
Prognostic significance of controlling nutritional status score-based nomogram for hepatocellular carcinoma within Milan criteria after radiofrequency ablation
Introduction
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related death worldwide (1). About 50% of HCC cases and deaths worldwide occur in China (2). The therapeutic options for the treatment of HCC include surgical resection, liver transplantation, interventional therapy, chemotherapy, targeted therapy, and immunotherapy (3-5). The clinical decision to use any particular therapy alone or in combination depends on tumor stage, liver function, and performance status (4,5). Radiofrequency ablation (RFA) induces coagulative necrosis in tumor tissue by transforming radiofrequency energy into thermal ablative energy (4-6). Current Guidelines for the Diagnosis and Treatment of Primary Liver Cancer in China recommended RFA as one of the first-line therapies for HCC patients within Milan criteria (single nodule ≤5 cm or no more than three nodules ≤3 cm, without vascular invasion or extrahepatic metastasis) (7).
The nutritional and immunological status of the patient has been reported to be important prognostic factors for various malignancies, including HCC (8). Controlling nutritional status (CONUT) score, a novel immuno-nutritional biomarker, is easy to be calculated based on the results of routine laboratory tests including serum albumin, total lymphocytes, and cholesterol. It serves as a useful tool for the nutritional assessment of hospitalized patients (9,10). Serum albumin level directly reflects hepatic function, which is considered to be an important prognostic factor in patients with HCC (11). Lymphocyte count is a verified and reliable biomarker of nutritional and immune status in patients with HCC (12). Meanwhile, previous study indicated that cholesterol level is a prognostic predictor of oncological outcomes in HCC patients (13). The CONUT score was established as an effective combination of these three biomarkers. High CONUT scores are associated with poor prognosis in malignancies such as HCC, gastric carcinoma, colorectal carcinoma, and urothelial carcinoma (14-17). To the best of our knowledge, no previous study has reported the relationship between CONUT score and prognosis of HCC patients within Milan criteria after ablation treatment.
Therefore, a retrospective study was conducted to investigate the prognostic significance of preoperative CONUT score for HCC within the Milan criteria after RFA treatment. Meanwhile, two nomograms based on CONUT score were developed to predict the probability of disease-free survival (DFS) and overall survival (OS), which can directly help clinical oncologists to formulate adjuvant therapeutic and preventive strategies. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/jgo-20-225).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Boards of the National Cancer Center and First Hospital of Shanxi Medical University (NCC2019KZ-010). Written informed consent for treatment was obtained from each patient. The need for written informed consent to publish the data was waived by the Institutional Review Boards, since the personal details of these patients were kept confidential. Patients who refused to undergo surgery, or those who were unfit for surgery received RFA treatment after the diagnosis.
In this retrospective study, HCC patients who underwent RFA at the National Cancer Center and the First Hospital of the Shanxi Medical University during the period from January 2010 to December 2014 were included when they satisfied the following criteria: (I) HCC confirmed by histology or noninvasive diagnostic criteria according to the European Association for the Study of Liver (EASL) (18); (II) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (III) solitary tumor with a diameter of ≤5 cm or multiple tumors of ≤3, each with a diameter of ≤3 cm (Milan criteria); (IV) no extrahepatic metastasis or major vessel invasion; (V) complete tumor ablation with RFA. The exclusion criteria were: (I) patients who underwent their first RFA at other centers; (II) patients who underwent surgery as the primary treatment and RFA for recurrence; and (III) patients with tumor(s) located in unfavorable locations. Unfavorable locations were defined as sites where the tumor margin was <0.5 cm from the important structures, including the major vessels, intrahepatic bile duct, gallbladder, diaphragm, pericardium and gastrointestinal tract, which were difficult to achieve complete ablation (19).
Preoperative CONUT score were calculated based on serum albumin levels, total lymphocyte count, and total cholesterol (as measured 3 days before RFA). Serum albumin levels were classified as normal (3.5–4.5 g/dL, score =0), mild (3.0–3.49 g/dL, score =2), moderate (2.5–2.99 g/dL, score =4), or severe (<2.5 g/dL, score =6). Total lymphocyte count was scored as normal (>1,600/mL, score =0), mild (1,200–1,599/mL, score =1), moderate (800–1,199/mL, score =2), or severe (<800/mL, score =3). The scoring criteria for total cholesterol was normal (>180 mg/dL, score =0), mild (140–180 mg/dL, score =1), moderate (100–139 mg/dL, score =2), or severe (<100 mg/dL, score =3). The CONUT score reflects all three of these clinical parameters. Scores are classified as normal (score 0–1), mild (score 2–4), moderate (score 5–8), or severe (score 9–12) malnutrition (10).
