
Influence of first line chemotherapy strategy depending on primary tumor location in metastatic colorectal cancer
Introduction
Colorectal cancer (CRC) is a major public health issue and stands as the third most frequent cancer throughout the world with 1.8 million new cases diagnosed every year. It is the second cause of cancer related death with, according to GLOBOCAN estimates, 880,000 deaths per year (1).
Between 20% and 30% of patients have metastases at the time of diagnosis (2). For the patients with an initially localized CRC, 20–40% will secondarily develop metastases. For 75–90% of patients with metastatic CRC (mCRC), the cancer is unresectable and they will receive palliative chemotherapy. In the 1990’s, the only available chemotherapy was 5-fluorouracil (5FU) with leucovorin (LV) and patients’ overall survival (OS) was disappointing, ranging from 11 to 13 months (3). The prognosis of these patients has been profoundly changed by modern chemotherapy. In the early 2000s, large phase III trials validated the use of bichemotherapy regimens with either oxaliplatin or irinotecan associated to 5FU as the first line of treatment for mCRC. These drug combinations allowed higher OS rates, ranging from 17 to 23 months (4,5).
In 2004, a phase III study published by Tournigand et al. investigated two chemotherapy sequences: folinic acid, 5FU, and irinotecan (FOLFIRI) followed by folinic acid, 5FU, and oxaliplatin (FOLFOX) or FOLFOX followed by FOLFIRI. There was no difference in terms of OS between the 2 groups with respectively 21.5 and 20.6 months of OS, P=0.99 (6). In 2005, Colucci et al.’s team had the same findings in 2005 (7).
More recently, targeted therapies, such as epidermal growth factor receptor (EGFR) inhibitors and vascular endothelial growth factor (VEGF) inhibitors, have proved their efficiency. Firstly associated with bichemotherapy regimens and secondly with trichemotherapy (5FU, oxaliplatin and irinotecan) regimens, targeted therapies have shown to be superior in terms of PFS and OS for patients with unresectable mCRC (8-11). In 2020, the TRIBE 2 trial showed the superiority of the use of a trichemotherapy regimen associated to bevacizumab compared to a pre-planned sequential strategy FOLFOX plus bevacizumab followed by FOLFIRI plus bevacizumab after disease progression (12). However, despite these new advances not all patients are fit to undergo a trichemotherapy regimen. The matter of the best chemotherapy sequence between the use of FOLFIRI or FOLFOX in combination with target therapies as first line of treatment stays of interest, especially for this population of patients and remain a matter of debate.
It is now well established that primary tumor location (PTL) is a major prognostic factor in mCRC, right-sided mCRC’s having a poorer prognosis than left-sided mCRCs (13-15). The prognosis of rectal cancers, is similar to the prognosis of left-sided CRCs (16). To our knowledge, there is no data exploring the PFS rates according to the first line of chemotherapy and depending on PTL.
The aim of this retrospective study, carried out at the Centre Georges-François Leclerc Hospital in Dijon, was to evaluate the outcome, in terms of PFS and OS, depending on the use of either FOLFOX or FOLFIRI as first line of chemotherapy for mCRC.
We present the study in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jgo-20-593).
Methods
Study population
Data was collected retrospectively from all consecutive patients treated at the Centre Georges-François Leclerc Hospital for mCRC between 31st of January 2000 and the 20th of December 2018. Patients with either synchronous, metachronous, resectable or non resectable metastases were included. Patients were excluded from this study if the tumor sidedness was not specified in the medical file, if the CRC was not the first or the only malignancy diagnosed, in case of appendicle cancer. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval from the local ethics committee was not required, in accordance with French legislation governing strictly observational studies using medical files. Because of the retrospective nature of the study, the requirement for informed consent was waived.
Data collection
The following parameters were retrospectively collected in the patients’ medical file: gender, age, performance status (PS), liver surgery or liver radiofrequency, lung surgery or lung radiofrequency, surgery of the primary tumor, tumor location (right colon cancer included right sided and transverse colon cancers; left colon cancers included left sided, sigmoid), synchronous or metachronous disease, number of metastatic sites, RAS and BRAF mutations, type of medical treatment, levels of lactate dehydrogenase, carcinoembryonic antigen.
