
Gastric cancer: lessons learned from high-incidence geographic regions
Introduction
Worldwide, gastric cancer is the fifth most commonly diagnosed cancer and is the third leading cause of oncological deaths (1,2). Adenocarcinomas comprise 95% of all gastric cancers and can be subdivided into cardia and non-cardia according to their anatomical location (1). Unless otherwise specified, gastric cancer refers to gastric adenocarcinomas throughout this article.
There is a marked geographical variability in the incidence of gastric cancer. Incidence is highest in East and Central Asia followed by Latin America and Eastern Europe (3). In contrast, the incidence is low in the United States and other Western countries, where gastric cancer is usually diagnosed at late stages due to non-specificity of symptoms and lack of population-based intervention programs (4). Though current treatment options have yielded modest benefits in terms of survival improvement, patients with gastric cancer generally have low survival rates (3). Prevention and early diagnosis may be effective strategies to reduce morbidity and mortality (1,3). H. pylori eradication and dietary modification are the main prevention strategies in use. Endoscopic screening has become the mainstay screen for early diagnosis.
Incidence and prognosis
In 2018, there were over 1,000,000 new cases and approximately 783,000 deaths related to gastric cancer worldwide (2). Incidence of gastric cancer varies greatly with geographic location, race, gender and socioeconomic status (1).
Geographic location
East Asia carries most of the world’s gastric cancer burden that accounts for approximately 75% of new cases worldwide (4). In 2012, the age-standardized incidence rates (ASIR) of gastric cancer in Korea, Japan and China were estimated at 41.8, 29.9 and 22.7 per 100,000, respectively (3). Other areas of high incidence are Central and Eastern Europe and Central and South America (3). Conversely, North America, Australia, New Zealand, South Asia, Northern Europe, and North and East Africa have a low incidence (3,5). Gastric cancer incidence in the United States is among the lowest in the world with an ASIR of 3.9 per 100,000 (3,4,6) (Figure 1).
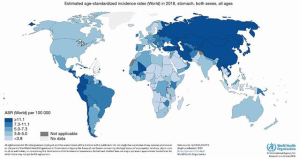
The incidence of gastric cancer subtypes differs among geographic regions. Non-cardia gastric cancer is the predominant subtype globally and throughout East and Central Asia and Eastern Europe (3). Conversely, cardia gastric cancer is the most common subtype in Western countries. This is secondary to a decrease in the incidence of non-cardia gastric cancer, while rates of cardia cancer have remained unchanged (3).
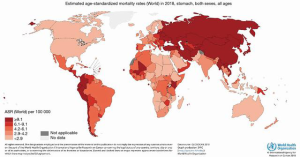
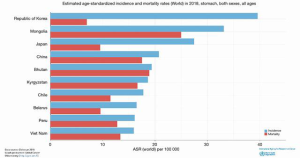
Mortality rates from gastric cancer are higher in areas of high incidence such as East and Central Asia and Latin America (1,6) (Figure 2). Japan and South Korea exhibit the highest incidence of gastric cancer and the greatest number of cumulative deaths, even though the mortality-incidence ratio is remarkably low due to increased survival rates (1,3,6,7) (Figure 3). Five-year gastric cancer survival rates of 67% and 69% have been reported in Korea and Japan, respectively (7-9). These survival rates are significantly better than the 5-year 20% relative survival rate seen in most parts of the world (8).
Race and ethnicity
There is marked variability in the incidence of gastric cancer between Eastern and Western nations. In the United States, Caucasian population have decreased risk of gastric cancer compared to Asian and Pacific Islanders, American Indian and Alaska Natives, African Americans, and Hispanics. Japanese and Korean Americans have an especially high rate of gastric cancer (1,8). However, Asian patients in the United States tend to have a better prognosis than Caucasians, with a 12% higher 5-year survival rate after adjusting for age, gender and stage (1).
Genetic polymorphisms among various ethnic groups are partly responsible for varying rates of gastric cancer (10). For example, polymorphisms in interleukin-10 and -17 genes are common in Asian populations. These polymorphisms appear to impact H. pylori infection and are associated with an increased risk of gastric cancer (10). Nevertheless, a strong environmental component is considered the greatest factor for the geographic variability of gastric cancer incidence (1).
