
Immunohistochemical features of the gastrointestinal tract tumors
Esophagus
Squamous cell carcinoma (SCC)
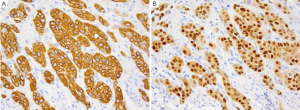
SCC of the esophagus has been associated with various geographic, ethnic and lifestyle risk factors. Compared to adenocarcinoma of the esophagus which is the more common tumor in the United States, SCC is much more common in Asian countries, where up to 40% have been linked to HPV infection (1). SCC is more common in males, particularly African American males and lifestyle risk factors such as smoking and alcohol are believed to increase the risk of SCC up to 90% (1,2). Patients may present with dysphagia, odynophagia and weight loss. Although SCC can develop in any part of the esophagus but are more commonly found in the middle and lower third portions of the esophagus (3,4). On gross examination the tumor is usually circumferential with sharp margin and are often ulcerate. Polypoid forms may also be seen (1). Microscopically, the tumors resemble their counterparts in the skin and show varying degrees of squamous differentiation with extensive keratinization in the well-differentiated forms and lack of cohesiveness, with even a pseudoglandular configuration in poorly-differentiated forms. The immunohistochemical profile of SCC is similar to that of its skin counterpart: CK7-, CD20-, CK5/6+, CK10+ and CK14+ (Figure 1A). SCC is always positive for p63 (Figure 1B) (5-9). Additionally, most cases of esophageal SCC are also positive for p53, a finding not seen in normal esophageal mucosa (8). As mentioned before, HPV has been found to be associated with esophageal SCC, particularly in cases reported from China (10) and Africa (11) where up to 20-40% of esophageal cases have been shown to be positive for HPV, particularly type 11, 16 and 18. Many of these HPV cases have been found to be positive for p16 as well, much similar to cases of cervical SCC (12,13).
Intestinal metaplasia(IM)
IM (Barrett’s esophagus) is defined as the presence of specialized intestinal epithelium in the distal esophagus above the level of the lower esophageal sphincter (14,15), and according to the American College of Gastroenterology Barrett’s mucosa is defined as a change in the esophageal epithelium of any length that can be recognized by endoscopy and is confirmed to be intestinal metaplasia (IM) by biopsy. Most patients with IM are adults, although this condition may develop in children with gastroesophageal reflux and following chemotherapy (16,17). Gastroesophageal reflux is believed to play a role in IM as up to 10% of patients with IM suffer from reflux. The importance of IM lies mainly in its association with the development of adenocarcinoma since more than 80% of patients with adenocarcinoma have been shown to have associated IM. Histologically, IM is quite similar to normal small intestinal mucosa with the presence of absorptive cells, goblet cells and Paneth cells. IM is further classified into three categories based on the degree of dysplasia: negative for dysplasia, indefinite for dysplasia and positive for dysplasia (low-grade and high grade). These are based on evaluation of surface maturation in comparison to underlying glands, architecture of the glands, cytologic features, inflammation and the presence of erosions/ulcers (18). In additional to its unique morphologic features, IM also shows a unique immunohistochemical profile. Greater than 95% cases of IM have characteristic superficial CK20 staining pattern along with a strong superficial and deep CK7 staining (19-21). Unlike normal gastric mucosa where cells are positive for MUC1, MUC5AC and MUC6 but negative for MUC2 the cells in IM/Barrett’s esophagus are positive for MUC2. The intensity of MUC2 staining varies according to the number of goblet cells, being higher in complete IM and lower in incomplete IM. Other monocolonal antibodies which are specific to gastric or colonic mucosa have also been used to confirm the diagnosis of IM such as Das-1 antibody which binds to colonic epithelial protein in absorptive cells, and HepPar-1 which is expressed in small intestinal mucosa but not normal gastric and colonic mucosa. Another marker that has been found to be useful in distinguishing the degree of dysplasia is AMACAR. AMACAR is a marker for prostate adenocarcinoma, and is also expressed in normal small intestinal and colorectal mucosa. AMCAR has also been found to be expressed in cases of IM with dysplasia, with an incidence of 20%, 40% and 80% in cases of indefinite, low grade and high-grade dysplasia, respectively (22) but is negative in cases of IM without dysplasia (22,23). p53 expression can also aid in the classification of dysplasia as up to 60% of cases of high-grade dysplasia and carcinoma express p53 in comparison to just 30% of cases classified as indefinite for dysplasia and low grade dysplasia (24,25). The increase in p53 expression is accompanied by increased Ki-67 labeling (26,27). IM with and without dysplasia can also be separated by using a combination of the markers described above. IM without dysplasia is usually positive for HepPar-1 and MUC2 and negative for AMCAR, whereas IM with dysplasia and adenocarcinoma often express AMCAR but not HepPar-1 or MUC2 (28,29).
Esophageal adenocarcinoma is rapidly increasing in incidence in the United States (30,31). Predisposing factors include male gender, white race, obesity, Barrett’s esophagus, smoking and alcohol consumption (32). Most cases of esophageal adenocarcinoma involve the lower one third of the esophagus and show glandular differentiation. These tumors usually express CK7, variable CK20, AMACAR, and weak focal CDX-2, an immunohistochemical pattern similar to that of gastric adenocarcinoma. P16 is negative in esophageal adenocarcinoma unlike SCC (26).
Stomach
Gastric epithelial dysplasia (GED)
GED most commonly occurs in men in their fifth to seventh decades, and is more common in westernized countries. There is often no gross features which can be recognized endoscopically but microscopically there may be glandular crowding, branching, budding, cytologic atypia, decreased apical mucin and frequent mitoses. GED may arise in either native gastric or intestinalized gastric epithelia, and is divided into three categories: indefinite for dysplasia, low-grade and high-grade dysplasia (33). Studies have show that 15% of low-grade dysplasia may show progression to carcinoma while cases of carcinoma from high-grade dysplasia is seen in 80-85% (34,35). Immunohistochemical stains may assist in the assessment of dysplasia as p53 expression and Ki-67 positive dysplastic cells also increase as dysplasia increases (36).
Gastric intestinal metaplasia (GIM)
GIM is similar histologically and immunohistochemically to Barrett’s esophagus/esophageal adenocarcinoma. It is defined as the development of goblet and/or Paneth cells within the normal gastric mucosa. The two main types of GIM are the complete type (type I) characterized by its resemblance to normal small intestinal mucosa with absorptive cells, Paneth cells and goblet cells; and the incomplete type (types II and III) where there are columnar and goblet cells. Most cases of gastric carcinoma arise within areas of incomplete GIM, and show CK7 and CK20 in the superficial and deep crypt cells (37-39). GIM is also positive for HepPar-14 and CDX-2 (40). The complete type of GIM is negative for MUC1 and MUC5AC but positive for MUC2 while incomplete GIM is positive for both (22). H. pylori infection has been found in over 80% of patients with GIM (37) which can then be identified by using the Das-1 antibody which stains H. pylori in gastric associated GIM (36).
Gastric adenocarcinoma (GA)
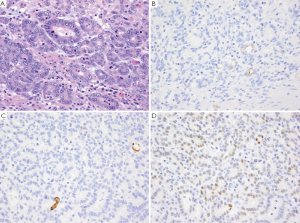
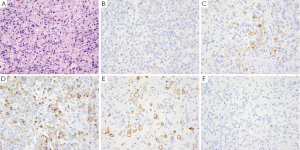
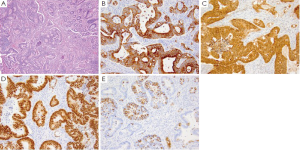
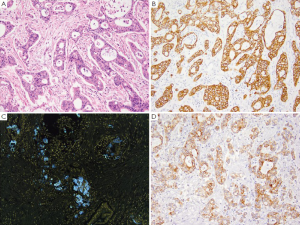
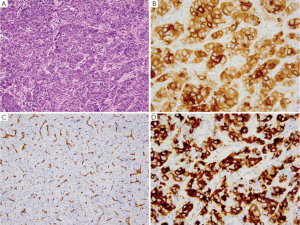
GA is the second most common cancer worldwide with the highest rates in Asia. It is more common in males and has been associated with risk factors such as low socioeconomic status, cigarette smoking, nitrites, chronic gastritis and H. pylori (41-43). The majority of gastric adenocarcinomas is located in the pylorus and antrum (50-60%), followed by the cardia (25%), and the body or fundus (15-25%) and may be exophytic, flat or ulcerated. There are two classifications of GA, the intestinal type, which has well-formed glands lined by columnar to cuboidal epithelial cells (Figure 2), and the diffuse type which shows single to poorly formed nests of cells growing in an infiltrate pattern (signet ring cell carcinoma) (Figure 3A) (43,44). Intestinal type GA shows variable expression of CK7 (Figure 2B), CK20 (Figure 2C), CDX-2 (Figure 2D), MUC1, and MUC5AC (45-47). Diffuse type of GA usually develops de novo, and is not associated with H. pylori induced IM. Over 70% of cases of the diffuse type of GA are positive for CDX-2 (Figure 3B), CK7 (Figure 3C), HepPar-1 (Figure 3D) and variable expression of CK20 (Figure 3E), MUC2 and MUC5AC, but negative for MUC1 and E-cadherin (Figure 3F) (48,49). Cases of poorly differentiated adenocarcinoma with prominent lymphoplasmacytic stroma may also be positive for EBV (50,51).
Tumors of the upper gastrointestinal tract such as Barrett’s esophagus, esophageal adenocarcinoma and gastric adenocarcinoma may show similar immunohistochemical findings, Table 1 compares each of their unique immunohistochemical profile (52,53).