All clinically relevant variables and cut-off values before the RFA were collected based on the results of previous studies (20,21), including age, gender, maximum tumor size, number of tumors, tumor differentiation, cirrhosis, hepatitis B virus (HBV) infection, Child-Pugh class, serum alpha-fetoprotein (AFP), aspartic transaminase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), serum γ-glutamyl transpeptidase (γ-GT) levels and CONUT score. The three components of CONUT score were not included separately in clinically relevant variables to avoid bias in analyses. Tumor differentiation was assessed according to the WHO classification of digestive system tumor by two pathologists with at least 5 years of experience (22).
RFA procedure
All RFA procedures were performed under the computed tomography (CT) guidance by doctors with at least 5 years of RFA experience using radiofrequency generator system (S-1500, MedSphere International Inc., CA, USA). Local anesthesia was given before RFA. The type of electrode to be used (monopolar or/and umbrella electrode) (MedSphere International Inc., CA, USA), the power of the generator, and ablation strategy were determined by the operator based upon the tumor burden and the manufacturer’s recommendations and so on. Expanding or overlapping ablation was performed such that all tumors were ablated with a safety margin of at least 0.5 cm. After the lesions were ablated, the ablation path was cauterized to avoid tumor seeding and hemorrhage during the RFA procedure. After RFA treatment, contrast-enhanced CT was immediately performed to confirm complete ablation. If residual tumor(s) were found, additional ablation treatment would be conducted to achieve complete ablation. Technical success was defined as the complete tumor ablation in single session according to the treatment protocol (23).
Follow-up
Patients were followed-up at 1, 3, 6, 9, and 12 months during the first year after RFA and every 6 months in subsequent years until tumor recurrence or death. The last follow-up date for this study was September 30th, 2019. Measurements of serum AFP levels and CE imaging were performed at each follow-up visit. DFS was defined as the duration from first RFA treatment to tumor recurrence or death. OS was defined as the duration between RFA treatment and death from any cause or the last follow-up examination. The multidisciplinary approach including surgical resection, repeat RFA, transarterial chemoembolization (TACE), or systemic therapy (i.e., sorafenib), was adopted to treat the recurrence (7).
Statistical analysis
Categorical variables were analyzed with Fisher’s exact test or the χ2 test. Continuous variables were analyzed with the t-test or the Mann-Whitney U-test. The Kaplan-Meier method was used to evaluate the cumulative incidence rates of DFS and OS, and the log-rank test was used to evaluate differences between groups. Univariate and multivariate Cox proportional hazards models were used to determine the risk factors for OS and DFS. The best cut-off value for CONUT score was determined by X-tile 3.6.1 software (Yale University School of Medicine, New Haven, CT, USA) (24). Spearman’s correlation analysis was used to detect potential correlations between risk factors. A two-tailed 95% confidence interval (CI) was applied to reveal the accuracy of the hazard ratio (HR), and a P value <0.05 was considered to be statistically significant. All statistical analyses were performed by using GraphPad Prism 7.0 software (GraphPad Software Inc., La Jolla, CA, USA) and R software version 3.6.2 (http://www.r-project.org/).
The nomograms were subjected to 500 bootstrap resamples for internal validation of the analyzed database. The performance of models for predicting prognosis was evaluated by calculating the concordance index (C‐index). The value of the C‐index varied between 0.5 and 1.0, with 1.0 indicating perfect ability to correctly discriminate outcomes and 0.5 indicating a random chance. Calibration of the nomogram for 1-, 2‐, and 3‐year DFS and 1-, 2‐, 3-, and 5‐year OS was performed by comparing observed survival with predicted survival. The information gain method was used to calculate the importance of each factor in the nomogram with a Pareto Diagram. The area under the time-dependent receiver operating characteristic (t-ROC) curve (t-AUC) was calculated to comprehensively evaluate the predictive power of the model in comparison to the C‐index method, as calculation of the t-AUC involves computations of sensitivity and specificity.