First-generation immigrants from areas with high incidence of gastric cancer exhibit a higher risk than the population native to the low-incidence countries where they relocate. The standardized incidence ratios are 1.08- to 5.05-fold higher than the destination native-born population. Second-generation immigrants from same countries remain at higher risk, although lower than first-generation immigrants. The level of acculturation, including dietary modification, influences the risk of gastric cancer. This explains the risk equalization in subsequent generations and the different rate at which this occurs in different groups. For instance, Japanese Americans experience an accelerated decrease in risk due to increased acculturation after immigrating, as compared to Korean Americans (11).
Gender
Incidence rates of gastric cancer clearly reflect a decreased risk of gastric cancer in females (1,2). Gastric cancer is the leading cause of death in males in several Western Asian countries, including Iran, Turkmenistan, and Kyrgyzstan (1). Estrogen seems to be the determinant factor due to its protective effect based on observations that delayed menopause and increased fertility are associated with a reduction in gastric cancer risk (12). This effect is blunted with the use of antiestrogen drugs such as tamoxifen (12). After menopause, the risk of gastric cancer in females equalizes to that of age matched males (1).
There are various mechanisms that can explain the protective effect of estrogen. Animal studies show that 17β-estradiol mediates a protective effect in H. pylori-infected mice, presumably by increasing the expression of trefoil factor family (TFF) proteins, which protect the gastric epithelium from dysplastic changes and promote mucosal repair (13). High concentrations of estrogen inhibit the expression of estrogen receptor α36 and halts the proliferation of gastric cancer cells, while low concentrations promotes its expression and stimulate gastric carcinogenesis (14).
Socioeconomic level
Low socioeconomic status is associated with increased risk of gastric cancer (1,3,15). The ASIR of gastric cancer in economically developed regions is 15.6 per 100,000 in males and 6.7 per 100,000 in females compared to 18.1 per 100,000 for males and 7.8 per 100,000 for females living in less developed areas of the world (3). These observations are likely secondary to higher rates of H. pylori infection or decreased access to fresh food, fruits, and vegetables in low socioeconomic stratums (1).
Stage at time of diagnosis
Survival rates in gastric cancer are highly dependent on the stage at time of diagnosis and surgical intervention (16). Screening programs result in diagnosis of gastric cancer in its early stages, which may account for some of the differences in survival among geographic regions, since over 50% of cancers are diagnosed at an early stage in Japan, as opposed to only 20% in the United States (8). In the United States, the 5-year survival rate for stage IA tumors treated with surgery is 94%, with rates dropping to 18% in the case of stage IIIC tumors (1).
Risk factors for gastric cancer
Helicobacter pylori
H. pylori infection induces inflammation of the gastric mucosa that results in the development of peptic ulcers, chronic and atrophic gastritis, intestinal metaplasia, gastric lymphoma, and adenocarcinoma (17). Approximately 85–90% of non-cardia gastric cancer are associated with this pathogen (18). As non-cardia gastric cancer constitutes the majority of gastric cancer, H. pylori infection is associated with 75–78% of all gastric cancers (1,19). H. pylori reportedly carries an odds ratio (OR) of 5.9 for the development of gastric cancer within 10 years of infection (1).
The association between H. pylori and gastric cancer is stronger in certain regions of the world, such as East Asia. Incidentally, other Asian areas like India and Taiwan have high frequency of H. pylori infection with a corresponding low incidence of gastric cancer (5,20,21). This is likely related to the geographical distribution of different strains of the bacterium in addition to interactions between H. pylori and different environmental factors (5,21). The vacuolating cytotoxin A (VacA) and cytotoxin-associated gene A (CagA) are virulence factors that mediate cellular damage and oncogenesis in H. pylori infections. Patients infected with strains with an intact cag pathogenicity island develop a greater inflammatory response and are more susceptible to gastric cancer development. Most H. pylori isolates in Japan and South Korea possess both CagA and VacA with genetic variants associated with progression of gastric preneoplastic lesions and increased cancer risk (21). In contrast, Indian H. pylori strains have European origin and are less pathogenic than the strains that are predominant in Far East Asia (20,21).