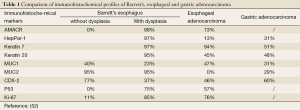
Full table
Gastrointestinal stromal tumor (GIST)
Stromal tumors comprise the majority of primary nonepithelial neoplasms in the stomach, and GIST is the most common GI mesenchymal neoplasm. GIST may occur anywhere within the GI tract but is most common in the stomach (60%) (53), with prognosis varying according to their location (54). Histologically, GISTs resemble smooth muscle tumors with spindle or epithelioid cells. Gastric GIST generally have a better prognosis compared to small intestinal GIST and may have either a predominantly spindle cell pattern or an epithelioid pattern. Features associated with a more aggressive behavior include a high mitotic rate (>5/per 50 hpf), large size (>5 cm), invasion, location within the fundus or gastrointestinal junction, coagulative necrosis, ulceration and epithelioid morphology (55,56). The vast majority of GISTs show a diffuse cytoplasmic staining with membranous accentuation of CD117 (KIT) (Figure 4A). CD117 is the product of the c-kit gene and is a type-3 tyrosine kinase receptor which is normally expressed in the interstitial cells of Cajal, mast cells, melanocytes, fetal endothelial cells and CD34-positive hematopoietic stem cells. CD117 is also positive in a variety of tumors such as mastocytoma, seminoma, pulmonary small cell carcinoma and blastic types of myeloid sarcoma just to name a few (57). Although CD117 positivity is present in most GIST, it is not required for diagnosis (58), since 5-10% of gastric GIST and 4% of small intestinal GIST may be negative for CD117 (57). Most CD117 negative GISTs are positive for another GIST marker-DOG-1 (Figure 4B). The diagnosis of GIST then requires examination of the morphologic, immunohistochemical and molecular PDGRFRA mutation analysis. Other immunohistochemical markers which may be positive in GIST include PDGFRA5, CD34 (80%), SMA (20%) (55), DOG1 (79%), and CK18 (59). Antibody cocktails for keratin such as AE1/AE3 are generally negative in gastric GIST as they are negative for CK7, CK17, CK19 and CK20. S-100 is also only positive in <1% of gastric GISTs (57). GFAP is negative in GIST and thus helps in differentiating from gastrointestinal schwannoma which is GFAP positive.
Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT)
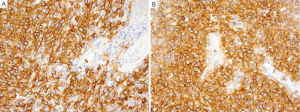
MALT is the most common type of lymphoma to occur in the stomach (60). Development of MALT has been associated with Helicobacter pylori infection with induction of remission reported by antibiotic treatment of the H. pylori (61). The lymphoma cells are B-cells and infiltrate the marginal zone around the preserved follicles. The cells are small to medium in size with a monocytoid appearance. Plasmacytic differentiation is often present in gastric MALT lymphomas (60). Tumor cells are positive for CD20, CD79a and Pax-5 but negative for CD5, CD10, and CD23. Aberrant CD5 co-expression has been described while co-expression of CD43 has been reported in one-third of cases (62). Cytogenetic abnormalities in MALT include t[11;18], t[1;14], t[14;18] and t[3;14] with t[11;18] being the most common translocation in MALT lymphomas involving the stomach (63,64).
Small intestine
The small intestine includes the duodenum, jejunum and ileum extending from the pylorus to ileocecal valve, yet neoplasms in the small intestine are extremely rare. Global incidence of small intestinal neoplasms is less than 1.0 per 100,000, and in the United States they represent only 0.4% of total cancers (65). The different types of primary small intestinal tumors include adenocarcinomas, carcinoid tumors, lymphomas and sarcomas (66,67).
Adenocarcinoma of the small intestine
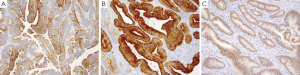
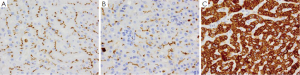
Adenocarcinoma of the small intestine is the most common type of primary malignancy in the small bowel, and generally presents in older males with a higher incidence in African Americans than Caucasians. Most cases are sporadic but reported risk factors include sporadic adenomatous polyps, familial adenomatous polyposis and Crohn’s disease. Presentation may include obstruction, jaundice, GI bleeding and abdominal pain, and often presents at an advanced stage. The most common locations for adenocarcinoma are the duodenum and proximal jejunum. Adenocarcinomas may present as polypoid, infiltrative or constricting lesions, with tumors in the duodenal and ampullary regions generally being exophytic in nature (67). Histologically, these tumors are similar to colorectal adenocarcinomas and are characterized by the degree of pleomorphism, complex glandular architecture, luminal necrosis and invasion. Small intestinal adenocarcinomas are CK7+ in more than half of all cases (Figure 5A), unlike normal small intestinal mucosa which is CK7- and colorectal adenocarcinomas which are CK7-/CK20+ (68). Adenocarcinomas of the small bowel are also positive for CK20 (Figure 5B), CDX-2 (Figure 5C), and villin (68).
Adenocarcinoma of ampulla of Vater
Adenocarcinoma of ampulla of Vater comprise about 5-6% of cancers arising (69) in the head of the pancreas. These tumors cause obstruction of the bile duct even at a very small size and hence patients often present early in the disease course with jaundice. Two major histologic types have been described: an intestinal type, arising from the overlying intestinal mucosa of the papilla (intestinal type adenocarcinoma of duodenal papillary origin) and a pancreatobiliary type, derived from the ductal epithelium which penetrates the duodenal muscularis propria (ampullary carcinoma of pancreatobiliary origin) (69). The intestinal type adenocarcinoma is much more common and has a much better prognosis (70), hence it is important to differentiate these two entities. Fortunately, the immunophenotype of these two types differ, with the intestinal type adenocarcinoma of duodenal papillary origin being positive for CK7, CK20, MUC2 and CDX-2 but negative for MUC1, MUC5AC and CK17; whereas ampullary carcinoma of pancreatobiliary origin is positive for MUC1, CK7, and CK17 but negative for MUC2 (69,70).
Gastrointestinal neuroendocrine tumors
Gastrointestinal neuroendocrine tumors are tumor derived from endocrine cells and can arise anywhere in the gastrointestinal tract. Most neuroendocrine tumors and carcinomas (carcinoids) in the GI tract are well differentiated. The location of these carcinoid tumors can be divided based on their embryologic derivation into carcinoids of the foregut (esophagus, stomach and duodenum), midgut (jejunum, ileum, appendix and ascending colon) and hindgut (transverse colon, descending colon, sigmoid and rectum) (71). Tumors from each different region of the gastrointestinal tract may secrete different hormones as well. Foregut and midgut carcinoid often produce serotonin and substance P while hindgut carcinoids may produce glucagon like peptide, pancreatic polypeptide, and polypeptide YY (72-78). In spite of these differences, these tumors share similar morphologic features such as clusters/sheets/nests of neuroendocrine cells with round to ovoid nuclei, “salt and pepper” chromatin and moderate amounts of clear cytoplasm. All gastrointestinal neuroendocrine tumors are positive for the generic markers of neuroendocrine differentiation such as chromogranin A, synaptophysin and NSE, as well as PGP 9.5, and CD56 (79). Determining the origin of the tumor may be challenging; however, immunohistochemical stains can be very helpful. Carcinoids from the foregut and midgut are generally positive for chromogranin A and CD56, while those from the hindgut are usually negative (73,80,81). Hindgut carcinoids on the other hand often express prostatic acid phosphatase (82). A less helpful marker is CDX-2, which although positive for most colorectal carcinomas has an immunoreactivity of about 40% in well differentiated carcinoids (83-87) but has a reported 80% expression rate in poorly differentiated carcinoids (80).
Carcinoid tumors make up about a third of the neoplasms in the small intestine. They most often occur in the ileum and rarely in the duodenum and can be separated by their location: duodenal and jejunoilieal carcinoids. Duodenal carcinoids, similar to any carcinoid in the gastrointestinal tract can be further divided by the type of cells which make up the tumor into gastrinomas (G-cell tumors), somatostatinomas (D-cell tumors) and a small percentage of the undefined type (88). Classification of neuroendocrine tumors is based on the degree of differentiation. Most carcinoids are well-differentiated carcinoid (50-75%), well-differentiated neuroendocrine carcinoma and poorly differentiated neuroendocrine carcinoma (<1-3%) (88). Carcinoid tumors usually show a monotonous proliferation of small bland polygonal cells with round nuclei, “salt and pepper” chromatin and moderate amounts of cytoplasm in either a nested (type A), trabecular (type B) or acinar (type C) pattern. Distinction between benign and malignant carcinoid is based on the presence or absence of metastasis rather than just on histology.
Colon and rectum
Colorectal cancer (CRC)
CRC is the third most common cancer diagnosed in the United States and third most common cause of cancer deaths. Risk for development of colorectal carcinoma increases significantly after the age of 40. In addition to age, lifestyle modifiers and genetic risk factors all play a role in CRC. Family history and genetics plays an important role as well, particularly in patients less than 50 years. Approximately 25% of CRC arise in patients with a family history of disease while 5% arise in the setting of an established familial syndrome (89). The genetic syndromes associated with CRC can be divided into the hereditary polyposis colon cancers (HPCC) and hereditary nonpolyposis colon cancers (HNPCC). Categories of HPCC include: (I) Familial adenomatous polyposis; (II) MUTYH-associated polyposis; (III) hyperplastic polyposis syndrome; (IV) Peutz-Jeghers syndrome; and (V) Juvenile polyposis syndrome (89). Of the polyposis CRC familial adenomatous polyposis (FAP) is the most common. FAP is an autosomal dominant disease with 100% penetrance. Patients with FAP develop hundreds to thousands of adenomatous colonic polyps starting in the second decade of life with a 100% risk of CRC (89,90). Another category of HPCC is the MUTYH - associated polyposis, an autosomal recessive colon cancer syndrome which accounts for 0.5% to 1% of all CRC (91,92). Patients with MUTYH - associated polyposis may have zero to thousands of polyps like FAP, with an estimated lifetime risk of CRC around 80% (92). Hyperplastic polyposis syndrome (HPS) is characterized by the development of numerous, large hyperplastic and sessile serrated polyps, with a 35% to 54% prevalence of CRC development (93). Peutz-Jeghers syndrome (PJS) is a rare autosomal dominant disease characterized by the development of pigmented macules on lips, mucosa, hands and feet, along with development of hamartomatous polyps as well as cancers in the CRC, stomach, small bowel, pancreas, breast, sex cord, uterus, cervix and skin. Patients with PJS have a 39% lifetime risk of CRC and 93% risk for any other malignancy (94). Juvenile polyposis syndrome (JPS) typically presents in childhood and has an associated 10-38% lifetime risk of developing colon cancer (95). Lynch syndrome/HNPCC is the most common autosomal dominant inherited colon cancer family syndrome responsible for 10% of colon cancer cases before the age of 50 years (96). The risk of CRC is related to the development of innumerable adenomas. Diagnosis of HNPCC is based on the Amsterdam criteria taking into account the extracolonic malignancies which are common in HNPCC involving the endometrium, stomach, ovary, urinary collecting system, skin, pancreatic and biliary tract (97). Patients with HNPCC have a seven fold increased risk of CRC and present at least 20 years younger than the general population (98).