Statistical analyses
The primary endpoint was to evaluate whether there was a benefit in favor of either FOLFIRI or FOLFOX plus target therapies as first line of metastatic in terms of PFS for patients depending PTL. Secondary endpoints were evaluating the OS, according to PTL, depending on the use of FOLFIRI or FOLFOX plus target therapies as the first line of metastatic treatment and PFS, according to PTL, depending on the use of mono, bi or trichemotherapy regimens as first line of metastatic treatment. All patients were followed until either their death or the date of last follow-up prior to the 31st of March 2020. PFS was defined as the interval between the time of diagnosis of metastatic disease and the date of progression to the first line of chemotherapy reported on medical record. For the PFS evaluation, survivors were censored at 2 years, for the OS evaluation survivors were censored at last follow-up.
The median follow-up was estimated using the reverse Kaplan-Meier method. PFS and OS were estimated using the Kaplan-Meier method, described using median with its 95% confidence interval (95% CI), and compared using log-rank test.
Statistical analyses were performed using SAS® software version 9.4.
Results
Patients’ characteristics
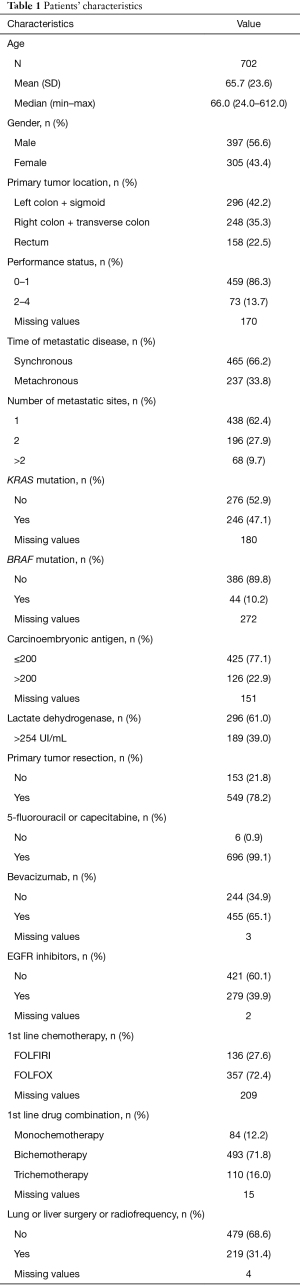
The data from 702 patients with mCRC was collected from the Centre Georges-Francois Leclerc Hospital database between January 31st 2000 and December 20th 2018. Median follow up was 8 years.
The male gender was slightly predominant (56.6%). The mean age was 65.7 years. PS was good for most patients with 86.3% of patients with 0 or 1 PS. The number of patients with right colic cancer, left colic cancer and rectal cancer was respectively 248 (35.3%), 296 (42.2%) and 158 (22.5%). The first line of chemotherapy was a bichemotherapy for 493 (71.8%) patients. One hundred and thirty-six patients received FOLFIRI, 357 patients received FOLFOX. For the remaining patients, 84 received a monochemotherapy, 110 received a trichemotherapy. The data was unknown for 15 patients. A high number of patients, 549, had undergone a primary tumor resection. A majority of patients received biotherapies with their chemotherapy (89%). Four hundred and fifty-five patients (65.1%) received at least antiangiogenic therapies and 279 patients (39.9%) received at least an anti-EGFR during follow-up. Only 8% of patients received anti EGFR in first line while 59% received bevacizumab in first line. Results shown in Table 1.
Full table
Association between PFS, PTL and efficacy 1st line of metastatic chemotherapy
The left-sided mCRC cohort who received either FOLFOX or FOLFIRI included 216 patients. Fifty-eight patients were treated with FOLFIRI, 158 patients were treated with FOLFOX. No particular clinical differences were seen between the FOLFOX and the FOLFIRI group for classical prognostic factors (not shown).
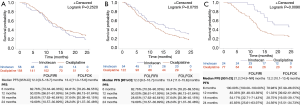
For the FOLFIRI group, the PFS was of 12.0 (95% CI: 9.5–16.7) months. It was of 13.4 (95% CI: 11.0–15.6) months for the FOLFOX group. The difference observed was not statistically significant with P=0.252. The right sided mCRC cohort who received either FOLFOX or FOLFIRI included 176 patients. Fifty-four patients were treated with FOLFIRI, 122 patients were treated with FOLFOX. For the FOLFIRI group, the PFS was of 14.9 (95% CI: 8.8–20.8) months. It was of 11.3 (95% CI: 8.4–13.2) months for the FOLFOX group. The difference observed of over 3 months of PFS was close to significance with P=0.0755. The rectal metastatic cancer cohort who received either FOLFOX or FOLFIRI included 101 patients. No particular differences were seen between rectal and colon cancer for classical prognostic factors (not shown).