Diet and environmental factors
Consumption of certain foods is associated with increased risk of gastric cancer. Ingestion of pickled vegetables, salted, smoked, cured or chemically preserved foods, together with a diet low in fresh fruits and vegetables, increase the risk of gastric cancer (5,21). Additionally, diets high in salt are implicated in the development of gastritis and potentiates the noxious effects of known gastric carcinogens such as N-methyl-N-nitro-N-nitrosoguanidine (MNNG) (22). Red meat, especially when preserved or cooked to high temperatures, is rich in N-nitroso compounds, heterocyclic amines (HCA) and polycyclic aromatic hydrocarbons (PAH), which have carcinogenic and pro-inflammatory effects (1,23,24). This effect is not seen with lamb or white meat, while consumption of fresh fish was found to be protective due to high content of polyunsaturated fatty acids (5,24). Furthermore, H. pylori inhibits the activity of ascorbic acid and favors the synthesis of N-nitroso compounds, increasing inflammation and the risk for gastric cancer (5).
Conversely, fruits and vegetables are rich in antioxidants like carotenoids, folate, phytochemicals and vitamin C that prevent metabolic damage (24,25). Higher intake of fruits and vegetables is associated with a 30–40% lower risk of gastric cancer (25). However, evaluation of the protective effect of non-dietary antioxidant sources, such as green tea and vitamin supplements have produced mixed results in preventing gastric cancer (1). Epidemiologic studies of Japanese immigrants to the United States who assimilated a Western diet exhibited a substantially lower rate of gastric cancer relative to those who did not (1,26).
Volcanic and high land regions are characterized by nitrous soils that lead to high concentrations of nitrate and nitrite in water and agricultural products. This is associated with a higher risk of gastric cancer in certain regions of Costa Rica, Chile, Japan and Iran (24). Soil containing low concentration of molybdenum may contribute to a higher risk of gastric cancer due to its inhibitory effect in the formation of nitrosamine compounds (24).
Atrophic gastritis and intestinal metaplasia
Atrophic gastritis and intestinal metaplasia are considered precursors of gastric cancer, and both are strongly associated with H. pylori. A Dutch cohort study found a direct association between the severity of premalignant lesions and risk for gastric cancer (8). Within 5 years of diagnosis, the annual incidence of gastric cancer is 0.1% in patients with atrophic gastritis, 0.25% for intestinal metaplasia, 0.6% in mild-moderate dysplasia, and increases to 6% in the case of severe dysplasia (8).
Family history of gastric cancer and familial syndromes
Approximately 1–3% of gastric cancers are believed to be related to familial syndromes (27). Having a first-degree family member with gastric cancer is associated with an OR of 2–10 for developing the disease (8).
Familial adenomatous polyposis, the most common form of familial intestinal gastric cancer, is an autosomal-dominantly inherited condition that predisposes individuals to adenomatous polyps caused by germline mutations in the APC gene (28). Nevertheless, the lifetime risk of developing gastric cancer among these patients is less than 1%, indicating that an environmental component is also necessary for oncogenesis (1).
Other familiar syndromes associated with increased risk of gastric cancer are Lynch syndrome, gastric adenocarcinoma and proximal polyposis of the stomach, and hereditary diffuse gastric cancer (1).
Trends
The incidence of gastric cancer has been steadily decreasing over the last 50 years (1). This phenomenon can be partially explained by reduction in rates of H. pylori infection and improved food preservation and produce availability (1,26). However, a decrease in H. pylori infection rates cannot be the sole driver of this trend, which preceded the inception of any eradication program (1).
