
High neutrophil-to-lymphocyte ratio following stereotactic body radiation therapy is associated with poor clinical outcomes in patients with borderline resectable and locally advanced pancreatic cancer
Introduction
Pancreatic cancer is the third most common cause of cancer related deaths in the US, accounting for over 48,000 deaths each year (1). Treatment usually involves a combination of chemotherapy, radiation therapy, and surgical resection (2). Even with aggressive therapy for patients with localized disease at presentation, prognosis remains guarded, with 5-year overall survival (OS) of less than 15%, for example, among patients with borderline resectable (BRPC) and/or locally advanced pancreatic cancer (LAPC) (1,3).
The role of radiation therapy for localized pancreatic cancer remains controversial. Currently, radiation therapy is administered for the purpose of margin sterilization and local recurrence risk reduction in the neoadjuvant setting and for improving local progression-free survival (LPFS) and preventing morbidity from disease progression in the unresectable setting (4-13). Two randomized studies have shown that neoadjuvant chemoradiation is associated with higher rates of R0 resection and improved OS in BRPC (14,15). However, the recent Alliance A021510 trial did not show a benefit of pre-operative chemoradiation versus pre-operative chemotherapy for patients with BRPC (16). Given such conflicting results, a more complete mechanistic understanding of the impact of radiation therapy, beyond classical radiobiologic DNA damage pathways, is critical to optimizing the manner in which radiation is delivered for this patient population. As an example, conformal, hypo-fractionation is often cited as a potential tool to promote antigen release and evoke pro-immunogenic pathways, but supporting data is lacking.
Inflammation has been associated with chronic diseases including diabetes, heart failure, chronic obstructive pulmonary disease, and autoimmune conditions (17-20). It has also been linked to a wide range of cancers including colorectal cancer, head and neck cancer, prostate cancer, esophageal cancer, and pancreatic cancer (21-24). Studies have shown that markers of systemic inflammation such as C-reactive protein and neutrophil-to-lymphocyte ratio (NLR) can predict outcomes, with high levels associated with poor outcomes (24-26). The exact mechanism is unknown, but inflammation is thought to promote tumor angiogenesis, epithelial to mesenchymal transition, and suppression of cytotoxic T lymphocytes (26). The NLR is a particularly attractive metric since it can be readily calculated from routine blood cell counts.
The prognostic value of NLR in pancreatic cancer treated with radiation therapy has been investigated in only a handful of reports (27-31). Current studies are limited by heterogeneity in clinical outcomes, radiation dose/fractionation, and time point of NLR (i.e., pre-radiation or post-radiation). Additionally, only two of these studies report on the role of NLR in pancreatic cancer patients treated with stereotactic body radiation therapy (SBRT), with conflicting findings (27,30). As SBRT continues to be explored in pancreatic cancer, an understanding of NLR dynamics and its association with survival outcomes may yield insight into potential immunologic mechanisms of radiation effect, which may have implications on both optimal radiation delivery as well as potential opportunities for combination strategies with immunotherapeutic agents. As such, we report on the association of pre-SBRT and post-SBRT NLR with survival outcomes in a cohort of patients with BRPC/LAPC treated with multi-agent induction chemotherapy followed by five-fraction SBRT. We present the study in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-513/rc).
Methods
Study design
This was a single institution review of patients with BRPC or LAPC treated with multi-agent induction chemotherapy followed by five-fraction SBRT from August 2016 to January 2019. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the institutional review board of Johns Hopkins University (No.: IRB00285919). Informed consent was not taken for this study because it was retrospective in nature and no human experimentation/involvement was taken place.
Inclusion criteria for the study were as follows: (I) biopsy confirmed diagnosis of pancreatic cancer; (II) BRPC or LAPC per NCCN guidelines (2); (III) treatment with multi-agent induction chemotherapy followed by five-fraction SBRT; (IV) complete blood count values before and/or after SBRT available for review.