The histopathologic types of CRC recognized by the World Health Organization include adenocarcinoma, mucinous adenocarcinoma, signet ring carcinoma, small cell carcinoma, adenosquamous carcinoma, squamous cell carcinoma and undifferentiated carcinoma.
Colorectal adenocarcinoma (CA)
CA may be polypoid, exophytic, ulcerative, or infiltrative, and can present anywhere within the colon. Polypoid tumors are more common in the cecum and right colon, while ulcerative tumors are more common in the left colon and rectum. Microscopically, CA form glands with mucin (Figure 6A) and are classified as well, moderate or poorly differentiated. In addition to the genetic syndromes discussed previously, sporadic CAs have been associated with mutations in the APC, K-ras and p53. CA is characterized by a CK7 negative and CK20 positive (Figure 6B) immunophenotype, and thus can be differentiated from non-ampullary small intestinal adenocarcinomas by their lack of expression of CK7 and positivity for CK20,Table 2 summarizes several key immunohistochemical stains which can help in distinguishing these two entities.

Full table
Appendiceal adenocarcinomas with mucinous differentiation, as well as rectal adenocarcinomas on the other hand may also show expression of CK7 and thus differentiation from metastatic ovarian mucinous tumors is required. Other markers for CA include villin which is positive in 80% of CA (Figure 6C), CDX-2 which is positive (Figure 6D) in almost all well differentiated CA and adenomas but less than 10-20% of poorly differentiated adenocarcinomas may be weakly positive or negative (99). CDX-2 is a transcription factor involved in the proliferation and differentiation of intestinal epithelial cells, and the incidence of CDX-2 expression in adenocarcinoma of the gastrointestinal tract increases from esophagus to rectum and in cases where it is positive all tumor cells show a strong staining pattern. In addition to expression in CA, CDX-2 may also be expressed in ovarian mucinous adenocarcinomas and bladder adenocarcinomas (99-101). CA with mucinous features have an immunophenotype similar to conventional CA with tumor cells positive for CK20, CDX-2, MUC2 (Figure 6E) and β-catenin (102), while the signet ring type of CA is also positive for CDX-2, CK20 and MUC2.
Appendix
The appendix may develop any of the tumors described above from the small and large intestines; however, there are a few unique entities at this site including mucinous neoplasms and goblet cell carcinoid tumors.
Goblet cell carcinoid of the appendix (GCC)
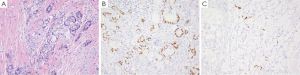
GCC is a distinct type of carcinoid tumor within the colorectum which exhibits both neuroendocrine and intestinal-type goblet cell morphology (103). Up to 50% of patients with GCC present with disseminated disease hence it is an important differential to consider particularly in female patients, where an ovarian primary is often one of the first considerations (104). GCC arise from the base of the epithelial crypts of the appendiceal mucosa without associated epithelial dysplasia, and show a submucosal growth pattern with invasion of the appendiceal wall comprised of goblet or signet ring cells in clusters or glands separated by smooth muscle stroma (Figure 7A) (105). The signet ring cells are positive for PAS, mucicarimine, pancytokeratin, CDX-2 (Figure 7B), CK20, MUC2 and CEA; as well as focally positive for chromogranin (Figure 7C) and synaptophysin. Up to 25% of cases are negative for neuroendocrine markers (106,107).
Mucinous neoplasms of the appendix
Mucinous neoplasms of the appendix are the most common type of epithelial neoplasms in the appendix. These neoplasms present in a wide spectrum ranging from mucinous cystadenoma, low-grade mucinous neoplasm, and disseminated peritoneal adenomucinosis or cystadenocarcinoma, mucinous carcinoma, and peritoneal mucinous carcinomatosis (108). These tumors are associated with pseudomyxoma peritonei, a clinical condition of gelatinous ascites, commonly also seen in ovarian mucinous neoplasms (109-112). The classification of mucinous neoplasms within the appendix remains a controversial issue. Broadly speaking, mucinous neoplasms of the appendix can be divided into two major types: those that resemble conventional colonic adenocarcinoma with potential for destructive growth, nodal or solid organ metastasis; and those, which are predominantly low-grade mucinous neoplasms with potential for peritoneal dissemination (108). Their immunophenotype is similar to that of other mucinous tumors in the lower gastrointestinal tract being positive for MUC-2, CK20, CDX-2 and beta-catenin, but with lower expression of CDX-2 and beta-catenin. In addition, mucinous adenocarcinomas of the appendix with positivity for CK7 (113), hence differentiation from upper GI and mucinous neoplasms from other areas is necessary.
Anal tumors
The anal canal is defined as the region located between the junction of the colorectal-type glandular mucosa and the junction between the squamous mucosa lined distal portion.
Despite its short length, the anal canal produces a wide variety of tumor types. Tumors within the anal canal include: (I) squamous cell tumors including condyloma acuminatum, flat squamous dysplasia, invasive squamous cell carcinoma and its variants; (II) adenocarcinoma rectal type, anal gland adenocarcinoma, fistula-related mucinous adenocarcinoma and intraepithelial adenocarcinoma (Paget disease); (III) neuroendocrine neoplasms; (IV) melanoma; (V) mesenchymal tumors and (VI) lymphoma.
Squamous cell carcinoma
Squamous cell carcinoma is the most common type of tumor within the anal canal. The incidence of SCC of the anal region is higher in females (114). There is also an increase in incidence in high-risk patient population (HIV positive patients) and an association with HPV (115). Three distinctive subtypes are recognized based on their distinctive histologic features: verrucous carcinoma, squamous cell carcinoma with mucinous microcysts and small cell (anaplastic) carcinoma (116).
Adenocarcinoma of the anal canal
Adenocarcinoma of the anal canal is much less common, accounting for about 10% of all anal cancers (117). Similar to squamous cell carcinoma of the anal canal, adenocarcinomas in this region have been associated with high-risk HPV types. Other risk factors include inflammatory conditions such as Crohn’s disease and chronic anal fistulas (118).
Of the various types of adenocarcinomas in this region, Paget disease is the one most likely to cause difficulties in diagnosis. Paget disease of the anal canal may arise from an underlying anal gland adenocarcinoma, adnexal (eccrine gland) adenocarcinoma or an underlying visceral malignancy, most commonly a colorectal adenocarcinoma. The use of immunohistochemistry can help differentiate these as those arising from anal gland adenocarcinoma would be CK7+/CK20+/CDX-2+/GCDFP-15- (119,120), from adnexal adenocarcinoma would be CK7+/CK20-/CDX-2-/GCDFP-15+ and that from a colorectal adenocarcinoma would be CK7-/CK20+/CDX-2+/GCDFP-15+- (119-124). These tumors may also need to be differentiated from mammary Paget disease (CK7+/CEA+/EMA+/HER-2/neu+/MUC1+/ER+/CK20-/CDX-2-/GCDFP-15+) (125-133) and Paget disease of the vulva (CK7+/CEA+/EMA+/HER-2/neu-/MUC1+/ER-/CK20-/CDX-2-/GCDFP-15-) (133-136).
Pancreas
Although pancreatic tumors are one of the less common tumors within the gastrointestinal tract, it is the 4th leading cause of cancer mortality in the United States in both men and women (137). Due to the nature of the disease, pancreatic cancers often do not cause symptoms until the later stages. In fact, less than 10% of pancreatic cancers are detected at a stage where cure is possible. The overall survival for this group of cancers is only about 5% (137). Based on the histological features, pancreatic tumors can be divided into three main categories: exocrine neoplasms, neuroendocrine tumors and mixed exocrine-endocrine tumors.
Pancreatic ductal adenocarcinomas
Pancreatic ductal adenocarcinomas make up the majority (>95%) of pancreatic tumors. Pancreatic cancer is more common among the elderly, with a higher incidence in men than in women and more common in blacks compared to other races (137). Risk factors include cigarette smoking, family history, diabetes mellitus and obesity (138). Presentation often occurs late in the disease course as epigastric pain, weight loss, painless jaundice, light clay-colored stools, dark urine, pruritus, and nausea. Pancreatic ductal carcinomas often present as poorly defined masses involving the head of the pancreas (>60%) with variable degrees of necrosis which may lead to the formation of cysts (139). Depending on the degree of differentiation these tumors may show well formed glands in a haphazard pattern (Figure 8A) or individual cells forming sheets, single cell infiltration or poorly formed glands in poorly differentiated adenocarcinoma. Variants of adenocarcinoma included adenosquamous carcinoma, colloid carcinoma, hepatoid carcinoma, medullary carcinoma, signet ring cell carcinoma and undifferentiated carcinoma (139). Most cases show expression of CK7 (Figure 8B), while a subset focally express CK20 (40%) (Figure 8C), a feature which allow for differentiation from extra-pancreatobiliary non-mucinous adenocarcinomas. Pancreatic ductal carcinomas are also positive for CK8, CK17 (Figure 8D), CK18, CK19, CEA, CA19-9, Dupan-2, MUC1, MUC4 and MUC5AC (140-143).
Pancreatic intraepithelial neoplasia (PanIN)
Pancreatic intraepithelial neoplasia (PanIN) has been speculated to be the precursor lesion of pancreatic ductal adenocarcinomas for over fifty years, but it is only recently that its significance and role in pancreatic carcinoma has been established. It is one of three major categories of precursor lesions defined by ongoing epidemiological and molecular studies, the other two being intraductal papillary-mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) (144). PanIN is the most common and most defined precursor lesion of pancreatic ductal carcinoma (145). These lesions are often found at the same time as the diagnosis for pancreatic adenocarcinoma, and share similar genetic alterations such as K-ras mutation, inactivation of tumor suppressor genes and both show expression of MUC1 and MUC5AC but not MUC2 (143,146,147).
Mucin-producing cystic neoplasms of the pancreas
Mucin-producing cystic neoplasms of the pancreas comprise of two entities: mucinous cystic neoplasm (MCN) and intraductal papillary mucinous neoplasm (IPMN).