Results
Determination of the cut-off value for CONUT score
The preoperative CONUT score of each patient was calculated using the scoring system described above. CONUT =4 was showed the best discriminative performance for DFS and OS according to X-tile software analysis (24). The value of CONUT =4 was also the cut-off used to differentiate between normal-to-mild and moderate-to-severe malnutrition. Therefore, CONUT =4 was used as a cut-off value for clinical significance in subsequent analyses.
Baseline characteristics
Based on the selection criteria, a total of 403 HCC patients who underwent RFA at National Cancer Center (n=268), and the First Hospital of the Shanxi Medical University (n=135) were included in this study. There were 324 patients in low CONUT score group (normal-to-mild malnutrition, CONUT ≤4) and 79 patients in high CONUT score group (moderate to severe malnutrition, CONUT ≥5). The baseline characteristics were compared between the two CONUT score groups as shown in Table 1. The AST, ALT and TBIL levels were significantly different between the two groups. Technical success was 93.93% (573/610), and 37 residual tumors were found on the immediate post-RFA contrast-enhanced CT. In these situations, an additional RFA session was performed to achieve complete ablation.
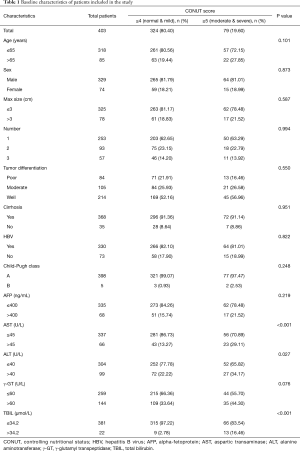
Full table
Prognostic factors affecting survival after RFA
Factors affecting DFS
Univariate analysis indicated that CONUT score (≥5, HR =1.449, 95% CI: 1.022–2.056, P=0.038), maximum tumor diameter (≤3 cm, HR =0.637, 95% CI: 0.418–0.972, P=0.037), number of lesions (two lesions, HR =2.639, 95% CI: 1.888–3.688, P<0.001; three lesions, HR =3.055, 95% CI: 2.101–4.443, P<0.001), degree of tumor differentiation (moderate, HR =3.314, 95% CI: 2.322–4.731, P<0.001; poor, HR =8.638, 95% CI: 5.921–12.600, P<0.001) and AFP levels (>400 ng/mL, HR =1.804, 95% CI: 1.265–2.573, P<0.001) were significantly associated with DFS (Table 2).
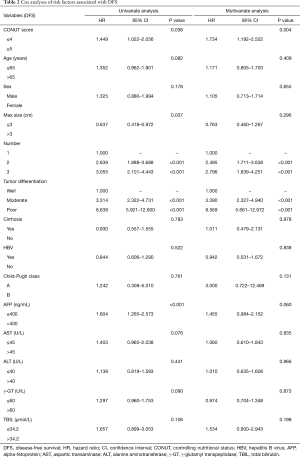
Full table
The results of multivariate analysis showed that CONUT score (≥5, HR =1.734, 95% CI: 1.192–2.522, P=0.004), number of lesions (two lesions, HR =2.495, 95% CI: 1.711–3.638, P<0.001; three lesions, HR =2.796, 95% CI: 1.839–4.251, P<0.001), and degree of tumor differentiation (moderate, HR =3.390, 95% CI: 2.327–4.940, P<0.001; poor, HR =8.569, 95% CI: 5.661–12.972, P<0.001) were independent predictors of DFS after RFA treatment (Table 2).
Factors affecting OS
The results of univariate analysis indicated that CONUT score (≥5, HR =2.151, 95% CI: 1.361–3.400, P<0.001), age (>65 years, HR =1.765, 95% CI: 1.107–2.841, P=0.017), number of lesions (two lesions, HR =1.756, 95% CI: 1.076–2.866, P=0.024; three lesions, HR =2.195, 95% CI: 1.270–3.792, P=0.005), degree of tumor differentiation (poor, HR =3.560, 95% CI: 2.177–5.820, P<0.001), AFP level (>400 ng/mL, HR =1.879, 95% CI: 1.157–3.051, P=0.011), and TBIL (>34.2 µmol/L, HR =3.058, 95% CI: 1.621–5.767, P<0.001) were significantly associated with OS (Table 3).
