
Resectable, borderline, and locally advanced pancreatic cancer—“the good, the bad, and the ugly” candidates for surgery?
Introduction
The prognosis of most cancer types has gradually improved over the past decades, but pancreatic cancer (PC) remains still among the few exceptions—a therapeutic riddle being difficult to solve (1,2). With its abundant stroma and hypoxic environment, shielding its cancer cells, pancreatic cancer highly resists the effect of chemotherapeutic agents, check-point inhibitors, and radiation (3-7). The only way to potentially defeat the tumor is to technically remove it by surgical resection (8,9). Surgery is the only treatment, causa sine qua non, required to achieve long-term survival. What complicates resection is the difficult anatomic location, challenging what is technically possible to do, and the risk for occult disease, giving rise to early recurrence despite attempt for curative treatment (10-12).
The overrule of surgery in relation to other oncologic therapy is perhaps the reason why pancreatic cancer has become a very “technical” disease. There is also the unique situation where the two widely used classifications of local tumor involvement do not completely overlap with each other—the T-staging in the TNM classification and the NCCN classification of local resectability (13,14). While TNM describes locally advanced pancreatic cancer (LAPC) as having arterial involvement of the celiac axis (CA) and the superior mesenteric artery (SMA), NCCN points out that even venous involvement can be a factor denominating advanced local involvement. Exactly how extensive this involvement should be to be counted as very poor prognostic factor is still a matter to some interpretation and experience—how much is technically feasible to do.
The chance for surgical resection plays inevitably a central role, at least as much as other biological factors, in patients’ long-term prognosis—median survival of 18–24 months compared to 7–8 months if resection is not performed (8,9,15-20). The categorization into resectable, borderline resectable PC (BRPC), and LAPC, summarizing the probability for resection, has been widely used for some time now and had led to the conviction that these are tumor groups having grossly different prognosis that is determined by the perivascular extension of the tumor. LAPC has been doomed as incurable disease, with a median and 5-year survival of 11–14 months and 0–7%, respectively, mainly because resection is precluded due to the involvement of CA, SMA or the hepatic arteries (21). Attempt for surgical resection of BRPC, besides resulting in lower resectability rate than for primary resectable cases, runs a much higher risk of leaving positive margins (22). Still the median survival of upfront resected BRPC, is better than when being treated by radiochemotherapy without resection: 3- and 5-year survival in a RCT was 20% and 10% versus 0, respectively (23-25). While in the broader public addressing BRPC by resection has been regarded as reasonable in the multimodality context, despite that for a long time the evidence behind neoadjuvant therapy in this matter has been scarce, the opinion against surgical approach in LAPC has been quite uniform (22).
Tumoral vascular infiltration has been seen as a negative prognostic factor for survival (26). Interestingly, the depth of invasion through the thickness of the vessel seems to be more clearly associated with prognosis—being worse only when intima was reached (27). Yet, other studies did not support neither one nor the other observation (18,19,28-30). Furthermore, histologic venous and arterial infiltration are generally reported in about 60% of resected specimen with en bloc vascular resection, despite that clinically they all appeared to have been adherent to the tumor (18,29,31,32). Should the patients with clinical but not histologic infiltration be denied resection then? Does “bad” location necessarily translate into “bad” biology? Critical evaluation of the watershed between what is technical challenge and aggressive biology is essential, particularly when potent oncologic treatments are now available that can potentially address occult systemic disease and lymph node spread, occurring more common in BRPC/LAPC than in primary resectable cases, and improve the prognosis of technically resectable disease (33-37).
This review aims to highlight the grey zones in the current classifications for local tumor involvement with respect to outcome in the current multimodality treatment era and initiates a call for a more comprehensive prognosis-related classification.
Geometry and biology
The introduction of the concept of “borderline” resectability, in between resectable and unresectable cancer, highlighted the evolution of the surgical possibility of resection (15,16,38). Delineating also the risk of leaving narrow resection margins, the borderline definition brought up the idea of the multimodality treatment to “compensate” for the space effect, regional, and potentially occult distant spread of the tumor. The grey-zone that borderline resectability implies, also challenged the prejudice that there is an absolute limit to surgery with extensive local involvement. The geometric description of vascular involvement, that splits borderline (potentially curable) from locally advanced (untreatable) disease, is somehow awkward to perceive as a true oncologic threshold.
From the standpoint of biology, it is not very intuitive how the cut-off of 180°-degree circumferential vascular involvement describes a clinical threshold for unfavorable prognosis (Figure 1A). This definition refers back to the 90 s, where the risk for vascular involvement discovered during surgery was significant whenever more than half of the circumference of the vessel (>180°) was surrounded by tumor on radiology (39,40). At this time, vascular resection was not an option. This was also the time when potent combination chemotherapy, like FOLFIRINOX or gemcitabine-N-paclitaxel, was not available. Therefore, what the NCCN classification of resectability reflects is that venous resections are generally feasible, but arterial resections are not.