Intraductal papillary mucinous neoplasms
Intraductal papillary mucinous neoplasms are more common in older men and are most often located at the head of the pancreas. These lesions comprise of an intraductal proliferation of mucinous epithelium within the main pancreatic and/or the branching ducts (155,156), usually in a papillary arrangement without ovarian-type stroma. The epithelial cells may be of intestinal type, pancreatobiliary type or null type (similar to gastric foveolar epithelium) or morphologically unclassifiable (150). Intestinal-type IPMN often show a colloid-type pattern of invasion and are frequently positive for CDX-2, and MUC2 but negative for MUC1, while the pancreatobiliary type is more aggressive and is negative for CDX-2, MUC2 and positive for MUC1 (150). The null type on the other hand is generally negative for MUC1, CDX-2 and MUC2 (157). Mucinous carcinomas which arise from IMPN are frequently positive for MUC1 but less often positive for MUC5AC (158).
Solid-pseudopapillary neoplasm (SPN)
SPN is an uncommon pancreatic tumor most often found in young women (159). Patients present with nonspecific symptoms related to the intra-abdominal mass such as abdominal pain and early satiety. SPNs are generally large, well circumscribed tumors which can occur anywhere within the pancreas (160). Microscopically, they form dense nests of uniform eosinophilic cells surrounding delicate vasculature resembling ependymal rosettes. The tumor cells often have nuclei with grooves and clear vacuolated cytoplasm (159). Slide preparations from material obtained by fine-needle aspirate biopsy show a distinct “Chinese character-like” appearance due to the branching capillaries are surrounded by small uniform tumor cell and show prominent nuclear grooves and/or inclusions in the tumor cells and background of metachromatic myxoid material (161). The tumor cells are positive for alpha-1-antitrypsin, vimentin, NSE, ER-β, PR, CK8/18, CD10, CD56 and synaptophysin (153,162,163). These tumors have a mutation of the β-catenin gene and show a diffuse cytoplasmic and nuclear positivity in virtually all cases by immunohistochemistry (164). Because the β-catenin complex activates transcription of cyclin D1, nuclear cyclin D1 immunoreactivity is detected in up to 75% of SPNs (165). SPNs have also been found to show a loss of cell-cell adhesion molecule and thus are negative for E-cadherin (166).
Serous cystic neoplasms (SCN)
SCN are neoplasms composed of glycogen-rich, ductular-like epithelial cells. Most SCNs are benign while others may be precursors to invasive cancer. Correlation with the patient’s age, gender, relationship between cysts and larger pancreatic ducts, cysts contents (serous fluid, mucin or necrotic debris), lining cell and nature of the stroma are all required in evaluation. Serous cystadenomas are more common in females and often present with nonspecific symptoms such as pain, nausea, weight loss. These tumors are well-circumscribed masses which on sectioning shows innumerable small cysts with a “honeycomb” appearance and often a central scar (167). The cells have a central round to oval nuclei, inconspicuous nucleoli and clear cytoplasm and are positive on periodic acid-Schiff (PAS) stain due to the abundant intracytoplasmic glycogen. SCNs are positive for low molecular weight keratins (CK7, CK8, CK18 and CK19), MUC1, EMA (168), alpha inhibin, NSE, and MUC6 but negative for MUC5A, CK17 and CEA (markers positive in pancreatic ductal adenocarcinoma) as well as chromogranin, synaptophysin, vimentin, PR, and β-catenin (169).
Pancreatic neuroendocrine tumors (PNETs)
Pancreatic neuroendocrine tumors (PNETs) are rare neoplasms with an incidence of 1 per 100,000 individuals per year and comprising just 1-2% of all pancreatic tumors (170). Pancreatic neuroendocrine tumors can present at any age but are most common during the 4th to 6th decades of life with no sex predilection (170). Although most tumors are sporadic there is an association with hereditary endocrinopathies such as multiple endocrine neoplasia type I (MEN I), von Hippel-Lindau syndrome, neurofibromatosis and tuberous sclerosis. PNETs can be broadly divided into functional and nonfunctional tumors. Functional neuroendocrine tumors are tumors which produce a variety of clinical syndromes due to an excess in hormones and include insulinoma, gastrinoma, glucagonoma, VIPoma, and somatostatinoma (171). The non-functional PNETs may also produce hormones but generally do not have symptoms due to the hormone production. These tumors are classified according to the WHO classification into well differentiated endocrine tumor, well differentiated endocrine carcinoma and poorly differentiated endocrine carcinoma based on size, mitotic count, Ki-67 proliferation index, angioinvasion and metastasis. PNETs are diffusely positive for synaptophysin consistently while chromogranin A may show a more focal staining pattern of variable intensity (170). They also express CD56, CD57, PDG 9.5 and NSE (172,173), as well a CK8 and 18. In differentiating PNETs from neuroendocrine tumors from other primary sites, CDX-2 may also be helpful as it has been reported to be positive in 20-30% of PNET cases (83,84). Other markers shown to be positive in pancreatic endocrine tumors include trypsin, chymotrypsin and lipase (174,175).
Pancreatoblastoma
Pancreatoblastoma is the most common pancreatic neoplasm of childhood. Most cases occur in children less than 10 years of age (176), and there is a slight male predominance and association with Beckwith-Weidemann syndrome (177). These tumors are generally large, and may arise in either the head or the tail of the pancreas as well-circumscribed and lobulated masses. Histologically, the tumor has a lobular appearance with well-defined islands of small epithelial cells separated by fibrous bands with a geographic pattern of lighter and darker staining cells due to the different cell types present. The tumor cells in the darker staining areas are small with centrally placed nuclei and prominent nucleoli with scant cytoplasm, while cells in the lighter areas have abundant eosinophilic cytoplasm and may be spindled in shape with a whorling pattern. The presence of occasional squamoid nests is characteristic for this lesion (178). The immunophenotype of the tumor cells often shows acinar differentiation as they are PAS-positive with diastase resistant cytoplasmic granules and positive for trypsin, chymotrypsin and lipase. Despite the presence of squamoid nests, pancreatoblastomas are negative for squamous markers (negative for high molecular weight keratins CK14, CK5/6, and CK17) and CK7 (179) but positive for CK8, CK18, CK19, EMA and cytoplasm and membranous β-catenin (180). Up to half of the tumors may exhibit neuroendocrine differentiation with focal chromogranin and synaptophysin positivity while the cells of ductal differentiation are highlighted by their production of mucin, CEA and CA19 positivity (181). Pancreatoblastomas have also been found to show alterations in the β-catenin/APC pathway in up to 80% of cases, hence its positivity by immunohistochemistry (180).
Acinar cell carcinoma
Acinar cell carcinoma is more common in adults and presents with non-specific gastrointestinal symptoms such as abdominal pain, nausea and weight loss. Some patients may have subcutaneous fat necrosis and polyarthralgia due to increase levels of serum lipase (159). These tumors are often large and can occur anywhere within the pancreas but are more often found at the head of the pancreas. Microscopically, acinar cell carcinomas show nests of pyramidal cells arranged in solid or acinar patterns. Tumor cells have basally oriented nuclei, single prominent nucleoli and granular cytoplasm. Acinar cell carcinomas are positive for pancytokeratin, CK8, CK18, zymogen, trypsin, chymotrypsin and lipase, but negative for CK7 and CK19 (182,183). Scattered cells positive for neuroendocrine markers are present in one-third of cases. A few cases may demonstrate the APC/β-catenin gene mutation (184).
Mixed exocrine-endocrine tumors
Mixed exocrine-endocrine tumors are defined as malignant epithelial neoplasms where the ductal and endocrine cells are intimately mixed in the primary tumor with at least one-third to one-half of tumor cells showing positivity for endocrine markers (185). Ductal differentiation is defined as ductular formation and mucin production (174) and presence of ductal markers like CEA, CK19 and CA19.9, while ductal acinar cells can be highlighted by pancreatic enzymes like trypsin, chymotrypsin and lipase (186,187). Endocrine cells can be characterized by positivity for endocrine markers chromogranin A and synaptophysin. These mixed tumors generally behave as ductal adenocarcinomas (187). It is important to remember that 40-80% of usual ductal adenocarcinomas may contain endocrine cells, but the metastases from these tumors generally lack endocrine cells (174,187).
Liver
Primary tumors of the liver are divided into epithelial and non-epithelial (mesenchymal) lesions and then further into benign and malignant categories. The majority of the mass lesions within the liver are benign lesions such as focal nodular hyperplasia (FNH), regenerative nodules, adenoma, cirrhosis, and vascular lesions. Of the malignant lesions metastatic tumors are far more common than primary hepatocellular carcinomas.
Hepatocellular carcinoma (HCC)
HCC is the sixth most common malignancy and third most common cause of mortality from cancer worldwide (188). Risk factors for HCC include chronic liver disease such as chronic hepatitis C virus, hepatitis B virus, cirrhosis, obesity related liver disease and alcohol-related liver disease (189). Symptoms of HCC include abdominal pain, fullness, mass or signs and symptoms of cirrhosis, with the most helpful indicator being elevated serum levels of alpha fetal protein (AFP). On gross examination, HCC presents as a single large mass which may or may not have satellite nodules. Histologically, well-differentiated HCC is difficult to differentiate from normal liver as the polygonal cells resemble hepatocytes and are arranged in trabecular pattern lined by sinusoids mimicking normal liver but have intracytoplasmic bile (Figure 9A) (188). Differentiation between HCC and normal/benign liver is therefore very difficult especially on small needle-core biopsies, and immunohistochemical stains are thus very helpful (189,190). Two immunohistochemical stains that can differentiate HCC from normal/benign liver are Glyican-3 (Figure 9B), a marker which is exclusively expressed in neoplastic processes and not normal tissue in humans (191), and CD34 (Figure 9C) a vascular marker which highlights the increased vascularity seen in HCC (192-196). Another marker which is only expressed in HCC and not normal liver is AFP (Figure 9D) (197). Other markers for HCC include CD10 (Figure 10A), polyclonal CEA (Figure 10B) which highlights the canaliculi (198,199), HepPar-1 (Figure 10C) which reacts with both neoplastic and normal liver tissue. and AFP (200). HCC express only a limited number of keratin markers, namely CK8 and CK18 and thus most metastatic carcinomas can be excluded as they generally express a larger variety of keratin markers such as CK5/6, CK7, CK14 or CK20 in comparison to HCC (201).