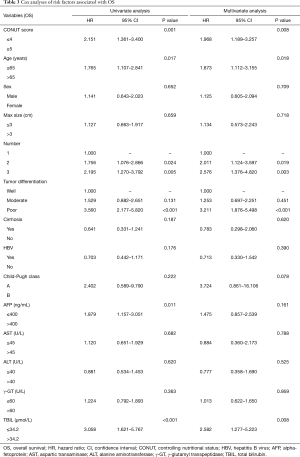
Full table
The results of multivariate analysis showed that CONUT score (≥5, HR =1.968, 95% CI: 1.189–3.257, P=0.008), age (>65 years, HR =1.873, 95% CI: 1.112–3.155, P=0.018), number of lesions (two lesions, HR =2.011, 95% CI: 1.124–3.597, P=0.019; three lesions, HR =2.576, 95% CI: 1.376–4.820, P=0.003), tumor differentiation (poor, HR =3.211, 95% CI: 1.876–5.498, P<0.001), and TBIL (>34.2 µmol/L, HR =2.582, 95% CI: 1.277–5.223, P=0.008) were significantly associated with OS after RFA treatment (Table 3).
Survival analysis of HCC patients after RFA
Among 324 patients with normal-to-mild malnutrition (CONUT ≤4), 142 patients developed recurrence (43.83%), and 59 patients died (18.21%). In 79 patients with moderate-to-severe malnutrition (CONUT ≥5), 41 patients developed recurrence (51.90%), and 27 patients died (34.18%). Median DFS and OS were 21.5 (1.6–77.1) months and 36.8 (2.9–89.0) months, respectively.
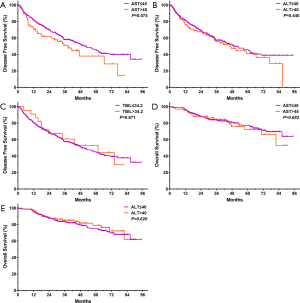
The cumulative rates of 1-, 2- and 3-year DFS rates were 81.73%, 70.02%, and 58.90%, respectively, in CONUT ≤4 group and 74.30%, 64.09%, and 48.46%, respectively, in CONUT ≥5 group. The cumulative DFS rates were significantly higher in CONUT ≤4 group, compared with the CONUT ≥5 group (P=0.033, Figure 1A). During the follow-up, among 183 patients with recurrence, nine patients underwent surgical resection, 107 patients received a second RFA treatment, 52 patients received TACE, and 15 patients received TACE combined with sorafenib.
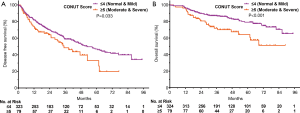
The cumulative rates of 1-, 2-, 3- and 5-year OS rates were 96.60%, 89.54%, 86.97%, and 79.17% in CONUT ≤4 group respectively, and 96.20%, 80.63%, 71.83%, and 64.23% in CONUT ≥5 group respectively. The cumulative OS rates were significantly higher in CONUT ≤4 group, compared with the CONUT ≥5 group (P<0.001, Figure 1B).
Further survival analysis was performed to investigate differences between higher and lower CONUT score groups in baseline clinical variables (AST, ALT, and TBIL). There were no significant differences in DFS that correlated with levels of AST, ALT, or TBIL levels (P=0.074, P=0.440, and P=0.871, respectively), nor were there any significant differences in OS that correlated with AST or ALT levels (P=0.682 and P=0.620) (Figure S1 for review but not for publication). However, abnormal TBIL levels (TBIL >34.2 µmol/L) were associated with poor OS (P<0.001, Figure 2A).
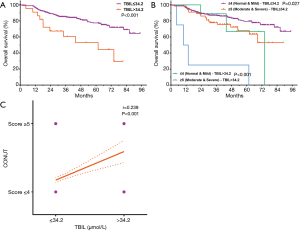
Further survival analysis was performed for CONUT scores stratified by TBIL. In patients with normal TBIL (≤34.2 µmol/L), significant differences in OS were observed between those classified as CONUT ≤4 and those classified as CONUT ≥5 (Figure 2B, P=0.027). Among patients with abnormal TBIL (>34.2 µmol/L), OS differed significantly between the two CONUT score groups (Figure 2B, P<0.001). The results of Spearman correlation analysis supported a positive correlation between CONUT and TBIL (Figure 2C, r=0.239, 95% CI: 0.145–0.329, P<0.001).