Treatment details
Patients were treated with multi-agent induction chemotherapy consisting of FOLFIRINOX (FFX), gemcitabine/nab-paclitaxel (GnP), FFX and GnP, or other regimens. Duration of upfront chemotherapy was at the discretion of the treating medical oncologist but was primarily based on response on interval computed tomography (CT) scans as well as tolerability. Following completion of chemotherapy, patients without distant progression were recommended for five-fraction SBRT. Prior to simulation, patients underwent endoscopic ultrasound-guided fiducial placement to assist with daily setup using cone beam CT (CBCT). At time of simulation, patients were positioned supine with their arms above their head in a Vac-Lok device (CIVCO Medical Solutions, Coralville, IA, USA) for immobilization. Thin-sliced CT scans with intravenous contrast were obtained for treatment planning. Respiratory motion was managed with active breathing control (ABC, Elekta, Stockholm, Sweden). In patients who could not tolerate ABC, a free breathing 4-dimensional (4D)-CT scan was acquired to account for respiratory motion, with an internal target volume (ITV) generated from peak inspiratory and expiratory phases. Target volumes and organs at risk were contoured using Pinnacle Treatment Planning Software (Phillips Radiation Oncology Systems, Fitchburg, WI, USA). The clinical target volume (CTV) consisted of gross disease seen on imaging and the full circumference of involved vasculature. A planning target volume (PTV) was generated by adding a 2–5 mm isotropic expansion to the CTV in breath-hold cases and to the ITV in free-breathing cases. Pre-treatment and intrafraction CBCT scans were acquired to verify patient positioning. Patients were aligned to bone and then shifted to align to fiducials. All patients were treated on an Elekta linear accelerator unit (Elekta, Stockholm, Sweden). Restaging CT scans were obtained approximately 4 weeks after completion of SBRT. Patients without distant progression and with local vascular involvement that was potentially amenable to complete surgical resection were considered for surgical exploration. Administration of adjuvant chemotherapy was at the discretion of the treating medical oncologist.
Laboratory values
Laboratory values included hematocrit, platelets, absolute neutrophil count (ANC), and absolute lymphocyte count (ALC). The NLR was obtained by dividing ANC by ALC. The platelet-to-lymphocyte ratio (PLR) was obtained by dividing platelet count by ALC. Laboratory values were collected within 4 weeks prior to the start of SBRT and 1–6 weeks after completion of SBRT. If multiples values existed, the value closest to start of SBRT and closest to 4 weeks after completion of SBRT was recorded.
Statistical analysis
Patient, disease, and treatment characteristics including age, sex, Karnofsky Performance Status, disease extent, chemotherapy duration/regimen, SBRT dose/fractionation, resection status, and laboratory values were recorded. Differences in median pre-/post-SBRT laboratory values were determined by Mann-Whitney U test. Receiver operating characteristic (ROC) curves were generated to identify the optimal pre-SBRT NLR, post-SBRT NLR, and change in (Δ) NLR cutoff values using the Youden index. These NLR cutoff values and median values for all other continuous variables were used in statistical analysis. Univariate Cox regression was performed to identify variables associated with OS, LPFS, distant metastasis-free survival (DMFS), and progression-free survival (PFS) from time of SBRT. OS was defined as time from SBRT to death. LPFS and DMFS were defined as time from SBRT to radiographic evidence of local progression and distant progression, respectively. PFS was defined as time from SBRT to radiographic evidence of any progression or death. Variables significant (P<0.05) on univariate Cox regression were included in multivariable Cox regression. Kaplan-Meier curves were generated for time to event outcomes, and statistical significance was determined by the log-rank test. A P value <0.05 was considered significant throughout the study, with all P values being 2-sided. Statistical analyses were performed with JMP version 14.0 (SAS institute, Cary NC, USA) and SPSS version 25.0 (IBM Corporation, Armonk NY, USA).
Results
Patient, disease, and treatment characteristics
Patient, disease, and treatment characteristics are shown in Table 1. From August 2016 to January 2019, 156 patients were treated with multi-agent induction chemotherapy followed by five-fraction SBRT. Median age was 66.4 years (range, 41.7–84.1 years), and 52% of patients were male. Borderline resectable disease and LAPC were seen in 41% (64/156) and 59% (92/156) of patients, respectively. The median baseline CA 19-9 was 216.8 U/mL (range, <1.0–7,358.4 U/mL), and median duration of upfront chemotherapy was 4 months (range, 1–18 months). Chemotherapy regimens consisted of FFX (94/156, 60.2%), GnP (32/156, 21%), FFX plus GnP (20/156, 13%), FFX plus other (3/156, 2%), GnP plus other (5/156, 3%), and other (2/156, 1%). All patients were treated with five-fraction SBRT. The most common dose/fractionation was 33 Gy/5 fractions (150/156, 96%), followed by 30 Gy/5 fractions (3/156, 2%), 36 Gy/5 fractions (2/156, 1%), and 30.5 Gy/5 fractions (1/156, 1%). The majority (106/156, 67%) of patients underwent surgical resection with Whipple procedure (69/106, 65%), distal pancreatectomy (34/106, 32 %), or total pancreatectomy (3/106, 3%). Post-SBRT/surgery chemotherapy was administered to 58 patients (37.2%) for a median duration of 2 months (range, 1–6 months).