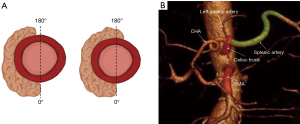
But what about the other dimensions of geometric venous involvement? Imamura et al. showed that even the length of venous contact predicts impaired survival—23.3 versus 39.3 months if the contact was longer or shorter than 20 mm on preoperative CT (41). With an appropriate mobilization using the Cattell-Braasch maneuver, even long venous segments, above 5 cm, can be safely resected with primary anastomosis, without graft interposition (32). Resection of the first jejunal branch of the superior mesenteric vein (SMV) is feasible and not mentioned particularly anymore in the NCCN classification (42). Resection might be achieved even in case of cavernous transformation of the portal vein (43,44). Whether a short vein involvement represents the same stage of disease and should be treated by the same clinical algorithm as long venous infiltration, extending into the root of SMV, with occlusion, but technically reconstructable, is uncertain, but currently it altogether goes under the rubrics of BRPC.
Another peculiar observation is that the exact spot of arterial anatomical contact by the adjacent tumor plays a role for the aggressive behavior of the tumor. While contact with the common hepatic artery (CHA) defines the tumor as borderline, the same or even broader contact with the splenic artery (SpA) classifies the tumor as clearly resectable (Figure 1B). Both CHA and SpA are same-grade branches of the celiac axis (CA), so how come they reflect different biology? Apparently, there is a clear technical issue rather than pure biology involved—the latter being easily resectable, while the former needs special attention and safe reconstruction.
Both the TNM and NCCN classifications evolved to follow the surgical achievements that pushed the limits of resectability. T4 stage emerged in the 5th edition of TNM classification to define involvement of CA and superior mesenteric artery (SMA) and separated from “adjacent vessels” in the 4th edition, thus recognizing that venous involvement no longer puts a stop to surgical resection (45). The NCCN classification no longer mentions specific anatomic landmarks of venous involvement. The “unresectable” tumor group changed name to “locally advanced”. Could it be that we are standing at the verge of a new conceptual definition of advanced pancreatic cancer?
The ugly (locally advanced pancreatic cancer)
The increase in pancreatic tumor size is associated with other poor prognostic factors for survival, such as higher risk for lymph node metastases, higher lymph node ratio, higher risk for perineural invasion, lower grade of tumor cell differentiation, higher probably to be classified as T stage 3 or 4, higher serum CA19-9 (37,46,47). Tumor size by itself (T1-3) according to the 8th TNM edition is distinctively predictive of survival, even irrespective of the lymph node status (47). T4 stage, though, stands alone, irrespective of tumor size, determined by infiltration of CA and SMA (47). Arterial involvement usually requires that the tumor grows beyond the pancreas, deeper into the surrounding tissue. So, is it really the arterial involvement per se or the tumor size that predicts the generally worse prognosis in locally advanced PC? And can it be overcome?
Arterial resection with pancreatectomy has for long been banned as risky procedure, bringing very little survival benefit, just slightly better than palliation, without 5-year survivors (27,48). Recent retrospective series challenge this prejudice and show that arterial resections can be performed safely with pancreatectomy in selected cases (18,49,50). In-hospital mortality as low as 2.9% has been reported (18). Most interestingly, long-term survivors, beyond the 5-year limit, have been observed, in some cases, even without preceding neoadjuvant therapy (18,50). In the context of multimodality neoadjuvant therapy, though, Tee et al. observed a 5-year survival close to the impressive for PC 40% with en bloc arterial resections (50). The evidence of performing arterial resections with pancreatectomy is nowadays low, but the first steps have been done. The technique and other surgical decisions, such as extend of pancreatectomy, perioperative care, etc., still need to be standardized before larger scale trials can be planned. Similarly, this was the bumpy way that venous resections went through. Even without randomized controlled trial, showing their feasibility versus no resection at all, venous resections have become standard in pancreatic surgery.