Complications
No treatment-related deaths and/or major complications occurred in two groups. The most common complications were fever and haemorrhage. The complication rates associated with RFA were 3.2% (13/403) in the overall data (nine in CONUT ≤4 group and four in CONUT ≥5 group, P=0.29).
Construction of a nomogram to predict DFS and OS
According to the results of multivariate Cox regression analysis, CONUT score, number of lesions, and degree of tumor differentiation were found to be potential prognostic factors affecting DFS after RFA treatment. For OS, the potential prognostic factors were CONUT score, age, number of lesions, degree of tumor differentiation, and TBIL level. We constructed a nomogram using these prognostic factors to predict DFS and OS after RFA treatment (Figure 3A,B). The results of analysis with the information gain method showed that CONUT was the second most important factor studied in the nomograms for predicting DFS and OS by Pareto Diagram (Figure 3C,D).
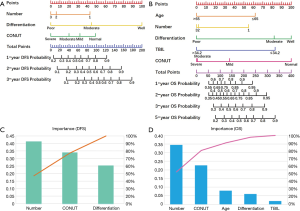
The probability of 1-, 2-, and 3-year DFS were predicted with a C-index of 0.798 (95% CI: 0.743–0.852). The t-AUC for the nomograms predicting 1-, 2-, and 3-year DFS were 0.884 (sensitivity =0.803, specificity =0.835, Figure 4A), 0.888 (sensitivity =0.774, specificity =0.840, Figure 4B), and 0.835 (sensitivity =0.798, specificity =0.712, Figure 4C).
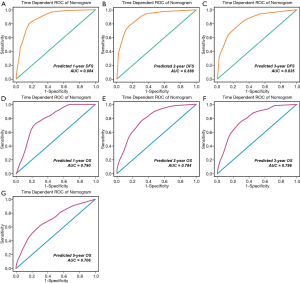
The probabilities of 1-, 2-, 3-, and 5-year OS were predicted with a C-index of 0.757 (95% CI: 0.708–0.806). The t-AUC for the nomograms predicting 1-, 2-, 3- and 5-year OS were 0.790 (sensitivity =0.729, specificity =0.747, Figure 4D), 0.794 (sensitivity =0.769, specificity =0.681, Figure 4E), 0.796 (sensitivity =0.758, specificity =0.699, Figure 4F), and 0.706 (sensitivity =0.618, specificity =0.697, Figure 4G).
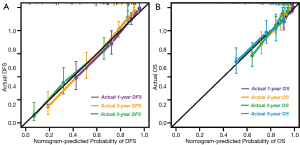
In the calibration curve for predicting DFS after RFA at 1, 2, and 3 years with the validation dataset, the X-axis presented the nomogram-predicted probability of DFS, and the Y-axis presented actual DFS. The calibration lines fit the 45° reference for 1-, 2-, and 3-year DFS (Figure 5A). In the calibration curve used with the validation dataset to predict OS after RFA at 1, 2, 3, and 5 years, the X-axis presents the nomogram-predicted probability of OS, and the Y-axis shows actual OS. The calibration lines fit the 45° reference for 1-, 2-, 3- and 5-year OS (Figure 5B).
Discussion
Thermal ablation has been recognized as an effective therapeutic option for HCC patients within Milan criteria (7). In this study, number of lesions, tumor differentiation, and CONUT score were identified as independent prognostic factors affecting DFS and OS. Age and TBIL were identified as prognostic factors for OS but not for DFS. Beyond well-recognized prognostic factors for HCC after RFA treatment such as age, number of lesions, maximum tumor diameter, degree of tumor differentiation, and AFP levels, we propose immune status and nutritional status to be important factors affecting the prognosis of patients with HCC (25,26). The CONUT score is based on measurements of serum albumin, total lymphocyte count, and total cholesterol. It has been shown to be an effective tool to determine and monitor the immunological and nutritional status of hospitalized patients (9,10).
HCC is an inflammation-related cancer. More than 90% of HCC patients have chronic inflammation caused by infection with HBV and cirrhosis (27). HBV-induced cirrhosis is the leading cause of HCC in China. Most patients with cirrhosis have liver dysfunction, hypoalbuminemia, malnutrition, and even cachexia (2). In this study, a total of 368 patients (91.32%) had cirrhosis, and 330 patients (81.89%) had HBV infection.