Cholangiocarcinoma (CC)
CC make up approximately 3% of all gastrointestinal cancers worldwide (202). These tumors are more common in elderly men and have been associated with cirrhosis, hepatitis C, infections by Clonorchis sinesis and Opisthorchisis viverrini, primary sclerosing cholangitis, Thorotrast exposure, genetic hemochromatosis, alpha-1-antitrypsin deficiency and contraceptive steroid use (202-204). CC arises from the intrahepatic bile duct epithelial cells and can be divided based on the location of origin, intrahepatic/peripheral CC arise at the confluence of the right and left hepatic ducts, while the extrahepatic CC arise between the ampulla of Vater and the hepatic hilium (205). Depending on their location the presenting symptoms may also differ. Histologically, CC is similar to ductal adenocarcinoma of the pancreas with tumor cells arranged in tubules and glands which may be cribriform or form nests, solid cords and papillary structures (205). CC is positive for CK7, CK17, mucin, CEA (cytoplasmic and luminal), CAM 5.2, CK19, EMA and CK20 (30-70%) (206).
Hepatoblastomas (HB)
HB are the most common primary liver tumors in children, with the majority occurring in children less than 2 years of age (207). These tumors have a slight predominance in males, low-birth weight infants and have been associated with familial adenomatous polyposis, and various chromosomal abnormalities as well as mutations in the β-catenin gene (208). HB generally present as solitary masses in the right lobe of liver and are classified based on their histology into six main patterns: fetal pattern, embryonal pattern, macrotrabecular pattern, small cell undifferentiated pattern, mixed epithelial and mesenchymal pattern and mixed pattern with teratoid features (209). Immunohistochemically HBs are positive for HepPar-1, AFP and EMA while those with the small cell pattern may be positive for cytokeratin with the mesenchymal areas being positive for vimentin (210).
Gallbladder
Benign bile duct proliferations: Benign bile duct proliferations such as bile duct hamartomas (also know as von Meyenburg complexes) and bile duct adenomas are usually small, incidental asymptomatic lesions identified at time of autopsy. Bile duct hamartomas are believed to be formed by failure of the embryonic ductal plates in the liver to involute. These lesions are often subcapsular, small white nodules that may require differentiation from metastatic adenocarcinoma or cholangiocarcinoma (211). Bile duct adenomas are also small subcapsular nodules consisting of acini and tubules and may be confused for a malignant lesion (212). Both of these benign bile duct proliferations have an immunohistochemical profile similar to that of pancreatic ductal adenocarcinoma and are positive for CK7, CK17, MUC1 and MUC5AC (213). They also share an immunophenotype with bile duct carcinoma, and are all positive for CK7, focally positive for CK20, and CDX-2; however, they are negative for p53 and monoclonal CEA which is positive in bile duct carcinoma (214). Hence, it is important to correlate with radiological and clinical findings.
Conclusions
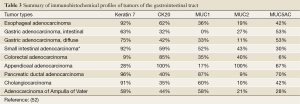
Tumors of the gastrointestinal tract are varied, yet can often prove to be diagnostically challenging. Understanding the unique immunohistochemical profiles of each entity will greatly assist in the diagnosis of these tumors. Table 3 provides a summary of the immunohistochemical profile of several key gastrointestinal tumors.
Full table
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sasajima K, Takai A, Taniguchi Y, et al. Polypoid squamous cell carcinoma of the esophagus. Cancer 1989;64:94-7. [PubMed]
- Brown LM, Hoover R, Silverman D, et al. Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol 2001;153:114-22. [PubMed]
- Gunnlaugsson GH, Wychulis AR, Roland C. Surg Gynecol Obstet 1970;130:997-1005. [PubMed]
- Sons HU, Borchard F. Esophageal cancer. Autopsy findings in 171 cases. Arch Pathol Lab Med 1984;108:983-8. [PubMed]
- Chu PG, Lyda MH, Weiss LM. Cytokeratin 14 expression in epithelial neoplasms: a survey of 435 cases with emphasis on its value in differentiating squamous cell carcinomas from other epithelial tumours. Histopathology 2001;39:9-16. [PubMed]
- Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol 2002;15:6-10. [PubMed]
- Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 2000;13:962-72. [PubMed]
- Tsubochi H, Suzuki T, Suzuki S, et al. Immunohistochemical study of basaloid squamous cell carcinoma, adenoid cystic and mucoepidermoid carcinoma in the upper aerodigestive tract. Anticancer Res 2000;20:1205-11. [PubMed]
- van Dorst EB, van Muijen GN, Litvinov SV, et al. The limited difference between keratin patterns of squamous cell carcinomas and adenocarcinomas is explicable by both cell lineage and state of differentiation of tumour cells. J Clin Pathol 1998;51:679-84. [PubMed]
- He D, Zhang DK, Lam KY, et al. Prevalence of HPV infection in esophageal squamous cell carcinoma in Chinese patients and its relationship to the p53 gene mutation. Int J Cancer 1997;72:959-64. [PubMed]
- Matsha T, Erasmus R, Kafuko AB, et al. Human papillomavirus associated with oesophageal cancer. J Clin Pathol 2002;55:587-90. [PubMed]
- Sturm I, Petrowsky H, Volz R, et al. Analysis of p53/BAX/p16(ink4a/CDKN2) in esophageal squamous cell carcinoma: high BAX and p16(ink4a/CDKN2) identifies patients with good prognosis. J Clin Oncol 2001;19:2272-81. [PubMed]
- Nakamura T, Ide H, Eguchi R, et al. Concomitant analysis of p16/INK4, cyclin D1, and retinoblastoma protein expression in esophageal squamous cell carcinoma. Hepatogastroenterology 2003;50:1321-6. [PubMed]
- Spechler SJ. Clinical practice.Barrett’s Esophagus. N Engl J Med 2002;346:836-42. [PubMed]
- Stein HJ, Siewert JR. Barrett’s esophagus: pathogenesis, epidemiology, functional abnormalities, malignant degeneration, and surgical management. Dysphagia 1993;8:276-88. [PubMed]
- Dahms BB, Greco MA, Strandjord SE, et al. Barrett’s esophagus in three children after antileukemia chemotherapy. Cancer 1987;60:2896-900. [PubMed]
- Dahms BB, Rothstein FC. Barrett’s esophagus in children: a consequence of chronic gastroesophageal reflux. Gastroenterology 1984;86:318-23. [PubMed]
- Montgomery E, Bronner MP, Goldblum JR, et al. Reproducibility of the diagnosis of dysplasia in Barrett esophagus: a reaffirmation. Hum Pathol 2001;32:368-78. [PubMed]
- Mohammed IA, Streutker CJ, Riddell RH. Utilization of cytokeratins 7 and 20 does not differentiate between Barrett’s esophagus and gastric cardiac intestinal metaplasia. Mod Pathol 2002;15:611-6. [PubMed]
- Shearer C, Going J, Neilson L, et al. Cytokeratin 7 and 20 expression in intestinal metaplasia of the distal oesophagus: relationship to gastro-oesophageal reflux disease. Histopathology 2005;47:268-75. [PubMed]
- Ormsby AH, Goldblum JR, Rice TW, et al. Cytokeratin subsets can reliably distinguish Barrett’s esophagus from intestinal metaplasia of the stomach. Hum Pathol 1999;30:288-94. [PubMed]
- Reis CA, David L, Correa P, et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res 1999;59:1003-7. [PubMed]
- Chinyama CN, Marshall RE, Owen WJ, et al. Expression of MUC1 and MUC2 mucin gene products in Barrett’s metaplasia, dysplasia and adenocarcinoma: an immunopathological study with clinical correlation. Histopathology 1999;35:517-24. [PubMed]
- Geboes K, Van Eyken P. The diagnosis of dysplasia and malignancy in Barrett’s oesophagus. Histopathology 2000;37:99-107. [PubMed]
- Kimura H, Konishi K, Kaji M, et al. p53 immunoreactivity in Barrett’s metaplasia, dysplasia, and adenocarcinoma--a case report. Hepatogastroenterology 2001;48:1662-4. [PubMed]
- Glickman JN, Yang A, Shahsafaei A, et al. Expression of p53-related protein p63 in the gastrointestinal tract and in esophageal metaplastic and neoplastic disorders. Hum Pathol 2001;32:1157-65. [PubMed]
- Fléjou JF, Svrcek M. Barrett’s oesophagus--a pathologist’s view. Histopathology 2007;50:3-14. [PubMed]
- Chu PG, Jiang Z, Weiss LM. Hepatocyte antigen as a marker of intestinal metaplasia. Am J Surg Pathol 2003;27:952-9. [PubMed]
- Dorer R, Odze RD. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus, ulcerative colitis, and Crohn’s disease. Am J Surg Pathol 2006;30:871-7. [PubMed]
- Blot WJ, Devesa SS, Fraumeni JF Jr. Continuing climb in rates of esophageal adenocarcinoma: an update. JAMA 1993;270:1320. [PubMed]
- Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am 2002;11:235-56. [PubMed]
- Hamilton SR, Smith RR, Cameron JL. Prevalence and characteristics of Barrett esophagus in patients with adenocarcinoma of the esophagus or esophagogastric junction. Hum Pathol 1988;19:942-8. [PubMed]
- Bearzi I, Brancorsini D, Santinelli A, et al. Gastric dysplasia: a ten-year follow-up study. Pathol Res Pract 1994;190:61-8. [PubMed]
- Di Gregorio C, Morandi P, Fante R, et al. Gastric dysplasia. A follow-up study. Am J Gastroenterol 1993;88:1714-9. [PubMed]
- Fertitta AM, Comin U, Terruzzi V, et al. Clinical significance of gastric dysplasia: a multicenter follow-up study. Gastrointestinal Endoscopic Pathology Study Group. Endoscopy 1993;25:265-8. [PubMed]