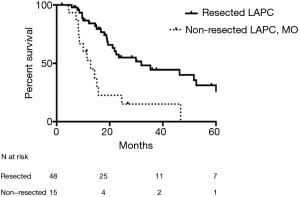
FOLFIRINOX significantly improved the prognosis of patient with LAPC, but only after resection could long-term survivors be observed (36,51-55). Comparing resected and unresected patients with LAPC is undoubtedly biased, since the unresected group includes even those with local disease progression and/or occurrence of distant metastases who apparently would exhibit a more sinister outcome. A current patient-level meta-analysis of patients with LAPC treated with FOLFIRINOX revealed a median survival of 24.2 months (56). Skeptically, that brings the question whether resection brings any benefit at all or is it just the potent effect of FOLFIRINOX that is camouflaged in it. In one of the studies focusing on resection of LAPC, statistical modeling revealed that resection as a time-varying covariate was associated with 69% lower mortality rate than non-resection (54,57). In a sub-analysis, resected patients with LAPC were compared to patients with LAPC who underwent exploration but were found to be unresectable for technical reasons only—lack of optimal vessel distance to achieve vascular reconstruction, but no distant metastases (not even to the paraaortic lymph nodes). Preoperatively both groups had radiologic stable disease or tumor regression and potential vascular margin for reconstruction that motivated surgical exploration (Figure 2). The resected LAPC group had significantly improved survival compared to the technically unresectable group—median and 5-year survival of 32 and 13 months and 26% and 0, respectively (57). This is probably the closest group comparison that can currently be achieved, showing the unequivocal benefit of surgical resection in LAPC. Of notice, in the same reported series, there was no statistically significant difference in survival among resected patients with BRPC and LAPC—something that current classifications cannot capture (54).
Interestingly, the feasibility of arterial resection is heavily discussed, yet vascular resections in general are not always necessary in resected patients with LAPC after neoadjuvant therapy—18–80.8% (20,51,54,58). Arterial resections in particular have been reported in up to 35% of the resected cases and about 60% of these had histologic arterial infiltration (18,54). That is because the radiological assessment after FOLFIRINOX can no longer distinguish between viable tumor and post-treatment fibrosis (38). Ferrone et al. pointed out that despite that radiology predicted persistent unresectability, still 92% of these patients could successfully have their tumors removed without tumoral involvement of the resection margin and having significantly improved prognosis (59). Tumor regression radiologically, according to the RECIST criteria, after NAT for PC is an unusual event—about 11%, while 83% of the patients show stable disease (52,60-62). Therefore, radiological re-assessment after NAT is not useful to judge on the possibility of resection and should not place a burden for it. Quite often after NAT, the arteries can be separated from the surrounding tissue without the necessity for resection. Perhaps that is why T4 was not found to be predictive of survival even in a recent large register-based trial from the US, summarizing data from all across the country and even from institutions not practicing arterial resections (63).
With current NAT, the problem with resected LAPC does not seem to be local anymore, but how to control the distant recurrence, that is much more commonly observed (52,54). Recurrence at distant sites has been seen in 73–80% of patients with BRPC/LAPC after resection, while local recurrence was observed in only 20–24% (10,54). How to select whom to resect after NAT, with lower risk for distant recurrence, is the more difficult question. FOLFIRINOX seems to be the chemotherapy able to lead the most patients to resection (52,53) and shows predicted 5-year survival of as high as 40% (50,54,57). Surgery, however, seems to bring survival benefit even if other, more tolerable, combination regimens have been used, or after dose reductions of the potent FOLFIRINOX, thus increasing the pool of patients who may benefit from multimodality treatment (54,55,58). Factors associated with improved survival have been explored after NAT, and most often seem to be found on the final pathological assessment—the degree of tumor regression in response to the cytotoxic drugs. Unfortunately, this information is available only after surgery and is not very helpful preoperatively, as a selection tool. CA19-9 is the only preoperative factor that so far has been associated with survival (20,36,52,58,64). Yet, there is no consensus as to which level should be the cut-off when resection should be recommended. Complete normalization or levels <100 have been suggested (55). Surgical resection seems to bring benefit for all values of elevated CA19-9 compared to no resection at all, yet, there needs to be a common sense in the balance between the magnitude of the survival gain and the consequences to extended resection. Circulating tumor cells and DNA has shown an association with response to NAT and survival, but further data is needed before this enters the wider practice (65-68).
Apparently, there is a continuously accumulating data series, intentionally selected, which show the proof of concept—patients with LAPC, in whom the systemic progression can be controlled, can be resected and have a long-term chance for cure. Furthermore, resection of LAPC seems to be even more cost-effective than palliation (69). Looking at their 5-year survival, that may resemble that of up-front resected primary resectable cases, apparently LAPC patients can hardly be seen anymore as the “ugly” candidates for surgery. How to select whom to take to the operating theatre is, however, the more difficult question. Since radiologic appearance does not clearly tell which patient with LAPC may benefit from resection, current practice is that everyone who does not progress (locally or systemically) during NAT should be offered a chance for surgical exploration and resection.