With respect to components of the CONUT score, serum albumin level is not only an important marker of hepatic functional reserve but also a reliable indicator of immunological and nutritional status. Low serum albumin levels reflect poor hepatic functional reserve, poor immunological status, and poor nutritional status (9,10). In the present study, we found that cumulative DFS and OS rates were significantly higher in the low CONUT score group, compared with the high CONUT score group (P=0.033, P<0.001). These results are similar to those reported previously (8,9).
Total lymphocyte count, another component of CONUT score, play a crucial role in host cell-mediated immunity. Meanwhile, cirrhosis-induced pancytopenia may reduce the lymphocyte count. Lymphocytes can be divided into T lymphocytes (T-cells), B lymphocytes (B-cells), and natural killer (NK) cells. Lymphocytes are closely related to the proliferation, invasion, and metastasis of cancer cells. For example, cytotoxic T-cells (CTL) activated by CD8+ T-cells can secrete perforin, granzyme B, interferon (INF), and tumor necrosis factor (TNF) to kill cancer cells; NK cells are effector cells that participate in antibody-dependent cell-mediated cytotoxicity (ADCC) (28). A low total lymphocyte count indicates poor immunological status and leads to a high CONUT score (9,10). Previous systematic reviews have verified that a low preoperative lymphocyte count is independently associated with poor prognosis in HCC patients (29,30). The current study also found that cumulative DFS and OS rates were significantly higher in low CONUT score group, compared with the high CONUT group (P=0.033, P<0.001). Nutritional status is closely associated with immunological status, and hypoalbuminemia is correlated with poor prognosis in HCC patients (31).
Cholesterol is a major component of the cell membrane that participates in cellular metabolism. Meanwhile, the liver plays an important role in cholesterol metabolism. Serum cholesterol level indicates the energy reserves available (10). One study demonstrated that low serum cholesterol levels limit the immunological competence of immune cells by altering the structure of immune cell membranes, which may explain the correlation between low cholesterol level and poor prognosis in patients with HCC (32).
Each component of the CONUT score is directly associated with liver disease (e.g., hepatitis, cirrhosis, HCC). A high CONUT score usually indicates low serum albumin, low cholesterol, and low lymphocyte count, indicating reduced immune capacity and poor nutritional status. Each component of the CONUT score is therefore considered to play a key role in the occurrence, development, and progression of HCC. Use of the CONUT score to account for the three biomarkers described above may provide clinicians with a more accurate and comprehensive index for immunological and nutritional status, which may explain the superiority of CONUT over other individual biomarkers as a prognostic factor for patients with liver disease.
In addition to CONUT score, TBIL level was also an independent prognostic factor for OS (P<0.001) but not for DFS, which is in accordance with the findings reported by N’Kontchou et al. (33). High levels of serum bilirubin (TBIL >34.2 µmol/L) is associated with liver cirrhosis (33). Hyperbilirubinemia reflect impairments in the transformation, and excretion of bilirubin caused by hepatocellular disease such as cirrhosis and cancer. When the excretion of bilirubin is blocked, it returns to the blood, resulting in increased serum levels of bilirubin. An increase in serum levels of bilirubin is associated with poor hepatic functional reserve and chronic inflammation in patients with HCC. Moreover, our study found a potential positive correlation between CONUT score and TBIL (r=0.239, P<0.001). This finding may reflect the fact that serum albumin level and total lymphocyte count are components of the CONUT score, and TBIL is an index for hepatic function. The specific mechanism and relationship between TBIL and prognosis in HCC patients remain to be elucidated.
The cut-off value (CONUT ≤4 vs. ≥5) obtained in this study by X-tile software is aligned with the clinical criteria used to differentiate mild malnutrition from moderate-to-severe malnutrition used in previous studies (9,10). The current study found that high CONUT score was associated with poor prognosis in HCC patients treated with RFA. One previous study found that malnutrition is an independent prognostic factor for HCC (8). The authors reported that nutritional interventions such as human serum albumin infusion and the intravenous infusion of branched-chain amino acids may have positive impacts on the oncological outcomes of patients with HCC who have undergone RFA (34). CONUT score may also be used as a nutritional marker to identify high-risk patients who may require nutritional interventions to obtain survival benefits.