Table 1
| Characteristics | N (%) or median [range] |
|---|---|
| No. of patients | 156 |
| Age (years) | 66.4 [41.7–84.1] |
| Sex | |
| Male | 81 (51.9) |
| Female | 75 (48.1) |
| KPS | |
| 90–100 | 116 (74.4) |
| 70–80 | 40 (25.6) |
| Histology | |
| Adenocarcinoma | 154 (98.8) |
| Acinar cell | 1 (0.6) |
| Undifferentiated carcinoma | 1 (0.6) |
| Location of primary tumor | |
| Head | 90 (57.7) |
| Other | 66 (42.3) |
| Disease extent | |
| Borderline resectable | 64 (41.0) |
| Locally advanced | 92 (59.0) |
| Baseline CA 19-9 (U/mL) | 216.8 [<1.0–7,358.4] |
| Induction chemotherapy duration (months) | 4 [1–18] |
| Induction chemotherapy | |
| FFX | 94 (60.2) |
| GnP | 32 (20.5) |
| FFX and GnP | 20 (12.8) |
| FFX plus other | 3 (1.9) |
| GnP plus other | 5 (3.2) |
| Other | 2 (1.3) |
| SBRT dose and fractionation | |
| 33 Gy in 5 fractions | 150 (96.2) |
| 30 Gy 5 fractions | 3 (1.9) |
| 36 Gy in 5 fractions | 2 (1.3) |
| 30.5 Gy in 5 fractions | 1 (0.6) |
| PTV (cm3) | 87.3 [13.1–381.6] |
| Surgically resected | 106 (67.1) |
| Whipple | 69 (65.1) |
| Distal | 34 (32.1) |
| Total pancreatectomy | 3 (2.8) |
| Post-SBRT/surgery chemotherapy | |
| Yes | 58 (37.2) |
| No | 91 (58.3) |
| Unknown | 7 (4.5) |
| Post-SBRT/surgery chemotherapy duration (months) | 2 [1–6] |
| Pre-SBRT counts | |
| Hct (%) | 33.3 [19.9–40.8] |
| Platelets (×1,000/μL) | 157.0 [40.0–457.0] |
| ANC (No./μL) | 3,715 [470–58,490] |
| ALC (No./μL) | 1,350 [340–4,980] |
| NLR | 2.6 [0.4–24.1] |
| PLR | 108.8 [17.2–1,269.4] |
| Post-SBRT counts | |
| Hct (%) | 35.9 [22.5–46.8] |
| Platelets (×1,000/μL) | 153.5 [42.4–416] |
| ANC (No./μL) | 2,980 [260–15,500] |
| ALC (No./μL) | 840 [180–1,990] |
| NLR | 3.3 [0.5–42.8] |
| PLR | 173.0 [33.9–944.4] |
KPS, Karnofsky Performance Status; CA 19-9, carbohydrate antigen 19-9; FFX, FOLFIRINOX; GnP, gemcitabine/nab-paclitaxel; SBRT, stereotactic body radiation therapy; PTV, planning target volume; Hct, hematocrit; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Laboratory values
Pre- and post-SBRT laboratory values are displayed in Table 1. Not all laboratory values were available for each patient. Missing values included pre-SBRT ALC (7/156, 4%), pre-SBRT ANC (7/156, 4%), pre-SBRT hematocrit (7/156, 4%), pre-SBRT platelets (7/156, 4%), post-SBRT ALC (40/156, 26%), post-SBRT ANC (40/156, 26%), post-SBRT hematocrit (22/156, 14%), and post-SBRT platelets (22/156, 14%). The median pre-SBRT and post-SBRT hematocrit values were 33.3% (range, 19.9–40.8%) and 35.9% (range, 22.5–46.8%), respectively. The median pre-SBRT and post-SBRT platelet counts were 157.0×1,000/μL (range, 40.0–457.0×1,000/μL) and 153.5×1,000/μL (range, 42.4–416×1,000/μL), respectively. The median pre-SBRT and post-SBRT PLR were 108.8 (range, 17.2–1,269.4) and 173.0 (range, 33.9–944.4), respectively. The median pre-SBRT ALC, ANC, and NLR were 1,350 No./μL (range, 340–4,980 No./μL), 3,715 No./μL (range, 470–58,490 No./μL), and 2.6 (0.4–24.1), respectively. The median post-SBRT ALC, ANC, and NLR were 840 No./μL (range, 180–1,990 No./μL), 2,980 No./μL (range, 260–15,500 No./μL), and 3.3 (0.5–42.8), with a change of −37.8% (P<0.001), −19.8% (P=0.014), and +26.9% (P=0.032), respectively (Figure 1).

Identification of NLR cutoff values
From ROC curves and Youden index, the optimal pre-SBRT NLR, post-SBRT NLR, and Δ NLR cutoff values in predicting OS were 2.3 (area under the curve: 0.614, sensitivity: 68.8%, specificity: 53.6%), 2.6 (area under the curve: 0.598, sensitivity: 84.4%, specificity: 43.1%), and −0.59 (area under the curve: 0.508, sensitivity: 82.0%, specificity: 34.4%) , respectively (Figure 2A-2C).