Twenty-four patients were treated with FOLFIRI, 77 patients were treated with FOLFOX. There was a statistically significant difference in terms of PFS in favor of the FOLFIRI group with a PFS of 21.2 (95% CI: 14.9–not estimable) months versus 12.2 (95% CI: 10.1–13.4) months for the FOLFOX group, P=0.009. Results shown in Figure 1. No difference in term of OS was observed between patients treated in first line with bevacizumab and anti EGFR whatever the sideness (not shown). We did not confirm previously reported influence of sidedness on efficacy of anti EGFR vs. antiangiogenic in our cohort (17,18).
Association between PFS, PTL and efficacy of mono-, bi- or trichemotherapy
In either left, right or rectal tumor monochemotherapy as first line gives poor PFS and OS in comparison to doublet or triplet. No particular differences were seen between patients treated with monotherapy, doublet or triplet for classical prognostic factors (not shown). Surprisingly, there is only in the rectal metastatic cancer cohort for which we were able to show that trichemotherapy was more efficient than bichemotherapy and monochemotherapy with PFSs of respectively 23.9 (95% CI: 15.2–not estimable) months, 13.3 (95% CI: 11.9–16.0) months, 9.5 (95% CI: 5.2–23.2) months, P=0.0392. We failed to show that benefice in the left and right-sided mCRC cohorts. Results shown in Figure 2.

Association between OS, PTL and efficacy of 1st line of metastatic chemotherapy
In our 3 groups (left-sided mCRC, rights-sided mCRC and rectal metastatic cancer) there was no difference in terms of OS whether the chemotherapy sequence began with FOLFIRI or with FOLFOX. The OS rates were for left-sided mCRC 2.6 (95% CI: 2.1–3.3) years for the FOLFIRI group and 3.2 (95% CI: 2.5–3.7) years for the FOLFOX group, P=0.6276; for right-sided mCRC 2.2 (95% CI: 1.4–2.7) years for the FOLFIRI group and 1.9 (95% CI: 1.4–2.6) years for the FOLFOX group, P=0.7852; for rectal metastatic cancer 3.6 (95% CI: 2.9–4.8) years for the FOLFIRI group and 2.9 (95% CI: 2.2–3.8) years for the FOLFOX group, P=0.1804. Results shown in Figure 3.

Discussion
Here, we report a large retrospective cohort of daily practice of chemotherapy prescription in patients treated for mCRC in a regional cancer center. This study is in favor of similar efficacy rates of either FOLFOX or FOLFIRI bichemotherapy regimens using in association with target therapies, as first line of treatment, in term of PFS and OS for both left and right colic tumors. Surprisingly, patients with rectal cancer seem to gain more benefit, in terms of PFS, when FOLFIRI is used first when compared to FOLFOX. However, this benefit is not significant when it terms of OS, despite a favorable trend for OS.