The good and the bad (resectable and borderline resectable pancreatic cancer)
Practically, BRPC should belong to the technically resectable group, but with possible positive microscopic margins, if the suggested definitions are to be followed (15,22). Yet, quite often in literature, BRPC is melted together with either primary resectable cancers or with LAPC, thus making it more difficult to draw conclusions of the feasibility of this classification.
How even small and technically resectable the tumor may appear to be on the preoperative staging CT, inevitably about 20–30% of the patients will develop early disease recurrence and will die within one year of surgery (70-72). Early recurrence, within 1 year is more often associated with distant metastases (10,73). For this group of patients, surgical resection has not been a good treatment strategy and may have postponed or eliminated the chance for oncologic treatment that could have potentially prolonged life. Among the predictors for early recurrence are elevated serum CA19-9, Charlson-Deyo comorbidity index, tumor diameter, differentiation grade, lymph node ratio, the former of these being available for consideration already in the preoperative setting (10,11,63,73). Possessing these factors that may lead to early recurrence or death, make the patients with resectable tumors not very “good” candidates for direct surgery. A recent consensus statement recognized that other factors than the sole radiologic appearance, such as CA19-9 and the patients’ condition, need to be taken into consideration to define how “advanced” the tumor is (74).
Metastases to the paraaortic lymph nodes (PALN, station 16b1 according to the Japanese classification), encountered in about 10–17% of resected patients, are considered M1 disease according to the TNM classification (75-80). Yet, PALN are not routinely sampled by most surgeons and used as guidance to make decision upon resection (81). While the opinion is uniform to bail out from surgery whenever liver metastases or peritoneal carcinosis are encountered, there is no consensus what the strategy should be in case of metastatic PALN in primary resectable tumors. The discussion is ongoing whether they should be regarded as M1 or more like N2 disease, as the prognosis can be improved to some extent by adjuvant chemotherapy (80,82). Most studies point out there are hardly any 5-year survivors among PALN+ patients, while the 3-year survivors are only about 0–10.6% (77-79,83-87). Thus, patients who are PALN+ should barely be considered “good” surgical candidates despite their radiologically resectable tumors. In comparison, the current series of arterial resection report better 3-year survival of about 13–25% (18,49,50) and yet have much more and stronger opponents regarding their oncological benefit compared to PALN+. Apparently, the opinion about feasibility in terms of oncologic prognosis of the two is refracted and perhaps distorted by the technical aspect. It is not only the prognosis that is being debated speaking about feasibility. The difference here is that while the arterial resection is more technically challenging, the resection of PALN is not. Looking at outcome, though, which of the two groups would make a better candidate for surgery?
Generally, the bigger the tumor, more often observed with BRPC, the higher risk for vascular invasion, lymph node metastases, and elevated CA19-9, all of them independently associated with worse survival (11,72,88). Resecting BRPC generally runs a higher risk for local recurrence, which is why NAT has been suggested, to attenuate this risk (15,22,88). Pretreatment with NAT in BRPC carries lower risk for superior mesenteric or portal vein invasion, lymphatic invasion, venous invasion, and lymph node metastasis (11). The benefit of NAT has evolved and amplified over the years, along with more potent chemotherapeutics being available (11,17,61,89-92) and was shown to improve the overall survival in BRPC patients compared to upfront resection in an intention-to-treat: median of 25.7 versus 19.0 months (11,24,90,92). This was confirmed also in the only randomized controlled trial available up to date on the feasibility of NAT in BRPC—median survival of 21 versus 12 months (61). The neoadjuvant approach also increases the chances that patients receive medical oncologic treatment compared to the adjuvant setting—96% versus 72%. In most studies comparing resectable to BRPC, the BRPC group had undergone NAT (15,17,20,38,57). After NAT, the survival of resected BRPC patients is comparable to that of upfront resectable PC and generally reported to be above 20 months (15,17,57,91). Thus, the “bad” BRPC tumors after NAT turn to be just as “good” candidates for resection as resectable tumors.
The immense impact of NAT on the possibility for resection of BRPC and LAPC and the following favorable long-term prognosis has awakened the interest in NAT even in resectable cases. In retrospective reviews and meta-analyses, NAT seems to improve the survival for both smaller (<2 cm) and bigger tumors (>2 cm) for treated per protocol patients (90,91,93,94). Recent randomized controlled trials showed the benefit of NAT in resectable PC, and others are ongoing (95-97). Yet the utility of NAT in intention-to-treat is yet to be determined (90,93). The notion behind NAT in PC should be to select the patients with favorable biology, which tumors tend to stay localized, irrespective of the magnitude of local tumor involvement, so that all patients who go to surgery are “good” candidates for resection.
