
Magnetic resonance elastography can predict the development of hepatocellular carcinoma: a meta-analysis and systematic review
Introduction
Liver cancer refers to a malignant tumor of the liver, and includes both primary and secondary liver cancers (1,2). The etiology of primary liver cancer is unknown. At present, it is believed to be related to a hepatitis viral infection, aflatoxin, and some chemical carcinogens. Secondary liver cancer is caused by the metastasis of other malignant tumors to the liver (3-5).
Magnetic resonance elastography (MRE) is a new method of MR examination that inputs shear waves into the brain and detects brain tissue elasticity by phase coding (6-8). As a novel and non-invasive imaging technology, MRE is a traditional mechanical and quantitative means of palpation that is not limited by the diagnosis site, and is thus known as “image palpation” (9-11).
Studies have shown that the measurement of non-tumor liver tissue hardness by MRE can indirectly achieve accurate and non-invasive evaluation of liver reserve function in hepatocellular carcinoma (HCC) patients, which can simplify the clinical liver function evaluation procedure and provide strong support for the selection of clinical treatment methods as well as the assessment of prognosis (12-15).
Considering the limited analysis of MRE imaging in the diagnosis of HCC, we conducted this research to determine the performance of MRE imaging in the diagnosis of HCC. This will allow for a deeper understanding of the overall diagnostic performance of MRE, enhance the imaging consistency technology, and help to improve the effectiveness of this diagnostic test as much as possible. We present the following article in accordance with the PRISMA-DTA reporting checklist (available at https://dx.doi.org/10.21037/jgo-21-196).
Methods
Literature search strategy
We retrieved relevant peer-reviewed articles published from 1990 to 2020 from the following databases: PubMed, EMBASE, Cochrane Central, and China National Knowledge Infrastructure, using the following keywords: “magnetic resonance elastography”, “MRE”, “liver cancer”, “liver tumor”, “hepatocellular carcinoma”, “HCC”, “liver cell carcinoma”, and “hepatic cell carcinoma”. No restrictions on the publication language were applied in the literature retrieval. The reference lists of retrieved articles and review articles were manually checked to identify further relevant studies that were not identified using the search strategy.
Study selection
The search strategy involved identifying potentially relevant articles, screening of identified papers based on their titles and abstracts, conducting a qualification review of the full text of potentially relevant studies, and requiring the included studies meet the following inclusion criteria:
- Using MRE;
- Patients with HCC;
- The full text is provided.
Based on the following exclusion criteria, we systematically excluded studies that did not meet the inclusion criteria:
- Research focused on other health problems;
- Patients receiving other diagnostic technologies;
- A lack of research on available data.
Data extraction and quality assessment
Two researchers carefully reviewed the full texts of all eligible studies and independently extracted relevant data, including the name of the first author, year of publication, country, sample size, study period, age and gender of patients, etc. The overall quality of the study was evaluated using the Cochrane bias risk assessment tool.
Statistical analysis
The results of classified variables were presented as a risk ratio (RR) and 95% confidence interval (CI), while the results of continuous variables were presented as a mean difference (MD) and 95% CI. The Cochrane Q-test index was used for detecting the existence of heterogeneity between the results of the primary studies and I-square index (I2) determined the degree of the heterogeneity in meta-analysis based on I2 value of 25%, 50%, and 75% were nominally regarded as low, moderate, and high estimates, respectively. A random effect model was used for high heterogeneity, and a fixed effect model was employed for low heterogeneity. A funnel plot was used to evaluate publication bias.
Sensitivity analysis was utilized to test the effect of each individual study on the stability of the overall results by deleting each study in turn. Stata 12.0 (StataCorp LP, College Station, TX, USA) was used for statistical analysis.
Results
Literature search
In total, the electronic search retrieved 886 potentially relevant articles. Based on a careful reading of the title and abstracts, 804 full-text papers were selected for full review. A total of 56 articles were excluded due to a lack of relevance, insufficient data, or other article types.
Finally, the meta-analysis was conducted using nine papers. Based on careful consideration of the purpose of the study, inclusion and exclusion criteria were established to guide the subsequent search process, as shown in Figure 1.