Clinical outcomes
Median follow-up time after SBRT for the entire cohort was 15.1 months (range, 0.3–42.4 months). At time of last follow-up, 85/156 patients (55%) had died. Of the patients who were alive, median follow-up time after SBRT was 20.1 months (range, 0.3–42.4 months). The median OS after SBRT was 17.6 months (range, 0.3–42.4 months), with 1-year, 2-year, and 3-year OS rates of 70.5%, 45.9%, and 26.7%, respectively. On univariate analysis (UVA), duration of induction chemotherapy, disease grade, resection status, pre-SBRT ANC (threshold 3.7 No./μL), pre-SBRT NLR (threshold 2.3), and post-SBRT NLR (threshold 2.6) were associated with OS (Table 2). Patients with post-SBRT NLR ≥2.6 had a median OS of 16.7 months versus median OS not yet reached in patients with post-SBRT <2.6 (P=0.009) (Figure 3A). On MVA, poorly differentiated disease (HR =1.82, 95% CI: 1.04–3.18, P=0.035), inability to undergo resection (HR =2.17, 95% CI: 1.25–3.70, P=0.006), and post-SBRT NLR ≥2.6 (HR =2.55, 95% CI: 1.20–5.45, P=0.015) were associated with inferior OS.
Table 2
| Variables | UVA | MVA | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (≥66 vs. <66 years) | 1.31 | 0.85–2.02 | 0.214 | ||||
| Sex (male vs. female) | 0.81 | 0.53–1.24 | 0.332 | ||||
| KPS (> 90 vs. ≤90) | 0.68 | 0.42–1.12 | 0.128 | ||||
| Disease extent (BRPC vs. LAPC) | 1.19 | 0.77–1.83 | 0.427 | ||||
| Tumor location (head vs. other) | 1.09 | 0.70–1.68 | 0.715 | ||||
| Induction CT duration (≥4 vs. <4 months) | 0.45 | 0.27–0.73 | 0.001 | 0.62 | 0.30–1.31 | 0.211 | |
| Induction CT (FFX vs. GnP) | 0.76 | 0.45–1.30 | 0.323 | ||||
| Grade (III vs. I/II) | 1.85 | 1.17–2.91 | 0.008 | 1.82 | 1.04–3.18 | 0.035 | |
| Resected (no vs. yes) | 2.86 | 1.85–4.55 | 0.001 | 2.17 | 1.25–3.70 | 0.006 | |
| PTV (≥87 vs. <87 cm3) | 1.40 | 0.90–2.17 | 0.130 | ||||
| Baseline CA 19-9 ( ≥216 vs. <216 U/mL) | 1.19 | 0.69–2.05 | 0.524 | ||||
| Pre-SBRT CA 19-9 (≥47 vs. <47 U/mL) | 1.23 | 0.79–1.91 | 0.368 | ||||
| Post-SBRT CA 19-9 (≥47 vs. <47 U/mL) | 1.69 | 0.83–3.46 | 0.151 | ||||
| Δ NLR (≥−0.59 vs. <−0.59) | 0.72 | 0. 43–1.21 | 0.220 | ||||
| Pre-SBRT counts | |||||||
| Hct (≥33.3% vs. <33.3%) | 0.85 | 0.55–1.32 | 0.463 | ||||
| Platelets (≥157 vs. <157 ×1,000/μL) | 0.83 | 0.44–1.58 | 0.570 | ||||
| ANC (≥3.7 vs. <3.7 No./μL) | 1.59 | 1.02–2.47 | 0.041 | 1.66 | 0.86–3.22 | 0.133 | |
| ALC (≥1.4 vs. <1.4 No./μL) | 1.12 | 0.72–1.74 | 0.608 | ||||
| NLR (≥2.3 vs. <2.3) | 1.78 | 1.11–2.84 | 0.017 | 1.00 | 0.50–2.01 | 0.986 | |
| PLR (≥108.8 vs. <108.8) | 1.17 | 0.75–1.82 | 0.483 | ||||
| Post-SBRT counts | |||||||
| Hct (≥35.9% vs. <35.9%) | 0.66 | 0.42–1.05 | 0.079 | ||||
| Platelets (≥153.5 vs. <153.5 ×1,000/μL) | 0.74 | 0.37–1.46 | 0.385 | ||||
| ANC (≥3.0 vs. <3.0 No./μL) | 1.02 | 0.62–1.66 | 0.950 | ||||
| ALC (≥0.8 vs. <0.8 No./μL) | 0.87 | 0.53–1.45 | 0.604 | ||||
| NLR (≥2.6 vs. <2.6) | 2.39 | 1.21–4.70 | 0.012 | 2.55 | 1.20–5.45 | 0.015 | |
| PLR (≥173.0 vs. <173.0) | 1.09 | 0.67–1.78 | 0.731 | ||||
KPS, Karnofsky Performance Status; CA 19-9, carbohydrate antigen 19-9; BRPC, borderline resectable pancreatic cancer; LAPC, locally advanced pancreatic cancer; CT, chemotherapy; FFX, FOLFIRINOX; GnP; gemcitabine/nab-paclitaxel; Δ, change; SBRT, stereotactic body radiation therapy; PTV, planning target volume; Hct, hematocrit; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
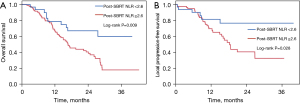