Conclusions
There is a continuously accumulating series of data providing proof that even patients with BRPC and LAPC can be resected and led to cure. The resected series contain certainly “selected” cases by chemotherapy, but rather seeing this as a bias, it would be more appropriate to look at it as a desired selection tool—to test whether systemic chemotherapy is able to control the local lymphatic and occult systemic spread that potentially all pancreatic cancers have, and to sieve out who might benefit most from the local treatment that surgery is. This selection concept is slowly moving forward even with primary resectable pancreatic cancer.
In the upcoming era of NAT, that strongly is making its way as the new standard in pancreatic surgery, we should probably no longer speak of “good”, “bad” and “ugly” candidates for resection based only the local perivascular extension of the primary tumor. We might recognize that we deal with a more uniform and easily understandable disease. It is either we encounter tumors with greater metastatic potential, unresponsive to systemic therapy, that would not benefit from resection how even small they are, or tumors that can stay localized and then it is the surgeons’ confrontation how to overcome the technical threshold for resection. How to select even better the “good” candidates and what strategies are helpful to convert the “bad and ugly” tumors into “good” ones are the next tasks that needs to be worked on.
Acknowledgments
Raffaella Pozzi-Mucelli for kindly providing an image from her own archive.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Gastrointestinal Oncology for the series “Surgery for Locally Advanced Pancreatic Cancer”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-2020-slapc-04). The series “Surgery for Locally Advanced Pancreatic Cancer” was commissioned by the editorial office without any funding or sponsorship. ER served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bilimoria KY, Talamonti MS, Sener SF, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg 2008;207:510-9. [Crossref] [PubMed]
- Socialstyrelsen. Cancer i siffror: populärvetenskapliga fakta om cancer. 2018.
- Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266-76. [Crossref] [PubMed]
- Neumann CCM, von Hörschelmann E, Reutzel-Selke A, et al. Tumor-stromal cross-talk modulating the therapeutic response in pancreatic cancer. Hepatobiliary Pancreat Dis Int 2018;17:461-72. [Crossref] [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [Crossref] [PubMed]
- Johnson JI, Decker S, Zaharevitz D, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer 2001;84:1424-31. [Crossref] [PubMed]
- Penchev VR, Rasheed ZA, Maitra A, et al. Heterogeneity and targeting of pancreatic cancer stem cells. Clin Cancer Res 2012;18:4277-84. [Crossref] [PubMed]
- Chakraborty S, Singh S. Surgical resection improves survival in pancreatic cancer patients without vascular invasion- a population based study. Ann Gastroenterol 2013;26:346-52. [PubMed]
- Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94. [Crossref] [PubMed]
- Groot VP, Gemenetzis G, Blair AB, et al. Defining and Predicting Early Recurrence in 957 Patients With Resected Pancreatic Ductal Adenocarcinoma. Ann Surg 2019;269:1154-62. [Crossref] [PubMed]
- Kurahara H, Maemura K, Mataki Y, et al. A Therapeutic Strategy for Resectable Pancreatic Cancer Based on Risk Factors of Early Recurrence. Pancreas 2018;47:753-8. [Crossref] [PubMed]
- Yamamoto Y, Ikoma H, Morimura R, et al. Optimal duration of the early and late recurrence of pancreatic cancer after pancreatectomy based on the difference in the prognosis. Pancreatology 2014;14:524-9. [Crossref] [PubMed]
- Network NCC. NCCN Clinical Practice Guidelines in Oncology. Pancreatic adenocarcinoma (Version 1.2020 - November 26, 2019). 2020.
- UICC. 8th Edition of the UICC TNM classification of malignant tumors. Pancreatic adenocarcinoma. Available online: uicc.org. 2016.