Decreases in incidence rates vary by geographic region. East Asia is experiencing a decrease in non-cardia cancer in relationship with a reduction in H. pylori infection rates (1). This phenomenon is not seen in Central and South America, where H. pylori infection rates have remained stable (1). Prevalence rates of H. pylori infection have notably decreased in younger populations in most areas of the world. For instance, in Japan, cross sectional studies reported a prevalence of H. pylori infection of 80% in people born before 1950 compared to 5% among people born after 1980, while the prevalence rate in Central and South America was estimated to be 70–85% in all age groups (3). This may be due to the lack of an effective campaign for infection prevention and treatment (1). In general, the survival rates of gastric cancer have also improved over the last 40 years due to earlier detection of disease and better treatment options (1).
Gastric cancer prevention
Helicobacter pylori testing and eradication
The eradication of H. pylori in the developing world remains a primary goal in the prevention of gastric cancer due to its strong association with non-cardia gastric cancer. H. pylori eradication effectively reduces gastric cancer rates, with a greater protective effect seen in patients with higher baseline gastric cancer risk and in high incidence areas (19).
Despite these results, there are currently no worldwide guidelines for screening and eradication of H. pylori in asymptomatic individuals (29). On a smaller scale, the Asia-Pacific Consensus Guideline recommends H. pylori screening and treatment in high-risk populations (grade A recommendation) (30) (Table 1). Additional studies conducted in Japan advocate for H. pylori eradication in patients who have undergone endoscopic resection of a gastric adenocarcinoma (31). These studies show a decrease in the incidence of metachronous lesions in patients who underwent H. pylori eradication when compared to the control groups (32). The Japanese Society for Helicobacter Research recommended H. pylori eradication as a measure for gastric cancer prevention (grade A recommendation) (33) (Table 2). The Europe Maastricht IV/Florence report recommends eradication of H. pylori in the following scenarios (grade A recommendation): populations at high-risk, first degree relatives of family members with gastric cancer, patients with previous gastric cancer treated with endoscopic or subtotal gastric resection, chronic gastric acid inhibition over 1 year, strong environmental risk factors (such as heavy smoking, occupational exposure), and per patient’s request (34) (Table 3).

Full table

Full table

Full table
H. pylori screening and eradication appears to be a viable and effective preventive strategy against gastric cancer. Reducing H. pylori infection rates would be a more cost-effective method for preventing gastric cancer in developing regions and low socioeconomic stratus compared to endoscopic screening due to high cost and increased need for resources (35-37). Mass screening and eradication would be especially beneficial in areas with high incidence of gastric cancer, but it could also be implemented in low-risk areas (19).
Lifestyle modifications
Dietary and lifestyle interventions represent a practical strategy to prevent gastric cancer, which can be implemented in the general population (26). A wholesome diet rich in fruits, vegetables, whole grains, and low in alcohol, salt, pickled foods, and processed, smoked, or cured meats lowers the risk of stomach inflammation and gastric cancer (1,5). As mentioned previously, there are mixed results regarding the benefits of vitamin supplementation and green tea consumption (1).
Other behaviors, such as regular exercise, may also have a protective effect against gastric cancer, especially non-cardia gastric cancer (38,39). Since tobacco use has been shown to increase the risk of gastric cancer and numerous other cancers, tobacco cessation programs would also benefit individuals by decreasing their risk of gastric cancer (1).
Prophylactic therapeutics
Non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to lower the risk of stomach cancer, especially non-cardia gastric cancer (28). However, NSAIDs also increase the risk of bleeding, and therefore are not recommended as a prophylactic measure for gastric cancer in the general population. Use of NSAIDs for gastric cancer prevention should be determined on a case-by-case basis (1).
Diagnosis
Screening for gastric cancer generally involves contrast radiography and endoscopy. Endoscopy is currently considered the gold standard for gastric cancer screening because it allows direct visual examination of the gastric mucosa, as well as biopsy for histologic evaluation (1). Conventional white light endoscopy is not sufficient for accurate diagnosis and surveillance of preneoplastic lesions. Newer technologies such as magnification chromoendoscopy and narrow band imaging increased the diagnostic yield of endoscopic screening. However, this technique is operator dependent and limited by the absence of standardized interpretation (29). Though less invasive, contrast radiography has low sensitivity for early gastric cancer diagnosis (40).