Given that post-SBRT NLR cutoff of 2.6 was significantly associated with OS on MVA, we next determined whether this cutoff value could predict LPFS. The median LPFS after SBRT for the entire cohort was 26.8 months, with 1-, 2-, and 3-year LPFS rates of 73.5%, 51.5%, and 48.9%, respectively. On UVA, baseline CA 19-9 (threshold 216 U/mL), pre-SBRT platelet level (threshold 157×1,000/μL), and post-SBRT NLR (threshold 2.6) were associated with LPFS (Table 3). Patients with post-SBRT NLR ≥2.6 had a median LPFS of 18.3 months versus median LPFS not yet reached in patients with post-SBRT NLR <2.6 (P=0.028) (Figure 3B). On MVA, only post-SBRT NLR ≥2.6 was associated with inferior LPFS (HR =3.22, 95% CI: 1.04–9.98, P=0.043) (Table 3). In the post-SBRT NLR ≥2.6 group, 38% (31/82) developed local progression compared to 19% (6/32) in the post-SBRT NLR <2.6 group (P=0.044) (Table 4).
Table 3
| Variables | UVA | MVA | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age (≥66 vs. <66 years) | 1.18 | 0.67–2.08 | 0.570 | ||||
| Sex (male vs. female) | 1.47 | 0.82–2.64 | 0.194 | ||||
| KPS (> 90 vs. <90) | 0.69 | 0.37–1.31 | 0.261 | ||||
| Disease extent (BRPC vs. LAPC) | 1.47 | 0.83–2.60 | 0.190 | ||||
| Tumor location (head vs. other) | 1.64 | 0.89–3.02 | 0.110 | ||||
| Induction CT duration (≥4 vs. <4 months) | 0.68 | 0.33–1.40 | 0.295 | ||||
| Induction CT (FFX vs. GnP) | 0.78 | 0.38–1.61 | 0.508 | ||||
| Grade (III vs. I/II) | 1.55 | 0.85–2.84 | 0.151 | ||||
| Resected (yes vs. no) | 1.49 | 0.78–2.86 | 0.230 | ||||
| PTV (≥87 vs. <87 cm3) | 0.91 | 0.51–1.63 | 0.752 | ||||
| Baseline CA 19–9 (≥216 vs. <216 U/mL) | 2.25 | 1.10–4.58 | 0.025 | 2.05 | 0.92–4.56 | 0.077 | |
| Pre–SBRT CA 19–9 (≥47 vs. <47 U/mL) | 1.54 | 0.86–2.76 | 0.150 | ||||
| Post–SBRT CA 19-9 (≥47 vs. <47 U/mL) | 2.23 | 0.90–5.52 | 0.085 | ||||
| Δ NLR (≥-0.59 vs. <−0.59) | 0.84 | 0.41–1.70 | 0.625 | ||||
| Pre-SBRT counts | |||||||
| Hct (≥33.3% vs. <33.3%) | 1.27 | 0.71–2.28 | 0.417 | ||||
| Platelets (≥157 vs. <157×1,000/μL) | 2.01 | 1.10–3.69 | 0.024 | 1.63 | 0.68–3.90 | 0.276 | |
| ANC (≥3.7 vs. <3.7 No./μL) | 1.15 | 0.64–2.06 | 0.640 | ||||
| ALC (≥1.4 vs. <1.4 No./μL) | 0.95 | 0.49–1.84 | 0.883 | ||||
| NLR (≥2.3 vs. <2.3) | 1.23 | 0.68–2.22 | 0.492 | ||||
| PLR (≥108.8 vs. <108.8) | 1.66 | 0.92–3.01 | 0.093 | ||||
| Post-SBRT counts | |||||||
| Hct (≥35.9% vs. <35.9%) | 1.02 | 0.56–1.85 | 0.953 | ||||
| Platelets (≥153.5 vs. <153.5×1,000/μL) | 1.06 | 0.58–1.91 | 0.858 | ||||
| ANC (≥3.0 vs. <3.0 No./μL) | 0.87 | 0.43–1.73 | 0.683 | ||||
| ALC (≥0.8 vs. <0.8 No./μL) | 1.27 | 0.63–2.53 | 0.505 | ||||
| NLR (≥2.6 vs. <2.6) | 2.60 | 1.07–6.28 | 0.034 | 3.22 | 1.04–9.98 | 0.043 | |
| PLR (≥173.0 vs. <173.0) | 1.23 | 0.64–2.37 | 0.529 | ||||
HR, hazard ratio; KPS, Karnofsky Performance Status; CA 19-9, carbohydrate antigen 19-9; BRPC, borderline resectable pancreatic cancer; LAPC, locally advanced pancreatic cancer; CT, chemotherapy; FFX, FOLFIRINOX; GnP; gemcitabine/nab-paclitaxel; Δ, change; SBRT, stereotactic body radiation therapy; PTV, planning target volume; Hct, hematocrit; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Table 4
| Variables | No. of patients | Local progression, n (%) | P value | |
|---|---|---|---|---|
| Yes | No | |||
| Post-SBRT NLR ≥2.6 | 82 | 31 (37.8) | 51 (62.2) | 0.044 |
| Post-SBRT NLR <2.6 | 32 | 6 (18.8) | 26 (81.2) | |
SBRT, stereotactic body radiation therapy; NLR, neutrophil-lymphocyte ratio.