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 846-8. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol 2011;18:619-27. [Crossref] [PubMed]
- Del Chiaro M, Rangelova E, Halimi A, et al. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB (Oxford) 2019;21:219-25. [Crossref] [PubMed]
- Ravikumar R, Sabin C, Abu Hilal M, et al. Impact of portal vein infiltration and type of venous reconstruction in surgery for borderline resectable pancreatic cancer. Br J Surg 2017;104:1539-48. [Crossref] [PubMed]
- Barnes CA, Chavez MI, Tsai S, et al. Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery 2019;166:277-85. [Crossref] [PubMed]
- Hartwig W, Werner J, Jäger D, et al. Improvement of surgical results for pancreatic cancer. Lancet Oncol 2013;14:e476-85. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Doi R, Imamura M, Hosotani R, et al. Surgery versus radiochemotherapy for resectable locally invasive pancreatic cancer: final results of a randomized multi-institutional trial. Surg Today 2008;38:1021-8. [Crossref] [PubMed]
- Cho IR, Chung MJ, Bang S, et al. Gemcitabine based neoadjuvant chemoradiotherapy therapy in patients with borderline resectable pancreatic cancer. Pancreatology 2013;13:539-43. [Crossref] [PubMed]
- Lillemoe KD, Cameron JL, Yeo CJ, et al. Pancreaticoduodenectomy. Does it have a role in the palliation of pancreatic cancer? Ann Surg 1996;223:718-25; discussion 725-8. [Crossref] [PubMed]
- Beltrame V, Gruppo M, Pedrazzoli S, et al. Mesenteric-Portal Vein Resection during Pancreatectomy for Pancreatic Cancer. Gastroenterol Res Pract 2015;2015:659730 [Crossref] [PubMed]
- Boggi U, Del Chiaro M, Croce C, et al. Prognostic implications of tumor invasion or adhesion to peripancreatic vessels in resected pancreatic cancer. Surgery 2009;146:869-81. [Crossref] [PubMed]
- Ratnayake CBB, Shah N, Loveday B, et al. The Impact of the Depth of Venous Invasion on Survival Following Pancreatoduodenectomy for Pancreatic Cancer: a Meta-analysis of Available Evidence. J Gastrointest Cancer 2020;51:379-86. [Crossref] [PubMed]
- Yekebas EF, Bogoevski D, Cataldegirmen G, et al. En bloc vascular resection for locally advanced pancreatic malignancies infiltrating major blood vessels: perioperative outcome and long-term survival in 136 patients. Ann Surg 2008;247:300-9. [Crossref] [PubMed]
- Hoshimoto S, Hishinuma S, Shirakawa H, et al. Reassessment of the clinical significance of portal-superior mesenteric vein invasion in borderline resectable pancreatic cancer. Eur J Surg Oncol 2017;43:1068-75. [Crossref] [PubMed]
- Bachellier P, Nakano H, Oussoultzoglou PD, et al. Is pancreaticoduodenectomy with mesentericoportal venous resection safe and worthwhile? Am J Surg 2001;182:120-9. [Crossref] [PubMed]
- Del Chiaro M, Segersvärd R, Rangelova E, et al. Cattell-Braasch Maneuver Combined with Artery-First Approach for Superior Mesenteric-Portal Vein Resection During Pancreatectomy. J Gastrointest Surg 2015;19:2264-8. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Michelakos T, Pergolini I, Castillo CF, et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann Surg 2019;269:733-40. [Crossref] [PubMed]
- Takahashi H, Akita H, Tomokuni A, et al. Preoperative Gemcitabine-based Chemoradiation Therapy for Borderline Resectable Pancreatic Cancer: Impact of Venous and Arterial Involvement Status on Surgical Outcome and Pattern of Recurrence. Ann Surg 2016;264:1091-7. [Crossref] [PubMed]
- Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [Crossref] [PubMed]
- Lu DS, Reber HA, Krasny RM, et al. Local staging of pancreatic cancer: criteria for unresectability of major vessels as revealed by pancreatic-phase, thin-section helical CT. AJR Am J Roentgenol 1997;168:1439-43. [Crossref] [PubMed]
- Saldinger PF, Reilly M, Reynolds K, et al. Is CT angiography sufficient for prediction of resectability of periampullary neoplasms? J Gastrointest Surg 2000;4:233-7; discussion 238-9. [Crossref] [PubMed]
- Imamura T, Yamamoto Y, Sugiura T, et al. Prognostic role of the length of tumour-vein contact at the portal-superior mesenteric vein in patients having surgery for pancreatic cancer. Br J Surg 2019;106:1649-56. [Crossref] [PubMed]
- Katz MH, Fleming JB, Pisters PW, et al. Anatomy of the superior mesenteric vein with special reference to the surgical management of first-order branch involvement at pancreaticoduodenectomy. Ann Surg 2008;248:1098-102. [Crossref] [PubMed]
- Gage MM, Reames BN, Ejaz A, et al. Pancreaticoduodenectomy with en bloc vein resection for locally advanced pancreatic cancer: a case series without venous reconstruction. Chin Clin Oncol 2018;7:7. [Crossref] [PubMed]
- Oehme F, Distler M, Müssle B, et al. Results of portosystemic shunts during extended pancreatic resections. Langenbecks Arch Surg 2019;404:959-66. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer 2007;110:738-44.
- Marchegiani G, Andrianello S, Malleo G, et al. Does Size Matter in Pancreatic Cancer?: Reappraisal of Tumour Dimension as a Predictor of Outcome Beyond the TNM. Ann Surg 2017;266:142-8. [Crossref] [PubMed]
- Saka B, Balci S, Basturk O, et al. Pancreatic Ductal Adenocarcinoma is Spread to the Peripancreatic Soft Tissue in the Majority of Resected Cases, Rendering the AJCC T-Stage Protocol (7th Edition) Inapplicable and Insignificant: A Size-Based Staging System (pT1: ≤2, pT2: >2-≤4, pT3: >4 cm) is More Valid and Clinically Relevant. Ann Surg Oncol 2016;23:2010-8.
- Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882-93. [Crossref] [PubMed]
- Bachellier P, Addeo P, Faitot F, et al. Pancreatectomy With Arterial Resection for Pancreatic Adenocarcinoma: How Can It Be Done Safely and With Which Outcomes?: A Single Institution's Experience With 118 Patients. Ann Surg 2020;271:932-40. [Crossref] [PubMed]
- Tee MC, Krajewski AC, Groeschl RT, et al. Indications and Perioperative Outcomes for Pancreatectomy with Arterial Resection. J Am Coll Surg 2018;227:255-69. [Crossref] [PubMed]
- Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153-9. [Crossref] [PubMed]
- Gemenetzis G, Groot VP, Blair AB, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg 2019;270:340-7. [Crossref] [PubMed]
- Hackert T, Sachsenmaier M, Hinz U, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg 2016;264:457-63. [Crossref] [PubMed]
- Rangelova E, Wefer A, Persson S, et al. Surgery Improves Survival After Neoadjuvant Therapy for Borderline and Locally Advanced Pancreatic Cancer: A Single Institution Experience. Ann Surg 2021;273:579-86. [Crossref] [PubMed]
- Truty MJ, Thomas RM, Katz MH, et al. Multimodality therapy offers a chance for cure in patients with pancreatic adenocarcinoma deemed unresectable at first operative exploration. J Am Coll Surg 2012;215:41-51; discussion 51-2. [Crossref] [PubMed]
- Suker M, Beumer BR, Sadot E, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta-analysis. Lancet Oncol 2016;17:801-10. [Crossref] [PubMed]
- E R. Borderline and locally advanced pancreatic cancer – redefining the biological and technical profile of the disease. Stockholm: Karolinska Institute; 2019.
- Macedo FI, Ryon E, Maithel SK, et al. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg 2019;270:400-13. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- van Veldhuisen E, van den Oord C, Brada LJ, et al. Locally Advanced Pancreatic Cancer: Work-Up, Staging, and Local Intervention Strategies. Cancers (Basel) 2019;11:976. [Crossref] [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- van Veldhuisen E, Vogel JA, Klompmaker S, et al. Added value of CA19-9 response in predicting resectability of locally advanced pancreatic cancer following induction chemotherapy. HPB (Oxford) 2018;20:605-11. [Crossref] [PubMed]
- Oba A, Croce C, Hosokawa P, et al. Prognosis Based Definition of Resectability in Pancreatic Cancer: A Road Map to New Guidelines. Ann Surg 2020; [Epub ahead of print]. [Crossref] [PubMed]
- Klaiber U, Schnaidt ES, Hinz U, et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann Surg 2021;273:154-62. [Crossref] [PubMed]
- Groot VP, Mosier S, Javed AA, et al. Circulating Tumor DNA as a Clinical Test in Resected Pancreatic Cancer. Clin Cancer Res 2019;25:4973-84. [Crossref] [PubMed]
- Patel H, Okamura R, Fanta P, et al. Clinical correlates of blood-derived circulating tumor DNA in pancreatic cancer. J Hematol Oncol 2019;12:130. [Crossref] [PubMed]
- Hugenschmidt H, Labori KJ, Brunborg C, et al. Circulating Tumor Cells are an Independent Predictor of Shorter Survival in Patients Undergoing Resection for Pancreatic and Periampullary Adenocarcinoma. Ann Surg 2020;271:549-58. [Crossref] [PubMed]
- McDuff S, Parikh AR, Hazar-Rethinam M, et al. Using circulating tumor DNA (ctDNA) to predict surgical outcome after neoadjuvant chemoradiation for locally advanced pancreatic cancer (LAPC). J Clin Oncol 2018;36:272. [Crossref]
- Gurusamy KS, Kumar S, Davidson BR, et al. Resection versus other treatments for locally advanced pancreatic cancer. Cochrane Database Syst Rev 2014;CD010244 [Crossref] [PubMed]
- Jones RP, Psarelli EE, Jackson R, et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg 2019;154:1038-48. [Crossref] [PubMed]
- Neoptolemos JP, Moore MJ, Cox TF, et al. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA 2012;308:147-56. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Nishio K, Kimura K, Amano R, et al. Preoperative predictors for early recurrence of resectable pancreatic cancer. World J Surg Oncol 2017;15:16. [Crossref] [PubMed]
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- E.S. Amin MB. AJCC Cancer Staging Manual . 8th ed, ed. E.S. Amin MB, Greene FL (Eds) In: FL G, editor. Chicago IL: Springe: Springer Nature, Springer International Publishing AG; 2018.