- DeMeester SR, Wickramasinghe KS, Lord RV, et al. Cytokeratin and DAS-1 immunostaining reveal similarities among cardiac mucosa, CIM, and Barrett’s esophagus. Am J Gastroenterol 2002;97:2514-23. [PubMed]
- Shen B, Ormsby AH, Shen C, et al. Cytokeratin expression patterns in noncardia, intestinal metaplasia-associated gastric adenocarcinoma: implication for the evaluation of intestinal metaplasia and tumors at the esophagogastric junction. Cancer 2002;94:820-31. [PubMed]
- Jovanovic I, Tzardi M, Mouzas IA, et al. Changing pattern of cytokeratin 7 and 20 expression from normal epithelium to intestinal metaplasia of the gastric mucosa and gastroesophageal junction. Histol Histopathol 2002;17:445-54. [PubMed]
- Schilling D, Spiethoff A, Rosenbaum A, et al. Does Cytokeratin7/20 immunoreactivity help to distinguish Barrett’s esophagus from gastric intestinal metaplasia? Results of a prospective study of 75 patients. Pathol Res Pract 2005;200:801-5. [PubMed]
- Kim HS, Lee JS, Freund JN, et al. CDX-2 homeobox gene expression in human gastric carcinoma and precursor lesions. J Gastroenterol Hepatol 2006;21:438-42. [PubMed]
- Dicken BJ, Bigam DL, Cass C, et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005;241:27-39. [PubMed]
- Ramón JM, Serra L, Cerdó C, et al. Dietary factors and gastric cancer risk. A case-control study in Spain. Cancer 1993;71:1731-5. [PubMed]
- Lambert R, Guilloux A, Oshima A, et al. Incidence and mortality from stomach cancer in Japan, Slovenia and the USA. Int J Cancer 2002;97:811-8. [PubMed]
- Vizcaino AP, Moreno V, Lambert R, et al. Time trends incidence of both major histologic types of esophageal carcinomas in selected countries, 1973-1995. Int J Cancer 2002;99:860-8. [PubMed]
- Taniere P, Borghi-Scoazec G, Saurin JC, et al. Cytokeratin expression in adenocarcinomas of the esophagogastric junction: a comparative study of adenocarcinomas of the distal esophagus and of the proximal stomach. Am J Surg Pathol 2002;26:1213-21. [PubMed]
- Flucke U, Steinborn E, Dries V, et al. Immunoreactivity of cytokeratins (CK7, CK20) and mucin peptide core antigens (MUC1, MUC2, MUC5AC) in adenocarcinomas, normal and metaplastic tissues of the distal oesophagus, oesophago-gastric junction and proximal stomach. Histopathology 2003;43:127-34. [PubMed]
- Gulmann C, Counihan I, Grace A, et al. Cytokeratin 7/20 and mucin expression patterns in oesophageal, cardia and distal gastric adenocarcinomas. Histopathology 2003;43:453-61. [PubMed]
- Lau SK, Weiss LM, Chu PG. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: an immunohistochemical study. Am J Clin Pathol 2004;122:61-9. [PubMed]
- Goldstein NS, Long A, Kuan SF, et al. Colon signet ring cell adenocarcinoma: immunohistochemical characterization and comparison with gastric and typical colon adenocarcinomas. Appl Immunohistochem Mol Morphol 2000;8:183-8. [PubMed]
- Shibata D, Tokunaga M, Uemura Y, et al. Association of Epstein-Barr virus with undifferentiated gastric carcinomas with intense lymphoid infiltration. Lymphoepithelioma-like carcinoma. Am J Pathol 1991;139:469-74. [PubMed]
- Shibata D, Weiss LM. Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol 1992;140:769-74. [PubMed]
- Dei Tos AP. The reappraisal of gastrointestinal stromal tumors: from Stout to the KIT revolution. Virchows Arch 2003;442:421-8. [PubMed]
- Chu PG, Weiss LM. Modern Immunohistochemistry. c2009. Chapter 6, Tumors of the Digestive System; New York: Cambridge University Press; p188-254.
- Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol 2000;13:1134-42. [PubMed]
- Greenson JK. Gastrointestinal stromal tumors and other mesenchymal lesions of the gut. Mod Pathol 2003;16:366-75. [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52-68. [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 2006;130:1466-78. [PubMed]
- West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. [PubMed]
- Doglioni C, Wotherspoon AC, Moschini A, et al. High incidence of primary gastric lymphoma in northeastern Italy. Lancet 1992;339:834-5. [PubMed]
- Wotherspoon AC, Doglioni C, Diss TC, et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993;342:575-7. [PubMed]
- Lai R, Weiss LM, Chang KL, et al. Frequency of CD43 expression in non-Hodgkin lymphoma. A survey of 742 cases and further characterization of rare CD43+ follicular lymphomas. Am J Clin Pathol 1999;111:488-94. [PubMed]
- Streubel B, Huber D, Wöhrer S, et al. Frequency of chromosomal aberrations involving MALT1 in mucosa-associated lymphoid tissue lymphoma in patients with Sjögren’s syndrome. Clin Cancer Res 2004;10:476-80. [PubMed]
- Remstein ED, Dogan A, Einerson RR, et al. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol 2006;30:1546-53. [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Chow JS, Chen CC, Ahsan H, et al. A population-based study of the incidence of malignant small bowel tumours: SEER, 1973-1990. Int J Epidemiol 1996;25:722-8. [PubMed]
- Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol 2009;19:58-69. [PubMed]
- Chen ZM, Ritter JH, Wang HL. Differential expression of alpha-methylacyl coenzyme A racemase in adenocarcinomas of the small and large intestines. Am J Surg Pathol 2005;29:890-6. [PubMed]
- Sessa F, Furlan D, Zampatti C, et al. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch 2007;451:649-57. [PubMed]
- Albores-Saavedra J, Henson DE, Klimstra DS. Tumors of Gallbladder, extrahepatic bile ducts, and ampulla of Vater. Third Series, Fascicle 27 ed. Washington DC: Armed Force Institute of Pathology; 2000.
- Williams ED, Sandler M. The classification of carcinoid tum ours. Lancet 1963;1:238-9. [PubMed]
- Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 2004;1014:13-27. [PubMed]
- Kimura N, Pilichowska M, Okamoto H, et al. Immunohistochemical expression of chromogranins A and B, prohormone convertases 2 and 3, and amidating enzyme in carcinoid tumors and pancreatic endocrine tumors. Mod Pathol 2000;13:140-6. [PubMed]
- Alumets J, Håkanson R, Ingemansson S, et al. Substance P and 5-HT in granules isolated from an intestinal argentaffin carcinoid. Histochemistry 1977;52:217-22. [PubMed]
- Alumets J, Alm P, Falkmer S, et al. Immunohistochemical evidence of peptide hormones in endocrine tumors of the rectum. Cancer 1981;48:2409-15. [PubMed]
- Yang K, Ulich T, Cheng L, et al. The neuroendocrine products of intestinal carcinoids. An immunoperoxidase study of 35 carcinoid tumors stained for serotonin and eight polypeptide hormones. Cancer 1983;51:1918-26. [PubMed]
- Märtensson H, Nobin A, Sundler F, et al. Endocrine tumors of the ileum. Cytochemical and clinical aspects. Pathol Res Pract 1985;180:356-63. [PubMed]
- Burke AP, Sobin LH, Federspiel BH, et al. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med 1990;114:700-4. [PubMed]
- Mertz H, Vyberg M, Paulsen SM, et al. Immunohistochemical detection of neuroendocrine markers in tumors of the lungs and gastrointestinal tract. Appl Immunohistochem 1998;6:175-80.
- Al-Khafaji B, Noffsinger AE, Miller MA, et al. Immunohistologic analysis of gastrointestinal and pulmonary carcinoid tumors. Hum Pathol 1998;29:992-9. [PubMed]
- Fahrenkamp AG, Wibbeke C, Winde G, et al. Immunohistochemical distribution of chromogranins A and B and secretogranin II in neuroendocrine tumours of the gastrointestinal tract. Virchows Arch 1995;426:361-7. [PubMed]
- Sobin LH, Hjermstad BM, Sesterhenn IA, et al. Prostatic acid phosphatase activity in carcinoid tumors. Cancer 1986;58:136-8. [PubMed]
- Saqi A, Alexis D, Remotti F, et al. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol 2005;123:394-404. [PubMed]
- Barbareschi M, Roldo C, Zamboni G, et al. CDX-2 homeobox gene product expression in neuroendocrine tumors: its role as a marker of intestinal neuroendocrine tumors. Am J Surg Pathol 2004;28:1169-76. [PubMed]
- La Rosa S, Rigoli E, Uccella S, et al. CDX2 as a marker of intestinal EC-cells and related well-differentiated endocrine tumors. Virchows Arch 2004;445:248-54. [PubMed]
- Moskaluk CA, Zhang H, Powell SM, et al. Cdx2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol 2003;16:913-9. [PubMed]
- Jaffee IM, Rahmani M, Singhal MG, et al. Expression of the intestinal transcription factor CDX2 in carcinoid tumors is a marker of midgut origin. Arch Pathol Lab Med 2006;130:1522-6. [PubMed]
- Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 2004;1014:13-27. [PubMed]
- Gala M, Chung DC. Hereditary colon cancer syndromes. Semin Oncol 2011;38:490-9. [PubMed]
- Bussey HJ, Veale AM, Morson BC. Genetics of gastrointestinal polyposis. Gastroenterology 1978;74:1325-30. [PubMed]
- Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 2004;96:1631-4. [PubMed]
- Jenkins MA, Croitoru ME, Monga N, et al. Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol Biomarkers Prev 2006;15:312-4. [PubMed]
- Boparai KS, Mathus-Vliegen EM, Koornstra JJ, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: a multicentre cohort study. Gut 2010;59:1094-100. [PubMed]
- Giardiello FM, Brensinger JD, Tersmette AC, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology 2000;119:1447-53. [PubMed]
- Howe JR, Mitros FA, Summers RW. The risk of gastrointestinal carcinoma in familial juvenile polyposis. Ann Surg Oncol 1998;5:751-6. [PubMed]
- Aaltonen LA, Salovaara R, Kristo P, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med 1998;338:1481-7. [PubMed]