Tables S1 and S2 show UVA and MVA for DMFS and PFS. On MVA, only poorly differentiated disease was associated worse DMFS (median DMFS: 7.5 vs. 11.5 months, HR =1.73, 95% CI: 1.09–2.75, P=0.019). On MVA, poorly differentiated disease (median PFS: 7.5 vs. 10.1 months, HR =1.65, 95% CI: 1.04–2.62, P=0.032) and post-SBRT hematocrit <35.9% (median PFS: 8.1 vs. 11.6 months, HR =0.52, 95% CI: 0.33–0.81, P=0.004) were associated with worse PFS.
Discussion
To our knowledge, this is the first study to report on the prognostic value of both pre- and post-SBRT NLR in a cohort of BRPC/LAPC patients treated with multi-agent induction chemotherapy and SBRT. We demonstrate that post-SBRT NLR is strongly associated with clinical outcomes. Specifically, post-SBRT NLR ≥2.6 predicted for worse OS and LPFS in this cohort.
The NLR has been identified as a prognostic factor in a wide range of malignancies, with high levels associated with poor outcomes (21-26). The exact mechanism is unknown. However, studies suggest that neutrophils have pro-tumorigenic effects through secretion of reactive oxygen species, which promote mutagenesis and chemokines/cytokines, which subsequently promote angiogenesis and tumor proliferation while suppressing cytotoxic T lymphocytes (32-34). In fact, a recent study of patients enrolled on LAP07, an international phase 3 trial that examined the role of consolidative chemoradiation after upfront chemotherapy in patients with LAPC, showed that baseline and pre-chemoradiation neutrophilia was associated with poor OS and decreased local control (35). Cytotoxic T lymphocytes, on the other hand, have anti-tumorigenic effects (36). A prior report from our institution demonstrated inferior OS in unresected LAPC patients who developed grade 3 lymphopenia following chemoradiation (37). These studies suggest that NLR, which takes into account both neutrophils and lymphocytes, may serve as a prognostic measure in pancreatic cancer treated with radiation therapy.
However, until now, few studies have investigated the prognostic role of post-radiation NLR, as opposed to pre-radiation NLR, given that the former may serve as a better measure of immunologic impact of radiation therapy for pancreatic cancer (27-31). A study by Pearson et al., published in abstract form, represents the only prior report on post-radiation NLR and found that post-SBRT NLR, and not pre-SBRT NLR, was predictive of OS (30). Our data corroborates these findings, with only post-SBRT NLR (≥2.6), and not pre-SBRT NLR, being predictive of outcomes in our cohort. Furthermore, while two prior studies have reported on the association of pre-treatment NLR with local response to therapy, our results are the first to suggest that post-radiation NLR is strongly associated with local control outcomes (29,31).
Our findings also suggest that classic radiobiological mechanisms of DNA damage may not fully encompass radiation effect in this setting and that exploration of radiation-induced immunologic mechanisms represents a key area of continued study (32). Radiation therapy can induce immunogenic death of cancerous cells but can also deplete intratumoral cytotoxic lymphocytes and induce formation of pro-tumorigenic neutrophil extracellular traps (32,34,38). This suggests that there is a fine balance between the anti- and pro-tumorigenic properties of radiation on the tumor microenvironment (TME). As such, further studies are warranted to better understand the complex interplay between radiation therapy and the pancreatic TME, with NLR serving as a potential biomarker of outcomes. Such understanding could have major implications for both optimal radiation design as well opportunities for combination therapy with immunotherapy. As an example, optimal radiation target volume design for pancreatic cancer remains controversial and highly variable, with some data supporting larger volume elective nodal irradiation (39). However, the impact of such volumes on dose to hematopoietic organs and circulating lymphocytes should be considered (40-42). Similarly, conformality and fractionation may also have impact in this regard. Indeed, in a recent study exploring the prognostic value of change in NLR in a cohort of patients with BRPC/LAPC, patients were treated with conventional fractionated radiation (median, 36 Gy/15 fractions) and experienced a mean increase in NLR of +99.7%, far greater than the median change in NLR of +26.9% experienced by patients in our cohort. Likewise, patients in the aforementioned study by Pearson et al. (30), in which SBRT was administered as well, also experienced a median increase in NLR of only +27.7%. These findings suggest that radiation technique may significantly impact NLR dynamics. Ultimately, further work to clarify such relationship mechanistically is warranted. Similarly, a better understanding of the manner in which radiation both promotes and disrupts immunologic processes may better inform those combination strategies that merit further study (43-47).