Before the development of biotherapies, Tournigand et al. demonstrated similar efficacy of the FOLFOX regimen followed by the FOLFIRI regimen or the reverse phase (6). However, the influence of PTL and chemotherapy efficacy was not address in this trial. In a retrospective study from SEER-Medicare similar OS were observed in patients treated with FOLFOX and not FOLFIRI in the era before target therapies. In this cohort sidedness did not impact the effect of chemotherapy doublet (19). Patients with right-sided tumors generally have a poorer prognosis than those with left-sided tumors (20-24). For RAS wildtype patients, treated with anti EGFR, a meta-analyze based on data from 7 randomized trials [CRYSTAL (25), PRIME (9), PEAK (26), FIRE-3 (27), CALGB 80405 (28) and TAILOR (29)] was performed. Subgroup analyses according to PTL ranked the efficacy of 7 different chemotherapy regimens (FOLFOX alone or with cetuximab, panitumumab, or bevacizumab and FOLFIRI alone or with cetuximab or bevacizumab). FOLFOX and FOLFIRI associated to cetuximab were more effective than the other drug combination in patients with RAS wildtype mCRC. For the patients with right-sided RAS wildtype mCRC, there was no significant difference amongst the seven regimens (30). Based on these results, it is thought that anti-EGFRs should be preferred as first line of treatment for left-sided RAS wildtype mCRCs, whereas bevacizumab should be preferred, in the same setting for right-sided mCRCs. The benefit in using bevacizumab combined to chemotherapy as first line of treatment for RAS wildtypes right-sided mCRCs was recently confirmed in another meta-analyze (31). However, these studies did not evaluate the impact, in terms of PFS and OS, of the chemotherapy doublet. Our results suggest, that in a population of patients treated with bichemotherapies regimens, with or without EGFR inhibitors or bevacizumab, similar efficacy is observed whether FOLFIRI or FOLFOX is used as first line of treatment and whether the primary tumor is left or right sided. However, this conclusion must be taken with caution for anti EGFR because few people in this series were treated with anti EGFR in first line. A surprising issue is that FOLFIRI doublet is more effective in rectal tumors. This could be explained by a higher sensitivity of rectal cancers to irinotecan (32), rectal cancers being enriched in CMS2 tumors (33).
Another surprising result is that only rectal cancers seem to gain benefit from trichemotherapy regimens. Previous studies underline that trichemotherapy regimens are more effective than bichemotherapy regimens in terms of OS and PFS (12). A recent meta-analysis confirmed that FOLFOXIRI + bevacizumab significantly and meaningfully improves the survival of patients with mCRC when compared to bichemotherapy regimens + bevacizumab and allows higher PFS rates, ORRs, and R0 resection rates at the price of a moderate increase in toxicity (34). This treatment seems effective in either left or right-sided mCRC. However, in our cohort of real-life patients, this data was not confirmed with the exception of rectal cancer. Such differences could be explained by the fact that only patients with life threatening diseases and probably with worst intrinsic prognostic are selected for trichemotherapy regimens. For rectal cancers, our data confirms the recent data from FFCD1102 trial which proved higher response rates, PFS rates and OS rates with induction therapy with FOLFIRINOX (35).
The limits to our study are of course its retrospective and mono centric design. However, we studied a large cohort of unselected patients which represent real life data. Our results in terms of outcome and population are similar to the results observed in clinical trials (36,37) or in studies evaluating survival in mCRC (38). However, we did not observe the expected benefit of trichemotherapy regimens probably because of patient selection biases. Other limitations to our study are that it compared a highly heterogeneous population of patients in terms of tumor burden and that the choice of the chemotherapy regimen was physician dependent and some differences in patients could have not been detected by our data collection.
Conclusions
Our results support that either FOLFIRI or FOLFOX regimens give similar efficacy in both left and right metastatic colic cancer. FOLFIRI and FOLFIRINOX regimens might be preferred for metastatic rectal carcinoma. Such data needs to be validated in prospective clinical trials.
Acknowledgments
We thank Zoe Tharin for English editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-593
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jgo-20-593
Peer Review File: Available at http://dx.doi.org/10.21037/jgo-20-593
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-593). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval from the local ethics committee was not required, in accordance with French legislation governing strictly observational studies using medical files. Because of the retrospective nature of the study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 2005;12:637-45. [Crossref] [PubMed]
- McQuade RM, Stojanovska V, Bornstein JC, et al. Colorectal Cancer Chemotherapy: The Evolution of Treatment and New Approaches. Curr Med Chem 2017;24:1537-57. [Crossref] [PubMed]
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355:1041-7. Erratum in: Lancet 2000 Apr 15;355(9212):1372. [Crossref] [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-47. [Crossref] [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [Crossref] [PubMed]
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol 2005;23:4866-75. [Crossref] [PubMed]
- Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [Crossref] [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [Crossref] [PubMed]
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306-15. [Crossref] [PubMed]
- Cremolini C, Marmorino F, Loupakis F, et al. TRIBE-2: a phase III, randomized, open-label, strategy trial in unresectable metastatic colorectal cancer patients by the GONO group. BMC Cancer 2017;17:408. [Crossref] [PubMed]
- Cremolini C, Antoniotti C, Rossini D, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2020;21:497-507. [Crossref] [PubMed]
- Venook AP. Right-sided vs left-sided colorectal cancer. Clin Adv Hematol Oncol 2017;15:22-4. [PubMed]
- Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of First-Line Chemotherapy Combined with Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 20 2017;317:2392-401.
- Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 2017;70:87-98. [Crossref] [PubMed]
- Tharin Z, Blanc J, Alaoui IC, et al. Influence of primary tumor location and resection on survival in metastatic colorectal cancer. World J Gastrointest Oncol 2020;12:1296-310. [Crossref] [PubMed]
- Grassadonia A, Di Marino P, Ficorella C, et al. Impact of primary tumor location in patients with RAS wild-type metastatic colon cancer treated with first-line chemotherapy plus anti-EGFR or anti-VEGF monoclonal antibodies: a retrospective multicenter study. J Cancer 2019;10:5926-34. [Crossref] [PubMed]
- Modest DP, Stintzing S, von Weikersthal LF, et al. Exploring the effect of primary tumor sidedness on therapeutic efficacy across treatment lines in patients with metastatic colorectal cancer: analysis of FIRE-3 (AIOKRK0306). Oncotarget 2017;8:105749-60. [Crossref] [PubMed]
- Neugut AI, Lin A, Raab GT, et al. FOLFOX and FOLFIRI Use in Stage IV Colon Cancer: Analysis of SEER-Medicare Data. Clin Colorectal Cancer 2019;18:133-40. [Crossref] [PubMed]
- Loupakis F, Yang D, Yau L, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015;107:dju427 [Crossref] [PubMed]
- Modest DP, Schulz C, von Weikersthal LF, et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). Anticancer Drugs 2014;25:212-8. [Crossref] [PubMed]
- Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995-2001. [Crossref] [PubMed]
- Zhang Y, Ma J, Zhang S, et al. A prognostic analysis of 895 cases of stage III colon cancer in different colon subsites. Int J Colorectal Dis 2015;30:1173-83. [Crossref] [PubMed]
- Ciombor KK, Goldberg RM. Highlights in Gastrointestinal (Colorectal) Cancer Treatment: The Primary Tumor Sidedness Debate and Advances in Immunotherapy. JAMA Oncol 2016;2:1537-8. [Crossref] [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [Crossref] [PubMed]
- Schwartzberg LS, Rivera F, Karthaus M, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014;32:2240-7. [Crossref] [PubMed]
- Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016;17:1426-34. [Crossref] [PubMed]
- Venook AP, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32: [Crossref]
- Qin S, Li J, Wang L, Xu J, et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients with RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J Clin Oncol 2018;36:3031-9. [Crossref] [PubMed]
- Zhou M, Fu L, Zhang L, et al. Ranking the efficacies of FOLFOX and FOLFIRI with or without anti-EGFR therapy or bevacizumab in wild-type-RAS metastatic colorectal cancer according to primary tumor location: A network meta-analysis. J Clin Oncol 2018;36: [Crossref]
- You XH, Jiang YH, Fang Z, et al. Chemotherapy plus bevacizumab as an optimal first-line therapeutic treatment for patients with right-sided metastatic colon cancer: a meta-analysis of first-line clinical trials. ESMO Open 2020;4:e000605 [Crossref] [PubMed]
- Stintzing S, Wirapati P, Lenz HJ, et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann Oncol 2019;30:1796-803. [Crossref] [PubMed]
- Loree JM, Pereira AAL, Lam M, et al. Classifying Colorectal Cancer by Tumor Location Rather than Sidedness Highlights a Continuum in Mutation Profiles and Consensus Molecular Subtypes. Clin Cancer Res 2018;24:1062-72. [Crossref] [PubMed]
- Cremolini C, Antoniotti C, Stein A, et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J Clin Oncol 2020; Epub ahead of print. [Crossref] [PubMed]
- Bachet JB, Lucidarme O, Levache CB, et al. FOLFIRINOX as induction treatment in rectal cancer patients with synchronous metastases: Results of the FFCD 1102 phase II trial. Eur J Cancer 2018;104:108-16. [Crossref] [PubMed]
- Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 2008;371:1007-16. [Crossref] [PubMed]
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609-18. [Crossref] [PubMed]
- Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677-83. [Crossref] [PubMed]