Implementation of national screening programs in high incidence regions
Japan and South Korea are countries with the highest incidence of gastric cancer (41). In 1983, Japan initiated an annual gastric cancer screening program for all residents 40 years and older that consisted of an upper gastrointestinal (UGI) series. In 2016, the Japanese guidelines were updated to allow screening with either endoscopy or UGI series (41). More recent guidelines recommend screening every 2–3 years in patients that are 50 years and older (8). South Korea also initiated a national gastric cancer screening program in 1999. Similar to Japan, endoscopy or UGI series is recommended every 2 years for individuals aged 40 years and older (1). As a result of these screening programs, early gastric cancer represents over 50% of the gastric cancer diagnoses in Japan and 46–67% in South Korea (42). The high rates of early gastric cancer improved patient outcomes and multiple studies have demonstrated a reduction in gastric cancer mortality (7,8).
Possible application of screening programs in low incidence regions
Gastric cancer screening could be implemented as mass screening or selectively for individuals at increased risk. Despite the success of gastric cancer screening programs in high-incidence countries, mass screening might not be cost-effective in low incidence areas such as the United States. Therefore, screening may be considered only in individuals at high risk for gastric cancer, such as immigrants from countries with high incidence of gastric cancer, individuals with family history of gastric cancer, and patients with H. pylori infection (7,29). Nevertheless, a 2011 study by Gupta, Bansal, and Wani found that the cost-effectiveness of performing an upper endoscopy for screening of upper GI tract cancers at the time of screening colonoscopy was comparable to other screening interventions routinely performed in the United States (43). The cost-effectiveness relationship would be expected to improve if this intervention is performed in a predefined high-risk population.
Regarding age for screening initiation, there is data to support screening initiation both at age 40 and 50 (8). Due to a trend of decreasing gastric cancer incidence between ages 40 to 49, the new national Japanese guidelines recommend screening in individuals that are 50 years or older (8).
However, the optimal interval for endoscopic screening has not been established (1,8). In a Japanese study, the 5-year survival rate was significantly higher in those patients that had undergone endoscopic screening within the previous two years when compared to patients that had not, suggesting that a 2-year interval for screening would be appropriate (44). Furthermore, a Korean study indicated that there was an increased rate of endoscopic resections in patients that had endoscopic screening every 2 years (45). Evidence is even more limited with regards to interval screening in Western countries. Two studies from England and Italy showed that almost two-thirds of newly diagnosed gastric cancers were in advanced stages despite a surveillance interval of 1 to 2 years (46,47). Annual endoscopic surveillance has been proposed in patients with intestinal metaplasia who present additional risk factors such as smokers, patients with a family history of gastric cancer, incomplete type intestinal metaplasia, or involvement of more than 20% of the gastric mucosa (48). In the United States, an interval screening ranging between 1–3 years has been proposed in patients with atrophic gastritis or intestinal metaplasia (7,49). The same recommendations may be considered for other high-risk gastric conditions.
Although gastric cancer incidence rates are low in the United States, due to lack of screening programs, cases of gastric cancer are generally diagnosed at a later stage when treatment options are limited. Therefore, there is a need for development of clear screening guidelines in western countries.
Conclusions
- Gastric cancer is an important cause of morbidity and mortality worldwide;
- High-incidence countries have laid the foundation for disease prevention and early diagnosis strategies;
- Gastric cancer survival is low in Western countries due to diagnosis at late stages;
- Prevention mainly involves dietary changes and H. pylori eradication. There are no current global guidelines, but some national or continental guidelines recommend H. pylori screening and eradication;
- Endoscopy is the preferred gastric cancer screening modality. Chromoendoscopy and narrow band imaging are favored. Japan and South Korea have well-established endoscopic screening programs that translate into early diagnosis and increased survival;
- There is a need for the development of comprehensive guidelines for gastric cancer screening and surveillance tailored to Western countries. Screening programs should be tailored to high-risk individuals to ensure cost-efficiency in the context of low incidence of the disease.