There are several limitations of this study including its single institution retrospective design, limiting generalizability of the findings. Furthermore, laboratory values were collected at varying times, anywhere from 1 day to 4 weeks prior to SBRT and from 1 week to 6 weeks after completion of SBRT. It is possible that these values may have fluctuated during these intervals. Additionally, patients received various upfront systemic therapy regimens including FFX, GnP, or a combination, which in turn, may have influenced laboratory values. Of note, upon subgroup analysis of patients who only received FFX and of patients who only received GnP, post-SBRT NLR still predicted for clinical outcomes. The strengths of this study are its large sample size, homogenous SBRT dose/fractionation regimen (150/156 receiving 33 Gy/5 fractions), and long follow-up time. Despite the limitations, these findings are in agreement with those from other studies and adds novel information about the role of NLR in pancreatic cancer treated with SBRT (27-31).
In conclusion, we demonstrate that post-SBRT NLR (threshold of ≥2.6) is associated with clinical outcomes following SBRT, with worse OS and LPFS observed in the high post-SBRT NLR group. These findings highlight the importance of further elucidating the immunologic effects of radiation therapy, which may have implications on radiation design and selection of combination strategies.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-513/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-513/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-21-513/coif). JMH is a former employee of Pancreatic Action Network and current employee of 1440 foundation. JMH serves as an unpaid editorial board member of Journal of the Gastrointestinal Oncology from January 2021 to December 2022. JM receives royalties from Uptodate and Springer and honoraria from Springer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Johns Hopkins University (No.: IRB00285919). Informed consent was not taken for this study because it was retrospective in nature and no human experimentation/involvement was taken place.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma. Version 2.2021). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf. Accessed 7/15/2021.
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 2019;10:10-27. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without Erlotinib: the LAP07 randomized clinical trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Cardillo N, Seible DM, Fero KE, et al. Clinical Impact of Local Progression in Pancreatic Cancer. J Natl Compr Canc Netw 2018;16:711-7. [Crossref] [PubMed]
- Sadot E, Doussot A, O'Reilly EM, et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol 2015;22:3512-21. [Crossref] [PubMed]
- Sherman WH, Chu K, Chabot J, et al. Neoadjuvant gemcitabine, docetaxel, and capecitabine followed by gemcitabine and capecitabine/radiation therapy and surgery in locally advanced, unresectable pancreatic adenocarcinoma. Cancer 2015;121:673-80. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Truty MJ, Kendrick ML, Nagorney DM, et al. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann Surg 2021;273:341-9. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18:543-8. [Crossref] [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a Prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 2018;268:215-22. [Crossref] [PubMed]
- Versteijne E, van Eijck CH, Punt CJ, et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials 2016;17:127. [Crossref] [PubMed]
- Katz MHG, Shi Q, Meyers JP, et al. Alliance A021501: Preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol 2021;39:377. [Crossref]
- Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016;138:16-27. [Crossref] [PubMed]
- Lopez-Candales A, Hernández Burgos PM, Hernandez-Suarez DF, et al. Linking Chronic Inflammation with Cardiovascular Disease: From Normal Aging to the Metabolic Syndrome. J Nat Sci 2017;3:e341. [PubMed]
- Duan L, Rao X, Sigdel KR. Regulation of Inflammation in Autoimmune Disease. J Immunol Res 2019;2019:7403796. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep 2019;9:19673. [Crossref] [PubMed]
- Murata M. Inflammation and cancer. Environ Health Prev Med 2018;23:50. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883-9. [Crossref] [PubMed]
- Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo) 2015;70:524-30. [Crossref] [PubMed]
- Kishi T, Nakamura A, Itasaka S, et al. Pretreatment C-reactive protein level predicts outcome and patterns of failure after chemoradiotherapy for locally advanced pancreatic cancer. Pancreatology 2015;15:694-700. [Crossref] [PubMed]