Characteristics of included studies
Table 1 summarizes the total number of patients. Relevant data were extracted from nine papers (16-24), including the first author’s name, year of publication, country, gender distribution (male/female), sample size, age range, and recruitment time. A total of 1,735 patients were selected for analysis. All included studies were journal articles published between 2009 and 2018. The study sample sizes ranged from 38 to 549 patients.
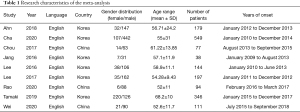
Full table
Quality assessment
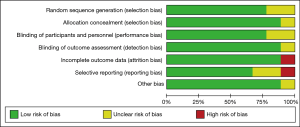
According to the results of the Cochrane risk of bias assessment tool, which included selection bias, detection bias, performance bias, loss bias, and reporting bias, the nine included trials exhibited no risk of bias (Figures 2,3).
Heterogeneity test
Assessment of heterogeneity
The nine studies included in the heterogeneity assessment showed significant heterogeneity (P=0.00), and the sensitivity (I2=92.44%) was relatively higher than the specificity (I2=67.86%).
Diagnostic accuracy
Nine studies with 1,735 patients were included in this meta-analysis. The sensitivity and specificity of these nine studies are shown in Figure 4. The variability of sensitivity (range, 31–100%) was relatively greater than that of specificity (range, 81–94%).
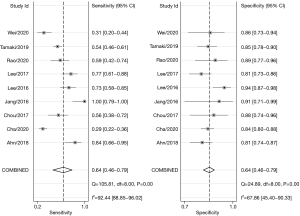
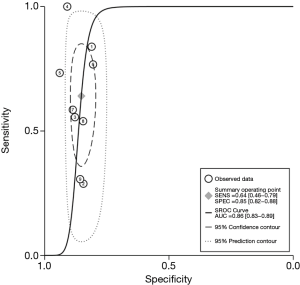
The diagnostic performance of MRE for HCC was estimated to have a sensitivity of 64% (95% CI: 46–79%) and a specificity of 85% (95% CI: 82–88%). Figure 5 shows the summary ROC (relative operating characteristic) curve, which had an area under the curve of 86%.
Sensitivity analysis and publication bias
A funnel plot was used to analyze publication bias. The funnel plot contained the nine included studies. Considering the good symmetry of the funnel plot, there is limited publication bias (Figure 6).
Discussion
In this study, nine eligible studies were included to evaluate the effectiveness of MRE in the diagnosis of HCC. Our meta-analysis of these studies showed a moderate sensitivity of 64% and a high specificity of 85%. The sensitivity of individual studies varied widely, ranging from 31% to 100%, while the specificity ranged from 81% to 94% (25,26). Our results were consistent with the findings of Qayyum et al. (12).
HCC is the main type of primary liver cancer, accounting for approximately 70–90%. It is the third most common cause of cancer-related mortality worldwide. Primary liver cancer refers to a malignant tumor originating from stem cells or intrahepatic bile duct epithelial cells, and includes HCC, intrahepatic cholangiocarcinoma (ICC), and HCC-ICC mixed type. Secondary liver cancer refers to a malignant liver tumor that is caused by the metastasis of another malignant tumor, typically from the respiratory tract, gastrointestinal tract, breast, and other non-liver parts, to the liver (27-29).
MRE is a non-invasive imaging method for the quantitative detection of soft tissue elasticity and structure. In the process of MRE detection, slight mechanical vibration (between 30 and 70 Hz) propagates to the tissue under investigation through an external vibration device, and the dynamic propagation of the vibration wave in the tissue is collected by a nuclear MRI (Magnetic Resonance Imaging) (30-32). During post-processing, we can quantify the hardness and softness of the tissue according to the appearance (wavelength and amplitude) of the vibration wave in the tissue.
Clinical examination of HCC includes serum alpha-fetoprotein, circulating tumor cells, CT (computed tomography), MRI, DSA (digital subtraction angiography), liver ultrasound, pathological examination, etc. The basic principle of MRE is to use MR technology to detect the particle displacement of tissues or organs in the body under the action of an external force, and obtain the MR phase image via the motion sensitive gradient. A distribution map of elastic coefficient of each point in the tissue or organ is obtained, and the elastic mechanical parameters of the tissue or organ are used as the basis of medical diagnosis (33-37).