- LH S. TNM Classification of Malignant Tumours (UICC). 5th ed. NY: John Wiley & Sons, New York; 1997.
- Kayahara M, Nagakawa T, Ohta T, et al. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: a significant indication for surgery? Cancer 1999;85:583-90. [Crossref] [PubMed]
- Liu C, Lu Y, Luo G, et al. Which patients with para-aortic lymph node (LN16) metastasis will truly benefit from curative pancreaticoduodenectomy for pancreatic head cancer? Oncotarget 2016;7:29177-86. [Crossref] [PubMed]
- Schwarz L, Lupinacci RM, Svrcek M, et al. Para-aortic lymph node sampling in pancreatic head adenocarcinoma. Br J Surg 2014;101:530-8. [Crossref] [PubMed]
- Sho M, Murakami Y, Motoi F, et al. Postoperative prognosis of pancreatic cancer with para-aortic lymph node metastasis: a multicenter study on 822 patients. J Gastroenterol 2015;50:694-702. [Crossref] [PubMed]
- Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156:591-600. [Crossref] [PubMed]
- Komo T, Murakami Y, Kondo N, et al. Prognostic Impact of Para-Aortic Lymph Node Micrometastasis in Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 2016;23:2019-27. [Crossref] [PubMed]
- Asano D, Nara S, Kishi Y, et al. A Single-Institution Validation Study of Lymph Node Staging By the AJCC 8th Edition for Patients with Pancreatic Head Cancer: A Proposal to Subdivide the N2 Category. Ann Surg Oncol 2019;26:2112-20.
- Asaoka T, Miyamoto A, Maeda S, et al. CA19-9 level determines therapeutic modality in pancreatic cancer patients with para-aortic lymph node metastasis. Hepatobiliary Pancreat Dis Int 2018;17:75-80. [Crossref] [PubMed]
- Choi SH, Kim SH, Choi JJ, et al. Clinical necessity of the immunohistochemical reassessment of para-aortic lymph nodes in resected pancreatic ductal adenocarcinoma. Oncol Lett 2013;6:1189-94. [PubMed]
- Hempel S, Plodeck V, Mierke F, et al. Para-aortic lymph node metastases in pancreatic cancer should not be considered a watershed for curative resection. Sci Rep 2017;7:7688. [Crossref] [PubMed]
- Sakai M, Nakao A, Kaneko T, et al. Para-aortic lymph node metastasis in carcinoma of the head of the pancreas. Surgery 2005;137:606-11. [Crossref] [PubMed]
- Yamada S, Fujii T, Sugimoto H, et al. Aggressive surgery for borderline resectable pancreatic cancer: evaluation of National Comprehensive Cancer Network guidelines. Pancreas 2013;42:1004-10. [Crossref] [PubMed]
- Andriulli A, Festa V, Botteri E, et al. Neoadjuvant/preoperative gemcitabine for patients with localized pancreatic cancer: a meta-analysis of prospective studies. Ann Surg Oncol 2012;19:1644-62. [Crossref] [PubMed]
- Versteijne E, Vogel JA, Besselink MG, et al. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg 2018;105:946-58. [Crossref] [PubMed]
- Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119:2692-700. [Crossref] [PubMed]
- Nagakawa Y, Sahara Y, Hosokawa Y, et al. Clinical Impact of Neoadjuvant Chemotherapy and Chemoradiotherapy in Borderline Resectable Pancreatic Cancer: Analysis of 884 Patients at Facilities Specializing in Pancreatic Surgery. Ann Surg Oncol 2019;26:1629-36. [Crossref] [PubMed]
- Lee YS, Lee JC, Yang SY, et al. Neoadjuvant therapy versus upfront surgery in resectable pancreatic cancer according to intention-to-treat and per-protocol analysis: A systematic review and meta-analysis. Sci Rep 2019;9:15662. [Crossref] [PubMed]
- Takahashi C, Shridhar R, Huston J, et al. Correlation of tumor size and survival in pancreatic cancer. J Gastrointest Oncol 2018;9:910-21. [Crossref] [PubMed]
- Labori KJ, Lassen K, Hoem D, et al. Neoadjuvant chemotherapy versus surgery first for resectable pancreatic cancer (Norwegian Pancreatic Cancer Trial - 1 (NorPACT-1)) - study protocol for a national multicentre randomized controlled trial. BMC Surg 2017;17:94. [Crossref] [PubMed]
- Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05. J Clin Oncol 2019;37:189. [Crossref]
- Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol 2020;38:1763-73. [Crossref] [PubMed]