- Zhou Y, Wei Q, Fan J, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in pancreatic cancer: A meta-analysis containing 8252 patients. Clin Chim Acta 2018;479:181-9. [Crossref] [PubMed]
- Alagappan M, Pollom EL, von Eyben R, et al. Albumin and Neutrophil-Lymphocyte Ratio (NLR) Predict Survival in Patients With Pancreatic Adenocarcinoma Treated With SBRT. Am J Clin Oncol 2018;41:242-7. [Crossref] [PubMed]
- Wolfe AR, Siedow M, Nalin A, et al. Increasing neutrophil-to-lymphocyte ratio following radiation is a poor prognostic factor and directly correlates with splenic radiation dose in pancreatic cancer. Radiother Oncol 2021;158:207-14. [Crossref] [PubMed]
- Hasegawa S, Eguchi H, Tomokuni A, et al. Pre-treatment neutrophil to lymphocyte ratio as a predictive marker for pathological response to preoperative chemoradiotherapy in pancreatic cancer. Oncol Lett 2016;11:1560-6. [Crossref] [PubMed]
- Pearson AL, Jin W, Mellon EA, et al. Post-Stereotactic Body Radiation Therapy (SBRT) Neutrophil-to-Lymphocyte Ratio (NLR) in Patients With Borderline Resectable Pancreatic Cancer (BRPC) May Be a Prognostic Biomarker. Int J Radiat Oncol Biol Phys. 2016;96:E153. [Crossref]
- Lee BM, Chung SY, Chang JS, et al. The Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio Are Prognostic Factors in Patients with Locally Advanced Pancreatic Cancer Treated with Chemoradiotherapy. Gut Liver 2018;12:342-52. [Crossref] [PubMed]
- Jarosz-Biej M, Smolarczyk R, Cichoń T, et al. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int J Mol Sci. 2019;20:3212. [Crossref] [PubMed]
- de Kleijn S, Langereis JD, Leentjens J, et al. IFN-γ-Stimulated Neutrophils Suppress Lymphocyte Proliferation through Expression of PD-L1. PLoS One. 2013;8:e72249. [Crossref] [PubMed]
- Wu L, Saxena S, Awaji M, et al. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers (Basel) 2019;11:564. [Crossref] [PubMed]
- Schernberg A, Vernerey D, Goldstein D, et al. Predictive Value of Neutrophils Count for Local Tumor Control After Chemoradiotherapy in Patients With Locally Advanced Pancreatic Carcinoma. Int J Radiat Oncol Biol Phys 2021;110:1022-31. [Crossref] [PubMed]
- Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: A review. J Cell Physiol 2019;234:8509-21. [Crossref] [PubMed]
- Wild AT, Ye X, Ellsworth SG, et al. The Association Between Chemoradiation-related Lymphopenia and Clinical Outcomes in Patients With Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol 2015;38:259-65. [Crossref] [PubMed]
- Shinde-Jadhav S, Mansure JJ, Rayes RF, et al. Role of neutrophil extracellular traps in radiation resistance of invasive bladder cancer. Nat Commun 2021;12:2776. [Crossref] [PubMed]
- Miller JA, Toesca DAS, Baclay JRM, et al. Pancreatic Stereotactic Body Radiation Therapy with or without Hypofractionated Elective Nodal Irradiation. Int J Radiat Oncol Biol Phys 2022;112:131-42. [Crossref] [PubMed]
- Deek MP, Benenati B, Kim S, et al. Thoracic Vertebral Body Irradiation Contributes to Acute Hematologic Toxicity During Chemoradiation Therapy for Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;94:147-54. [Crossref] [PubMed]
- Chadha AS, Liu G, Chen HC, et al. Does Unintentional Splenic Radiation Predict Outcomes After Pancreatic Cancer Radiation Therapy? Int J Radiat Oncol Biol Phys 2017;97:323-32. [Crossref] [PubMed]
- Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2016;94:571-9. [Crossref] [PubMed]
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03161379. A Phase II Clinical Trial of GVAX Pancreas Vaccine (With Cyclophosphamide) in Combination With Nivolumab and Stereotactic Body Radiation Therapy (SBRT) Followed by Definitive Resection for Patients With Borderline Resectable Pancreatic Adenocarcinoma. May 19, 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03161379
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02648282. A Phase II Study of GM-CSF Secreting Allogeneic Pancreatic Cancer Vaccine in Combination With PD-1 Blockade Antibody (Pembrolizumab) and Stereotactic Body Radiation Therapy (SBRT) for the Treatment of Patients With Locally Advanced Adenocarcinoma of the Pancreas. Available online: https://clinicaltrials.gov/ct2/show/NCT02648282
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03563248. A Randomized Phase 2 Study of Losartan and Nivolumab in Combination With FOLFIRINOX and SBRT in Localized Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03563248
- Bockorny B, Semenisty V, Macarulla T, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med 2020;26:878-85. [Crossref] [PubMed]
- Faget J, Peters S, Quantin X, et al. Neutrophils in the era of immune checkpoint blockade. J Immunother Cancer 2021;9:e002242. [Crossref] [PubMed]