Malignant tumors of the liver can increase tissue elasticity, which improves the applicability of MRE in the diagnosis of liver cancer. MRE uses a unique magnetic resonance technology to distinguish the transmission and change of mechanical waves in tissue and judge the evolution of tissue elasticity. MRE has been used to evaluate the pathological changes of patients with chronic liver disease, which has the advantages of high safety, high diagnostic accuracy, and non-invasive. MRE can be used to stage liver fibrosis instead of liver biopsy (35,36).
However, there were some limitations in this study that should be noted. Firstly, the comparison of different tumor sizes was not considered, and thus, further study is needed. Secondly, the details of different stages of the tumor were not taken into account, and should be analyzed in future research. In conclusion, MRE imaging has moderate sensitivity and excellent specificity in the detection of HCC, and can be used as a recommended diagnostic technique for HCC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-DTA reporting checklist. Available at https://dx.doi.org/10.21037/jgo-21-196
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jgo-21-196). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 2005;308:551-4. [Crossref] [PubMed]
- Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 2004;304:93-6. [Crossref] [PubMed]
- Bellec M. La Tigresse Du Muse Cernuschi: Mre De Lait Ou Dvoreuse Dhommes? Ligeia 2016;1:140. [Crossref]
- Burrel M, Llovet JM, Ayuso C, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology 2003;38:1034-42. [Crossref] [PubMed]
- Seror O, N'Kontchou G, Ganne N, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2011;254:837-author reply 837-8. [Crossref] [PubMed]
- Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology 2006;43:515-24. [Crossref] [PubMed]
- Imamura H, Tanaka E, Matsuyama Y, et al. Risk Factors Contributing to Early and Late Phases of Intrahepatic Recurrence of Hepatocellular Carcinoma (HCC) After Hepatectomy. Gastroenterology 2000;4:A916. [Crossref]
- Boyault S, Rickman DS, de Reyniès A, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology 2007;45:42-52. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Ma S, Lee TK, Zheng BJ, et al. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008;27:1749-58. [Crossref] [PubMed]
- Wang C, Ding ZW, Zheng CG, et al. COCH predicts survival and adjuvant TACE response in patients with HCC. Oncol Lett 2021;21:275. [Crossref] [PubMed]
- Qayyum A, Avritscher R, Morani A, et al. Immunotherapy Response Evaluation with MR Elastography (MRE) in Advanced HCC. J Clin Oncol 2019;4:230. [Crossref]
- Bansal G. The Application of Texture Analysis Pipeline on MRE Imaging for HCC Diagnosis. Dissertations & Theses - Gradworks 2013.
- Wang J, Shan Q, Liu Y, et al. 3D MR Elastography of Hepatocellular Carcinomas as a Potential Biomarker for Predicting Tumor Recurrence. J Magn Reson Imaging 2019;49:719-30. [Crossref] [PubMed]
- Liu Y, Hou N, Zhao F, et al. HBV infection downregulated Mre11 expression and induced genome instability. Wei Sheng Wu Xue Bao 2008;48:1031-4. [PubMed]
- Rao C, Wang X, Li M, et al. Value of T1 mapping on gadoxetic acid-enhanced MRI for microvascular invasion of hepatocellular carcinoma: a retrospective study. BMC Med Imaging 2020;20:43. [Crossref] [PubMed]
- Jang S, Lee JM, Lee DH, et al. Value of MR elastography for the preoperative estimation of liver regeneration capacity in patients with hepatocellular carcinoma. J Magn Reson Imaging 2017;45:1627-36. [Crossref] [PubMed]
- Tamaki N, Higuchi M, Kurosaki M, et al. Risk assessment of hepatocellular carcinoma development by magnetic resonance elastography in chronic hepatitis C patients who achieved sustained virological responses by direct-acting antivirals. J Viral Hepat 2019;26:893-9. [Crossref] [PubMed]