Acknowledgments
The authors thank Emily A. Andreae PhD for assisting with manuscript editing.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John F. Gibbs and Quyen D. Chu) for the series “Global GI Malignancies” published in Journal of Gastrointestinal Oncology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-2019-gi-05). The series “Global GI Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019;14:26-38. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Balakrishnan M, George R, Sharma A, et al. Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep 2017;19:36. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Kadar Z, Jung I, Orlowska J, et al. Geographic particularities in incidence and etiopathogenesis of sporadic gastric cancer. Pol J Pathol 2015;66:254-9. [Crossref] [PubMed]
- Arnold M, Rutherford M, Lam F, et al. ICBP SURVMARK-2 online tool: International Cancer Survival Benchmarking. Lyon, France: International Agency for Research on Cancer. Available online: http://gco.iarc.fr/survival/survmark. Accessed 15 December 2019.
- Suh YS, Yang HK. Screening and Early Detection of Gastric Cancer: East Versus West. Surg Clin North Am 2015;95:1053-66. [Crossref] [PubMed]
- Kim GH, Liang PS, Bang SJ, et al. Screening and surveillance for gastric cancer in the United States: Is it needed? Gastrointest Endosc 2016;84:18-28. [Crossref] [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Merchant SJ, Li L, Kim J. Racial and ethnic disparities in gastric cancer outcomes: More important than surgical technique? World J Gastroenterol 2014;20:11546-51. [Crossref] [PubMed]
- Pabla BS, Shah SC, Corral JE, et al. Increased Incidence and Mortality of Gastric Cancer in Immigrant Populations from High to Low Regions of Incidence: A Systematic Review and Meta-Analysis. Clin Gastroenterol Hepatol 2020;18:347-59.e5. [Crossref] [PubMed]
- Camargo MC, Goto Y, Zabaleta J, et al. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2012;21:20-38. [Crossref] [PubMed]
- Wang Z, Butler LM, Wu AH, et al. Reproductive factors, hormone use and gastric cancer risk: The Singapore Chinese Health Study. Int J Cancer 2016;138:2837-45. [Crossref] [PubMed]
- Ur Rahman MS, Cao J. Estrogen receptors in gastric cancer: Advances and perspectives. World J Gastroenterol 2016;22:2475-82. [Crossref] [PubMed]
- Uthman OA, Jadidi E, Moradi T. Socioeconomic position and incidence of gastric cancer: a systematic review and meta-analysis. J Epidemiol Community Health 2013;67:854-60. [Crossref] [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/csr/1975_2016/
- Carrasco G, Corvalan AH. Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer. Gastroenterol Res Pract 2013;2013:393015 [Crossref] [PubMed]
- Moss SF. The Clinical Evidence Linking Helicobacter pylori to Gastric Cancer. Cell Mol Gastroenterol Hepatol 2016;3:183-91. [Crossref] [PubMed]
- Lee YC, Chiang TH, Chou CK, et al. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016;150:1113-24.e5. [Crossref] [PubMed]
- Misra V, Pandey R, Misra SP, et al. Helicobacter pylori and gastric cancer: Indian enigma. World J Gastroenterol 2014;20:1503-9. [Crossref] [PubMed]
- Watari J, Chen N, Amenta PS, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol 2014;20:5461-73. [Crossref] [PubMed]
- Song JH, Kim YS, Heo NJ, et al. High Salt Intake Is Associated with Atrophic Gastritis with Intestinal Metaplasia. Cancer Epidemiol Biomarkers Prev 2017;26:1133-8. [Crossref] [PubMed]
- Dubrow R, Darefsky AS, Park Y, et al. Dietary components related to N-nitroso compound formation: a prospective study of adult glioma. Cancer Epidemiol Biomarkers Prev 2010;19:1709-22. [Crossref] [PubMed]
- Ghaffari HR, Yunesian M, Nabizadeh R, et al. Environmental etiology of gastric cancer in Iran: a systematic review focusing on drinking water, soil, food, radiation, and geographical conditions. Environ Sci Pollut Res Int 2019;26:10487-95. [Crossref] [PubMed]
- Epplein M, Shu XO, Xiang YB, et al. Fruit and vegetable consumption and risk of distal gastric cancer in the Shanghai Women's and Men's Health studies. Am J Epidemiol 2010;172:397-406. [Crossref] [PubMed]
- Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 2007;10:75-83. [Crossref] [PubMed]
- Carneiro F. Hereditary gastric cancer. Eur J Cancer 2018;92.