Nomograms have been widely used as a visualization tool in predicting the prognosis of patients with various types of tumors, including HCC (35). In this study, we constructed nomograms based on the prognostic factors indicated by multivariate Cox regression to predict survival outcomes in HCC patients after RFA treatment. The five prognostic factors included in the nomogram for OS were age, number of lesions, degree of tumor differentiation, TBIL, and CONUT score. The three prognostic factors included in the nomogram for DFS were number of lesions, degree of tumor differentiation, and CONUT score. The C-index, calibration plots and t-AUCs demonstrated that the nomograms developed in the present study have well discrimination and calibration. These preliminary results provide novel insights and tools for the postoperative management of HCC patients. However, our findings need to be prospectively validated in larger external cohorts.
The most common prognostic scoring systems for HCC are the Barcelona Clinic Liver Cancer (BCLC) system and albumin-bilirubin (ALBI) score system (36), which focus on tumor number, tumor size, liver function, and presence of metastasis. Nevertheless, the CONUT score focuses on nutritional and immune status, which is a novel biomarker for prognostic evaluation. This is the first study to identify the CONUT score as a prognostic factor of HCC and develop CONUT score-based nomograms for HCC patients within Milan criteria after RFA. Future studies will be conducted to compare the accuracy of CONUT score with other scoring systems such as BCLC system and ALBI score system.
Some limitations of the present study should be noted when interpreting the results of this study. First, it was a retrospective study, performed at two institutions, with limited sample size and potential selection bias. Second, more than 80% (330/403) of the patients included in the study had HBV infection. Hence, the results could not be extrapolated to other populations where alcohol is the main etiological factor of HCC, as observed in Western countries (37). Third, external validation was not performed because we need to ensure the availability of enough samples for constructing reliable and accurate nomograms, in order to predict prognosis.
In conclusion, the preliminary results of this two-center retrospective study indicate that high CONUT scores are associated with poor survival outcomes. Nomograms constructed using the CONUT score in combination with other prognostic factors may accurately predict DFS and OS in HCC patients after RFA treatment. Moreover, CONUT score may be used as a novel immuno-nutritional biomarker for the identification of high-risk patients likely to obtain survival benefits from nutritional interventions. Future prospective multicenter studies are warranted to validate the prognostic role of CONUT scores in HCC.
Acknowledgments
Funding: This study was funded by National Natural Science Foundation of China (30970839 & 31170957) and Research Project of Shanxi Province Health Commission (2017045).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-225
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jgo-20-225
Peer Review File: Available at http://dx.doi.org/10.21037/jgo-20-225
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-225). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Boards of the National Cancer Center and First Hospital of Shanxi Medical University (NCC2019KZ-010). Written informed consent for treatment was obtained from each patient. The need for written informed consent to publish the data was waived by the Institutional Review Boards, since the personal details of these patients were kept confidential.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Zheng R, Qu C, Zhang S, et al. Liver cancer incidence and mortality in China: temporal trends and projections to 2030. Chin J Cancer Res 2018;30:571-9. [Crossref] [PubMed]
- Yang Y, Si T. Yttrium-90 transarterial radioembolization versus conventional transarterial chemoembolization for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Cancer Biol Med 2018;15:299-310. [Crossref] [PubMed]
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391:1301-14. [Crossref] [PubMed]
- Villanueva A. Hepatocellular Carcinoma. N Engl J Med 2019;380:1450-62. [Crossref] [PubMed]
- Craig AJ, von Felden J, Garcia-Lezana T, et al. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2020;17:139-52. [Crossref] [PubMed]
- Department of Medical Administration, National Health and Health Commission of the People's Republic of China. Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition). Zhonghua Gan Zang Bing Za Zhi 2020;28:112-28. [PubMed]
- Schütte K, Tippelt B, Schulz C, et al. Malnutrition is a prognostic factor in patients with hepatocellular carcinoma (HCC). Clin Nutr 2015;34:1122-7. [Crossref] [PubMed]