- Cha DI, Jang KM, Kim SH, et al. Preoperative Prediction for Early Recurrence Can Be as Accurate as Postoperative Assessment in Single Hepatocellular Carcinoma Patients. Korean J Radiol 2020;21:402-12. [Crossref] [PubMed]
- Lee S, Kim SH, Lee JE, et al. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol 2017;67:526-34. [Crossref] [PubMed]
- Ahn SJ, Kim JH, Park SJ, et al. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY) 2019;44:539-48. [Crossref] [PubMed]
- Lee DH, Lee JM, Yi NJ, et al. Hepatic stiffness measurement by using MR elastography: prognostic values after hepatic resection for hepatocellular carcinoma. Eur Radiol 2017;27:1713-21. [Crossref] [PubMed]
- Wei H, Jiang H, Liu X, et al. Can LI-RADS imaging features at gadoxetic acid-enhanced MRI predict aggressive features on pathology of single hepatocellular carcinoma? Eur J Radiol 2020;132:109312 [Crossref] [PubMed]
- Chou CT, Chen RC, Wu WP, et al. Prospective Comparison of the Diagnostic Performance of Magnetic Resonance Elastography with Acoustic Radiation Force Impulse Elastography for Pre-operative Staging of Hepatic Fibrosis in Patients with Hepatocellular Carcinoma. Ultrasound Med Biol 2017;43:2783-90. [Crossref] [PubMed]
- Garner K, Levesque A, Eastman A. The Role of Chk1 and Mre11/Nbs1/Rad50 in S Phase Arrest Induced by DNA Damage. Cancer Res 2008;4233.
- Yoshimoto S, Loo TM, Hara E. Cellular Senescence and Liver Cancer: A Gut Microbial Connection. Inflammation & Regeneration 2015;3:106-13. [Crossref]
- Setiawan VW, Wei PC, Hernandez BY, et al. Disparity in liver cancer incidence and chronic liver disease mortality by nativity in Hispanics: The Multiethnic Cohort. Cancer 2016;122:1444-52. [Crossref] [PubMed]
- Wang Y, Chen F, Zhao M, et al. The long noncoding RNA HULC promotes liver cancer by increasing the expression of the HMGA2 oncogene via sequestration of the microRNA-186. J Biol Chem 2017;292:15395-407. [Crossref] [PubMed]
- Chen M, Wei L, Law CT, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology 2018;67:2254-70. [Crossref] [PubMed]
- Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res 2018;30:571-9. [Crossref] [PubMed]
- Ferretti S, Bossard N, Binder-Fouchard F, et al. Trends in net survival from liver cancer in six European Latin countries: results from the SUDCAN population-based study. Eur J Cancer Prev 2017;26 Trends in cancer net survival in six European Latin Countries: the SUDCAN study:S56-62.
- Lee JH, Kim HY, Kim YJ, et al. Barcelona Clinic Liver Cancer staging system and survival of untreated hepatocellular carcinoma in a hepatitis B virus endemic area. J Gastroenterol Hepatol 2015;30:696-705. [Crossref] [PubMed]
- Chen YJ, Wallig MA, Jeffery EH. Dietary Broccoli Lessens Development of Fatty Liver and Liver Cancer in Mice Given Diethylnitrosamine and Fed a Western or Control Diet. J Nutr 2016;146:542-50. [Crossref] [PubMed]
- Shen L, Zhang G, Lou Z, et al. Cryptotanshinone enhances the effect of Arsenic trioxide in treating liver cancer cell by inducing apoptosis through downregulating phosphorylated- STAT3 in vitro and in vivo. BMC Complement Altern Med 2017;17:106. [Crossref] [PubMed]
- Shalapour S, Lin XJ, Bastian IN, et al. Author Correction: Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2018;561:E1. Erratum for Nature 2017;551:340-5. [Crossref] [PubMed]
- Fang T, Lv H, Wu F, et al. Musashi 2 contributes to the stemness and chemoresistance of liver cancer stem cells via LIN28A activation. Cancer Lett 2017;384:50-9. [Crossref] [PubMed]
- Sun YY, Xiao L, Wang D, et al. Triptolide inhibits viability and induces apoptosis in liver cancer cells through activation of the tumor suppressor gene p53. Int J Oncol 2017;50:847-52. [Crossref] [PubMed]
(English Language Editor: A. Kassem)





