3874-82. [Crossref] [PubMed]
- Kim YW, Kim IY. Factors associated with postoperative complications and 1-year mortality after surgery for colorectal cancer in octogenarians and nonagenarians. Clin Interv Aging 2016;11:689-97. [Crossref] [PubMed]
- Wang R, Han L, Dai W, et al. Cause of death for elders with colorectal cancer: a real-world data analysis. J Gastrointest Oncol 2020;11:269-76. [Crossref] [PubMed]
- Turrentine FE, Wang H, Simpson VB, et al. Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg 2006;203:865-77. [Crossref] [PubMed]
- Zhou S, Wang X, Zhao C, et al. Laparoscopic vs open colorectal cancer surgery in elderly patients: short- and long-term outcomes and predictors for overall and diseasefree survival. BMC Surg 2019;19:137. [Crossref] [PubMed]
- Huang SH, Tsai WS, You JF, et al. Preoperative Carcinoembryonic Antigen as a Poor Prognostic Factor in Stage I-III Colorectal Cancer After Curative-Intent Resection: A Propensity Score Matching Analysis. Ann Surg Oncol 2019;26:1685-94. [Crossref] [PubMed]
- Maeda H, Okabayashi T, Ichikawa K, et al. Colorectal cancer surgery in patients older than 80 years of age: experience at one nonteaching hospital in Japan. Am Surg 2011;77:1454-9. [Crossref] [PubMed]
- Papamichael D, Audisio RA, Glimelius B, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 2015;26:463-76. [Crossref] [PubMed]
- van der Maarel-Wierink CD, Vanobbergen JN, Bronkhorst EM, et al. Oral health care and aspiration pneumonia in frail older people: a systematic literature review. Gerodontology 2013;30:3-9. [Crossref] [PubMed]
- Tanaka T, Takahashi K, Hirano H, et al. Oral Frailty as a Risk Factor for Physical Frailty and Mortality in Community-Dwelling Elderly. J Gerontol A Biol Sci Med Sci 2018;73:1661-7. [Crossref] [PubMed]
- Nishida T, Yamabe K, Honda S. Dysphagia is associated with oral, physical, cognitive and psychological frailty in Japanese community-dwelling elderly persons. Gerodontology 2020;37:185-90. [Crossref] [PubMed]
- Sura L, Madhavan A, Carnaby G, et al. Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging 2012;7:287-98. [PubMed]
- Gaitanidis A, Spathakis M, Tsalikidis C, et al. Risk factors for cardiovascular mortality in patients with colorectal cancer: a population-based study. Int J Clin Oncol 2019;24:501-7. [Crossref] [PubMed]
- Vergara-Fernandez O, Trejo-Avila M, Salgado-Nesme N. Sarcopenia in patients with colorectal cancer: A comprehensive review. World J Clin Cases 2020;8:1188-202. [Crossref] [PubMed]
- Trejo-Avila M, Bozada-Gutiérrez K, Valenzuela-Salazar C, et al. Sarcopenia predicts worse postoperative outcomes and decreased survival rates in patients with colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 2021;36:1077-96. [Crossref] [PubMed]
- Carli F, Bousquet-Dion G, Awasthi R, et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg 2020;155:233-42. [Crossref] [PubMed]
- Li Y, Wang S, Gao S, et al. Laparoscopic colorectal resection versus open colorectal resection in octogenarians: a systematic review and meta-analysis of safety and efficacy. Tech Coloproctol 2016;20:153-62. [Crossref] [PubMed]
- Devoto L, Celentano V, Cohen R, et al. Colorectal cancer surgery in the very elderly patient: a systematic review of laparoscopic versus open colorectal resection. Int J Colorectal Dis 2017;32:1237-42. [Crossref] [PubMed]