- Horii A, Nakatsuru S, Miyoshi Y, et al. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res 1992;52:3231-3. [PubMed]
- Compare D, Rocco A, Nardone G. Screening for and surveillance of gastric cancer. World J Gastroenterol 2014;20:13681-91. [Crossref] [PubMed]
- Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009;24:1587-600. [Crossref] [PubMed]
- Shiota S, Murakawi K, Suzuki R, et al. Helicobacter pylori infection in Japan. Expert Rev Gastroenterol Hepatol 2013;7:35-40. [Crossref] [PubMed]
- Siao D, Somsouk M. Helicobacter pylori: evidence-based review with a focus on immigrant populations. J Gen Intern Med 2014;29:520-8. [Crossref] [PubMed]
- Asaka M, Kato M, Takahashi S, et al. Japanese Society for Helicobacter Research.Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010;15:1-20.
- Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64. [Crossref] [PubMed]
- Roderick P, Davies R, Raftery J, et al. Cost-effectiveness of population screening for Helicobacter pylori in preventing gastric cancer and peptic ulcer disease, using simulation. J Med Screen 2003;10:148-56. [Crossref] [PubMed]
- Parsonnet J, Harris RA, Hack HM, et al. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet 1996;348:150-4. [Crossref] [PubMed]
- Lansdorp-Vogelaar I, Sharp L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract Res Clin Gastroenterol 2013;27:933-47. [Crossref] [PubMed]
- Abioye AI, Odesanya MO, Abioye AI, et al. Physical activity and risk of gastric cancer: a meta-analysis of observational studies. Br J Sports Med 2015;49:224-9. [Crossref] [PubMed]
- Kunzmann AT, Mallon KP, Hunter RF, et al. Physical activity, sedentary behaviour and risk of oesophago-gastric cancer: A prospective cohort study within UK Biobank. United European Gastroenterol J 2018;6:1144-54. [Crossref] [PubMed]
- Suzuki T, Kitagawa Y, Nankinzan R, et al. Early gastric cancer diagnostic ability of ultrathin endoscope loaded with laser light source. World J Gastroenterol 2019;25:1378-86. [Crossref] [PubMed]
- Hamashima C, Goto R. Potential capacity of endoscopic screening for gastric cancer in Japan. Cancer Sci 2017;108:101-7. [Crossref] [PubMed]
- Kula ZK, Zegarski W, Jóźwicki W. Diagnosis and treatment of early gastric cancer: experience of one center. Prz Gastroenterol 2018;13:200-5. [Crossref] [PubMed]
- Gupta N, Bansal A, Wani SB. Endoscopy for upper GI cancer screening in the general population: a cost-utility analysis. Gastrointest Endosc 2011;74:610-24.e2. [Crossref] [PubMed]
- Mori Y, Arita T, Shimoda K, et al. Effect of periodic endoscopy for gastric cancer on early detection and improvement of survival. Gastric Cancer 2001;4:132-6. [Crossref] [PubMed]
- Nam SY, Choi IJ, Park KW, et al. Effect of repeated endoscopic screening on the incidence and treatment of gastric cancer in health screenees. Eur J Gastroenterol Hepatol 2009;21:855-60. [Crossref] [PubMed]
- Whiting JL, Sigurdsson A, Rowlands DC, et al. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut 2002;50:378-81. [Crossref] [PubMed]
- Tava F, Luinetti O, Ghigna MR, et al. Type or extension of intestinal metaplasia and immature/atypical "indefinite-for-dysplasia" lesions as predictors of gastric neoplasia. Hum Pathol 2006;37:1489-97. [Crossref] [PubMed]
- Zullo A, Hassan C, Romiti A, et al. Follow-up of intestinal metaplasia in the stomach: when, how and why. World J Gastrointest Oncol 2012;4:30-6. [Crossref] [PubMed]
- Correa P, Piazuelo MB, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol 2010;105:493-8. [Crossref] [PubMed]