- Watson P, Lynch HT. The tumor spectrum in HNPCC. Anticancer Res 1994;14:1635-9. [PubMed]
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004;96:261-8. [PubMed]
- Werling RW, Yaziji H, Bacchi CE, et al. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol 2003;27:303-10. [PubMed]
- Raspollini MR, Nesi G, Baroni G, et al. Immunohistochemistry in the differential diagnosis between primary and secondary intestinal adenocarcinoma of the urinary bladder. Appl Immunohistochem Mol Morphol 2005;13:358-62. [PubMed]
- Suh N, Yang XJ, Tretiakova MS, et al. Value of CDX2, villin, and alpha-methylacyl coenzyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod Pathol 2005;18:1217-22. [PubMed]
- Nonaka D, Kusamura S, Baratti D, et al. CDX-2 expression in pseudomyxoma peritonei: a clinicopathological study of 42 cases. Histopathology 2006;49:381-7. [PubMed]
- Butler JA, Houshiar A, Lin F, et al. Goblet cell carcinoid of the appendix. Am J Surg 1994;168:685-7. [PubMed]
- Tang LH, Shia J, Soslow RA, et al. Pathologic classification and clinical behavior of the spectrum of goblet cell carcinoid tumors of the appendix. Am J Surg Pathol 2008;32:1429-43. [PubMed]
- Isaacson P. Crypt cell carcinoma of the appendix (so-called adenocarcinoid tumor). Am J Surg Pathol 1981;5:213-24. [PubMed]
- Gulubova MV, Yovchev Y, Vlaykova T, et al. Application of light microscopical and ultrastructural immunohistochemistry in the study of goblet cell carcinoid in the appendix. World J Surg Oncol 2008;6:15. [PubMed]
- Hristov AC, Young RH, Vang R, et al. Ovarian metastases of appendiceal tumors with goblet cell carcinoidlike and signet ring cell patterns: a report of 30 cases. Am J Surg Pathol 2007;31:1502-11. [PubMed]
- Tang LH. Epithelial neoplasms of the appendix. Arch Pathol Lab Med 2010;134:1612-20. [PubMed]
- Pai RK, Longacre TA. Appendiceal mucinous tumors and pseudomyxoma peritonei: histologic features, diagnostic problems, and proposed classification. Adv Anat Pathol 2005;12:291-311. [PubMed]
- Ronnett BM, Yan H, Kurman RJ, et al. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer 2001;92:85-91. [PubMed]
- Bradley RF, Stewart JH 4th, Russell GB, et al. Pseudomyxoma peritonei of appendiceal origin: a clinicopathologic analysis of 101 patients uniformly treated at a single institution, with literature review. Am J Surg Pathol 2006;30:551-9. [PubMed]
- Panarelli NC, Yantiss RK. Mucinous neoplasms of the appendix and peritoneum. Arch Pathol Lab Med 2011;135:1261-8. [PubMed]
- Chu PG, Chung L, Weiss LM, et al. Determining the site of origin of mucinous adenocarcinoma: an immunohistochemical study of 175 cases. Am J Surg Pathol 2011;35:1830-6. [PubMed]
- Welton ML, Sharkey FE, Kahlenberg MS. The etiology and epidemiology of anal cancer. Surg Oncol Clin N Am 2004;13:263-75. [PubMed]
- Joseph DA, Miller JW, Wu X, et al. Understanding the burden of human papillomavirus-associated anal cancers in the US. Cancer 2008;113:2892-900. [PubMed]
- Ryan DP, Mayer RJ. Anal carcinoma: histology, staging, epidemiology, treatment. Curr Opin Oncol 2000;12:345-52. [PubMed]
- Beal KP, Wong D, Guillem JG, et al. Primary adenocarcinoma of the anus treated with combined modality therapy. Dis Colon Rectum 2003;46:1320-4. [PubMed]
- Shia J. An update on tumors of the anal canal. Arch Pathol Lab Med 2010;134:1601-11. [PubMed]
- Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol 1998;22:170-9. [PubMed]
- Hamm H, Vroom TM, Czarnetzki BM. Extramammary Paget’s cells: further evidence of sweat gland derivation. J Am Acad Dermatol 1986;15:1275-81. [PubMed]
- Nowak MA, Guerriere-Kovach P, Pathan A, et al. Perianal Paget’s disease: distinguishing primary and secondary lesions using immunohistochemical studies including gross cystic disease fluid protein-15 and cytokeratin 20 expression. Arch Pathol Lab Med 1998;122:1077-81. [PubMed]
- Ito Y, Watanabe S, Usui Y, et al. Expression of cytokeratin antigen 20 in perianal Paget’s disease. Br J Dermatol 1999;140:1169-70. [PubMed]
- Ramalingam P, Hart WR, Goldblum JR. Cytokeratin subset immunostaining in rectal adenocarcinoma and normal anal glands. Arch Pathol Lab Med 2001;125:1074-7. [PubMed]
- Battles OE, Page DL, Johnson JE. Cytokeratins, CEA, and mucin histochemistry in the diagnosis and characterization of extramammary Paget’s disease. Am J Clin Pathol 1997;108:6-12. [PubMed]
- Cohen C, Guarner J, DeRose PB. Mammary Paget’s disease and associated carcinoma.An immunohistochemical study. Arch Pathol Lab Med 1993;117:291-4. [PubMed]
- Lundquist K, Kohler S, Rouse RV. Intraepidermal cytokeratin 7 expression is not restricted to Paget cells but is also seen in Toker cells and Merkel cells. Am J Surg Pathol 1999;23:212-9. [PubMed]
- Smith KJ, Tuur S, Corvette D, et al. Cytokeratin 7 staining in mammary and extramammary Paget’s disease. Mod Pathol 1997;10:1069-74. [PubMed]
- Vanstapel MJ, Gatter KC, De Wolf-Peeters C, et al. Immunohistochemical study of mammary and extra-mammary Paget’s disease. Histopathology 1984;8:1013-23. [PubMed]
- Mori O, Hachisuka H, Sasai Y. Immunohistochemical demonstration of epithelial membrane antigen (EMA), carcinoembryonic antigen (CEA), and keratin on mammary and extramammary Paget’s disease. Acta Histochem 1989;85:93-100. [PubMed]
- Filotico R, De Santis M, Filotico M. Morphologic and immunohistochemical observations on the mammary and extramammary Paget’s disease: implications for the histogenesis. Pathologica 1992;84:275-85. [PubMed]
- Brummer O, Stegner HE, Böhmer G, et al. HER-2/neu expression in Paget disease of the vulva and the female breast. Gynecol Oncol 2004;95:336-40. [PubMed]
- Keatings L, Sinclair J, Wright C, et al. c-erbB-2 oncoprotein expression in mammary and extramammary Paget’s disease: an immunohistochemical study. Histopathology 1990;17:243-7. [PubMed]
- Kuan SF, Montag AG, Hart J, et al. Differential expression of mucin genes in mammary and extramammary Paget’s disease. Am J Surg Pathol 2001;25:1469-77. [PubMed]
- Ohira S, Itoh K, Osada K, et al. Vulvar Paget’s disease with underlying adenocarcinoma simulating breast carcinoma: case report and review of the literature. Int J Gynecol Cancer 2004;14:1012-7. [PubMed]
- Ganjei P, Giraldo KA, Lampe B, et al. Is immunocytochemistry helpful in assessing the surgical margins? J Reprod Med 1990;35:1002-4. [PubMed]
- Olson DJ, Fujimura M, Swanson P, et al. Immunohistochemical features of Paget’s disease of the vulva with and without adenocarcinoma. Int J Gynecol Pathol 1991;10:285-95. [PubMed]
- Zhang J, Dhakal I, Yan H, et al. Trends in pancreatic cancer incidence in nine SEER Cancer Registries, 1973-2002. Ann Oncol 2007;18:1268-79. [PubMed]
- Gold EB, Goldin SB. Epidemiology of and risk factors for pancreatic cancer. Surg Oncol Clin N Am 1998;7:67-91. [PubMed]
- Hruban RH, Fukushima N. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod Pathol 2007;20:S61-70. [PubMed]
- Goldstein NS, Bassi D. Cytokeratins 7, 17, and 20 reactivity in pancreatic and ampulla of vater adenocarcinomas. Percentage of positivity and distribution is affected by the cut-point threshold. Am J Clin Pathol 2001;115:695-702. [PubMed]
- Park SY, Kim HS, Hong EK, et al. Expression of cytokeratins 7 and 20 in primary carcinomas of the stomach and colorectum and their value in the differential diagnosis of metastatic carcinomas to the ovary. Hum Pathol 2002;33:1078-85. [PubMed]
- Ji H, Isacson C, Seidman JD, et al. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous carcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol 2002;21:391-400. [PubMed]
- Chhieng DC, Benson E, Eltoum I, et al. MUC1 and MUC2 expression in pancreatic ductal carcinoma obtained by fine-needle aspiration. Cancer 2003;99:365-71. [PubMed]
- Hruban RH, Fukushima N. Pancreatic adenocarcinoma: update on the surgical pathology of carcinomas of ductal origin and PanINs. Mod Pathol 2007;20:S61-70. [PubMed]
- Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol 2008;1:306-16. [PubMed]
- Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol 2002;15:1087-95. [PubMed]
- Levi E, Klimstra DS, Andea A, et al. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol 2004;57:456-62. [PubMed]
- Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999;23:410-22. [PubMed]
- Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17-32. [PubMed]
- Volkan Adsay N. Cystic lesions of the pancreas. Mod Pathol 2007;20:S71-93. [PubMed]
- Lüttges J, Feyerabend B, Buchelt T, et al. The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am J Surg Pathol 2002;26:466-71. [PubMed]
- Wilentz RE, Albores-Saavedra J, Hruban RH. Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol 2000;17:31-42. [PubMed]
- He H, Luthringer DJ, Hui P, et al. Expression of CD56 and WT1 in ovarian stroma and ovarian stromal tumors. Am J Surg Pathol 2008;32:884-90. [PubMed]
- Handra-Luca A, Fléjou JF, Rufat P, et al. Human pancreatic mucinous cystadenoma is characterized by distinct mucin, cytokeratin and CD10 expression compared with intraductal papillary-mucinous adenoma. Histopathology 2006;48:813-21. [PubMed]
- Adsay NV, Conlon KC, Zee SY, et al. Intraductal papillary-mucinous neoplasms of the pancreas: an analysis of in situ and invasive carcinomas in 28 patients. Cancer 2002;94:62-77. [PubMed]