- Lin ZX, Ruan DY, Jia CC, et al. Controlling nutritional status (CONUT) score-based nomogram to predict overall survival of patients with HBV-associated hepatocellular carcinoma after curative hepatectomy. Clin Transl Oncol 2020;22:370-80. [Crossref] [PubMed]
- Takagi K, Yagi T, Umeda Y, et al. Preoperative controlling nutritional status (CONUT) score for assessment of prognosis following hepatectomy for hepatocellular carcinoma. World J Surg 2017;41:2353-60. [Crossref] [PubMed]
- Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550-8. [Crossref] [PubMed]
- Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res 2014;20:6212-22. [Crossref] [PubMed]
- Lee YL, Li WC, Tsai TH, et al. Body mass index and cholesterol level predict surgical outcome in patients with hepatocellular carcinoma in Taiwan - a cohort study. Oncotarget 2016;7:22948-59. [Crossref] [PubMed]
- Harimoto N, Yoshizumi T, Inokuchi S, et al. Prognostic significance of preoperative controlling nutritional status (CONUT) score in patients undergoing hepatic resection for hepatocellular carcinoma: a multi-institutional study. Ann Surg Oncol 2018;25:3316-23. [Crossref] [PubMed]
- Kuroda D, Sawayama H, Kurashige J, et al. Controlling Nutritional Status (CONUT) score is a prognostic marker for gastric cancer patients after curative resection. Gastric Cancer 2018;21:204-12. [Crossref] [PubMed]
- Tokunaga R, Sakamoto Y, Nakagawa S, et al. CONUT: a novel independent predictive score for colorectal cancer patients undergoing potentially curative resection. Int J Colorectal Dis 2017;32:99-106. [Crossref] [PubMed]
- Ishihara H, Kondo T, Yoshida K, et al. Preoperative controlling nutritional status (CONUT) score as a novel predictive biomarker of survival in patients with localized urothelial carcinoma of the upper urinary tract treated with radical nephroureterectomy. Urol Oncol 2017;35:539.e9-16. [Crossref] [PubMed]
- European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Xu Y, Shen Q, Liu P, et al. Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy and long-term outcomes. Eur Radiol 2017;27:3877-87. [Crossref] [PubMed]
- Chen Y, Zhao C, Yang Y, et al. Using the controlling nutritional status (CONUT) score for evaluating patients with early-stage hepatocellular carcinoma after radiofrequency ablation: a two-center retrospective study. Cardiovasc Intervent Radiol 2020;43:1294-304. [Crossref] [PubMed]
- Yang H, Yang Y, Dou J, et al. Cholecystectomy is associated with higher risk of recurrence after microwave ablation of hepatocellular carcinoma: a propensity score matching analysis. Cancer Biol Med 2020;17:478-91. [PubMed]
- WHO Classification of Tumours Editorial Board. WHO classification of digestive system tumor 5th edition. Geneva: World Health Organization, 2019:229-39.
- Song KD, Lee MW, Rhim H, et al. Percutaneous US/MRI Fusion-guided Radiofrequency Ablation for Recurrent Subcentimeter Hepatocellular Carcinoma: Technical Feasibility and Therapeutic Outcomes. Radiology 2018;288:878-86. [Crossref] [PubMed]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. [Crossref] [PubMed]
- Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol 2017;66:338-46. [Crossref] [PubMed]
- Kao WY, Su CW, Chiou YY, et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology 2017;285:670-80. [Crossref] [PubMed]
- Triolo M, Della Corte C, Colombo M. Impact of HBV therapy on the incidence of hepatocellular carcinoma. Liver Int 2014;34 Suppl 1:139-45. [Crossref] [PubMed]
- Sanmamed MF, Chen L. A paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 2019;176:677. [Crossref] [PubMed]
- Yao W, He JC, Yang Y, et al. The prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a systematic review and meta-analysis. Sci Rep 2017;7:7525. [Crossref] [PubMed]
- Ding W, Xu X, Qian Y, et al. Prognostic value of tumor-infiltrating lymphocytes in hepatocellular carcinoma: a meta-analysis. Medicine (Baltimore) 2018;97:e13301. [Crossref] [PubMed]
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010;6:149-63. [Crossref] [PubMed]
- Kritchevsky SB, Kritchevsky D. Serum cholesterol and cancer risk: an epidemiologic perspective. Annu Rev Nutr 1992;12:391-416. [Crossref] [PubMed]
- N'Kontchou G, Mahamoudi A, Aout M, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 2009;50:1475-83. [Crossref] [PubMed]
- Nojiri S, Fujiwara K, Shinkai N, et al. Effects of branched-chain amino acid supplementation after radiofrequency ablation for hepatocellular carcinoma: a randomized trial. Nutrition 2017;33:20-7. [Crossref] [PubMed]
- Chen SH, Wan QS, Zhou D, et al. A simple-to-use nomogram for predicting the survival of early hepatocellular carcinoma patients. Front Oncol 2019;9:584. [Crossref] [PubMed]
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol 2018;68:783-97. [Crossref] [PubMed]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]