- Murakami Y, Uemura K, Ohge H, et al. Intraductal papillary-mucinous neoplasms and mucinous cystic neoplasms of the pancreas differentiated by ovarian-type stroma. Surgery 2006;140:448-53. [PubMed]
- Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol 2004;28:839-48. [PubMed]
- Ueda M, Miura Y, Kunihiro O, et al. MUC1 overexpression is the most reliable marker of invasive carcinoma in intraductal papillary-mucinous tumor (IPMT). Hepatogastroenterology 2005;52:398-403. [PubMed]
- Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol 2007;20:S94-112. [PubMed]
- Buetow PC, Buck JL, Pantongrag-Brown L, et al. Solid and papillary epithelial neoplasm of the pancreas: imaging-pathologic correlation on 56 cases. Radiology 1996;199:707-11. [PubMed]
- Bardales RH, Centeno B, Mallery JS, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid-pseudopapillary tumor of the pancreas: a rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol 2004;121:654-62. [PubMed]
- Kosmahl M, Seada LS, Jänig U, et al. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch 2000;436:473-80. [PubMed]
- Notohara K, Hamazaki S, Tsukayama C, et al. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol 2000;24:1361-71. [PubMed]
- Tiemann K, Kosmahl M, Ohlendorf J, et al. Solid pseudopapillary neoplasms of the pancreas are associated with FLI-1 expression, but not with EWS/FLI-1 translocation. Mod Pathol 2006;19:1409-13. [PubMed]
- Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol 2002;160:1361-9. [PubMed]
- Tang WW, Stelter AA, French S, et al. Loss of cell-adhesion molecule complexes in solid pseudopapillary tumor of pancreas. Mod Pathol 2007;20:509-13. [PubMed]
- Campbell F, Azadeh B. Cystic neoplasms of the exocrine pancreas. Histopathology 2008;52:539-51. [PubMed]
- Compton CC. Serous cystic tumors of the pancreas. Semin Diagn Pathol 2000;17:43-55. [PubMed]
- Kosmahl M, Wagner J, Peters K, et al. Serous cystic neoplasms of the pancreas: an immunohistochemical analysis revealing alpha-inhibin, neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol 2004;28:339-46. [PubMed]
- Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol 1997;23:36-42. [PubMed]
- Milan SA, Yeo CJ. Neuroendocrine tumors of the pancreas. Curr Opin Oncol 2012;24:46-55. [PubMed]
- Lloyd RV, Mervak T, Schmidt K, et al. Immunohistochemical detection of chromogranin and neuron-specific enolase in pancreatic endocrine neoplasms. Am J Surg Pathol 1984;8:607-14. [PubMed]
- Portela-Gomes GM, Hacker GW, Weitgasser R. Neuroendocrine cell markers for pancreatic islets and tumors. Appl Immunohistochem Mol Morphol 2004;12:183-92. [PubMed]
- Kamisawa T, Tu Y, Egawa N, et al. Ductal and acinar differentiation in pancreatic endocrine tumors. Dig Dis Sci 2002;47:2254-61. [PubMed]
- Yantiss RK, Chang HK, Farraye FA, et al. Prevalence and prognostic significance of acinar cell differentiation in pancreatic endocrine tumors. Am J Surg Pathol 2002;26:893-901. [PubMed]
- Klimstra DS, Wenig BM, Adair CF, et al. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol 1995;19:1371-89. [PubMed]
- Drut R, Jones MC. Congenital pancreatoblastoma in Beckwith-Wiedemann syndrome: an emerging association. Pediatr Pathol 1988;8:331-9. [PubMed]
- Nishimata S, Kato K, Tanaka M, et al. Expression pattern of keratin subclasses in pancreatoblastoma with special emphasis on squamoid corpuscles. Pathol Int 2005;55:297-302. [PubMed]
- Tanaka Y, Kato K, Notohara K, et al. Significance of aberrant (cytoplasmic/nuclear) expression of beta-catenin in pancreatoblastoma. J Pathol 2003;199:185-90. [PubMed]
- Klimstra DS, Wenig BM, Adair CF, et al. Pancreatoblastoma. A clinicopathologic study and review of the literature. Am J Surg Pathol 1995;19:1371-89. [PubMed]
- Morohoshi T, Kanda M, Horie A, et al. Immunocytochemical markers of uncommon pancreatic tumors. Acinar cell carcinoma, pancreatoblastoma, and solid cystic (papillary-cystic) tumor. Cancer 1987;59:739-47. [PubMed]
- Hoorens A, Lemoine NR, McLellan E, et al. Pancreatic acinar cell carcinoma. An analysis of cell lineage markers, p53 expression, and Ki-ras mutation. Am J Pathol 1993;143:685-98. [PubMed]
- Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol 2002;160:953-62. [PubMed]
- Ballas KD, Rafailidis SF, Demertzidis C, et al. Mixed exocrine-endocrine tumor of the pancreas. JOP 2005;6:449-54. [PubMed]
- Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol 1994;18:765-78. [PubMed]
- Klöppel G. Mixed exocrine-endocrine tumors of the pancreas. Semin Diagn Pathol 2000;17:104-8. [PubMed]
- Bruno S, Silini E, Crosignani A, et al. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a prospective study. Hepatology 1997;25:754-8. [PubMed]
- Fattovich G, Giustina G, Schalm SW, et al. Occurrence of hepatocellular carcinoma and decompensation in western European patients with cirrhosis type B. The EUROHEP Study Group on Hepatitis B Virus and Cirrhosis. Hepatology 1995;21:77-82. [PubMed]
- Varma V, Cohen C. Immunohistochemical and molecular markers in the diagnosis of hepatocellular carcinoma. Adv Anat Pathol 2004;11:239-49. [PubMed]
- Wee A. Diagnostic utility of immunohistochemistry in hepatocellular carcinoma, its variants and their mimics. Appl Immunohistochem Mol Morphol 2006;14:266-72. [PubMed]
- White GR, Kelsey AM, Varley JM, et al. Somatic glypican 3 (GPC3) mutations in Wilms’ tumour. Br J Cancer 2002;86:1920-2. [PubMed]
- Ohmori S, Shiraki K, Sugimoto K, et al. Expression of CD34-positive sinusoidal endothelial cells in patients with HBV-associated chronic liver diseases. Int J Mol Med 2004;14:179-84. [PubMed]
- Scott FR, el-Refaie A, More L, et al. Hepatocellular carcinoma arising in an adenoma: value of QBend 10 immunostaining in diagnosis of liver cell carcinoma. Histopathology 1996;28:472-4. [PubMed]
- Ruck P, Xiao JC, Kaiserling E. Immunoreactivity of sinusoids in hepatocellular carcinoma. An immunohistochemical study using lectin UEA-1 and antibodies against endothelial markers, including CD34. Arch Pathol Lab Med 1995;119:173-8. [PubMed]
- Maeda T, Adachi E, Kajiyama K, et al. CD34 expression in endothelial cells of small hepatocellular carcinoma: its correlation with tumour progression and angiographic findings. J Gastroenterol Hepatol 1995;10:650-4. [PubMed]
- Park YN, Yang CP, Fernandez GJ, et al. Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol 1998;22:656-62. [PubMed]
- Christensen WN, Boitnott JK, Kuhajda FP. Immunoperoxidase staining as a diagnostic aid for hepatocellular carcinoma. Mod Pathol 1989;2:8-12. [PubMed]
- Chu P, Arber DA. Paraffin-section detection of CD10 in 505 nonhematopoietic neoplasms.Frequent expression in renal cell carcinoma and endometrial stromal sarcoma. Am J Clin Pathol 2000;113:374-82. [PubMed]
- Chu PG, Ishizawa S, Wu E, et al. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol 2002;26:978-88. [PubMed]
- Christensen WN, Boitnott JK, Kuhajda FP. Immunoperoxidase staining as a diagnostic aid for hepatocellular carcinoma. Mod Pathol 1989;2:8-12. [PubMed]
- Johnson DE, Herndier BG, Medeiros LJ, et al. The diagnostic utility of the keratin profiles of hepatocellular carcinoma and cholangiocarcinoma. Am J Surg Pathol 1988;12:187-97. [PubMed]
- Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [PubMed]
- Kobayashi M, Ikeda K, Saitoh S, et al. Incidence of primary cholangiocellular carcinoma of the liver in japanese patients with hepatitis C virus-related cirrhosis. Cancer 2000;88:2471-7. [PubMed]
- Boberg KM, Bergquist A, Mitchell S, et al. Cholangiocarcinoma in primary sclerosing cholangitis: risk factors and clinical presentation. Scand J Gastroenterol 2002;37:1205-11. [PubMed]
- Goodman ZD. Neoplasms of the liver. Mod Pathol 2007;20:S49-60. [PubMed]
- Rullier A, Le Bail B, Fawaz R, et al. Cytokeratin 7 and 20 expression in cholangiocarcinomas varies along the biliary tract but still differs from that in colorectal carcinoma metastasis. Am J Surg Pathol 2000;24:870-6. [PubMed]
- Reynolds P, Urayama KY, Von Behren J, et al. Birth characteristics and hepatoblastoma risk in young children. Cancer 2004;100:1070-6. [PubMed]
- Weinberg AG, Finegold MJ. Primary hepatic tumors of childhood. Hum Pathol 1983;14:512-37. [PubMed]
- Rowland JM. Hepatoblastoma: assessment of criteria for histologic classification. Med Pediatr Oncol 2002;39:478-83. [PubMed]
- Fasano M, Theise ND, Nalesnik M, et al. Immunohistochemical evaluation of hepatoblastomas with use of the hepatocyte-specific marker, hepatocyte paraffin 1, and the polyclonal anti-carcinoembryonic antigen. Mod Pathol 1998;11:934-8. [PubMed]
- Redston MS, Wanless IR. The hepatic von Meyenburg complex: prevalence and association with hepatic and renal cysts among 2843 autopsies Mod Pathol 1996;9:233-7. [corrected]. [PubMed]
- Bhathal PS, Hughes NR, Goodman ZD. The so-called bile duct adenoma is a peribiliary gland hamartoma. Am J Surg Pathol 1996;20:858-64. [PubMed]
- Chu PG, Schwarz RE, Lau SK, et al. Immunohistochemical staining in the diagnosis of pancreatobiliary and ampulla of Vater adenocarcinoma: application of CDX2, CK17, MUC1, and MUC2. Am J Surg Pathol 2005;29:359-67. [PubMed]
- Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol 2005;29:381-9. [PubMed]


