
Is my life going to change?—a review of quality of life after rectal resection
Introduction
Rectal resection is a common procedure for colorectal surgeons. The most frequent causes of rectal resection are cancer, inflammatory bowel disease, endometriosis, and rectovaginal or rectourethral fistulas, among which, rectal cancer is the most common. The incidence of colorectal carcinoma is high in the western world, probably due to differences in environment and diet, where it is the second cause of cancer death and the fourth worldwide (1). It affects men and women almost equally. Approximately one million new cases and 250,000 deaths occur each year worldwide (2).
In the last decades, rectal surgery has radically changed with the development of surgical techniques, and it has progressed from abdominoperineal resection (APR) with a permanent colostomy to the total mesorectal excision (TME) and sphincter-saving surgery (3). The use of sphincter-preserving procedures has increased and includes anterior resection, low anterior resection (LAR), ultra-LAR and intersphincteric resection (ISR). A study from Abraham et al. (4) reported a 10% decrease in the use of APR over time from 1989 to 2001. All these surgical techniques can lead to important sequels that modify the quality of life (QoL) of patients. Historically, surgical outcomes, such as complications, survival and recurrences, have been widely studied by surgeons. In the present day, surgical outcomes have improved, rectal cancer recurrence rate has decreased and survival has increased. For these reasons, QoL aspects of patient outcome have become important. Since an increase in survival is expected in the future, physicians have to include QoL aspects to a greater extent into their treatment recommendations (5).
Symptoms reported after resection of the rectum are very varied and include pelvic pain, defecatory, sexual or urinary dysfunctions. Defecatory dysfunctions, which vary from daily episodes of incontinence to obstructed defecation and constipation, are reported in up to 90% of patients after LAR for rectal cancer (6). After treatment, up to 30–40% of survivors may discontinue sexual activity and high percentages ranging from 23–69% of men and from 19–62% of women may experience new sexual dysfunction (7-9). As a consequence, nowadays there is a trend towards organ preservation in rectal cancer whenever possible, including watch and wait strategies in case of complete response to neoadjuvant therapies (10).
The aim of our study is to review the current literature to determine to what degree the QoL of patients who underwent a rectal resection decreases, which domains are the most affected and, in addition, to establish the influence of different surgical techniques and approaches on functional outcomes.
Materials and methods
Literature was searched in Cochrane Library databases, MEDLINE, PubMed, and EMBASE using the following keywords: quality of life, HRQoL, health status, rectal cancer, rectal cancer surgery, abdominoperineal resection, anterior resection, sphincter-saving surgery, ultralow anterior resections, total mesorectal excision, transanal TME. Additional searches were developed through the terms: low anterior resection syndrome, bowel dysfunction, incontinence, fecal incontinence, functional outcome, stoma. papers which included only adult patients were selected. The website of the World Health Organization has also been consulted. The bibliographic search was carried out by a single researcher. The review of the selected documents and the inclusion decision was made by all the researchers.
Global QoL
In recent years, aspects of QoL have been gaining importance within Medicine. Nevertheless, there is no consensus on the definition of this concept. World Health Organization defines QoL as: “An individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns. It is a broad ranging concept affected in a complex way by the person’s physical health, psychological state, personal beliefs, social relationships and their relationship to salient features of their environment” (11). On the other hand, Koller et al. (12) define QoL as: “an individual sense of well being in the somatic, emotional and social domains”. Most studies measure the QoL using the Short Form 36 health survey and the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaires. Among which, the most frequent are quality-of-life questionnaire CR29, quality-of-life questionnaire C30 and quality-of-life questionnaire CR38 (13-16).
In general terms, no differences were found between rectal cancer patients and general population on global health status (17). It is striking that in some studies the perception of well-being and some subscales of instruments were even better with respect to normal data (17-19). The relatively high QoL might be explained by the fact that the measurement followed their earlier diagnosis of a life-threatening disease, which changed their perceptions of the length of life, thereby shifting their expectations and priorities regarding life fulfilment. Successful treatment therefore might result in a higher QoL as reported by the patient (17,20,21). This effect, known as Rejoice, has been noted from the beginning of QoL research (22). An additional contributing factor might be the adaptation of the patients to their morbidity over time, a phenomenon that is also referred to as coping or “response shift” (20). Adaptation is defined as a change in the meaning of a respondent’s self-evaluation of QoL that results from changes in his or her internal standards, values, or conceptualization of QoL (20,23). Another explanation is that traumatic events seem to produce positive effects, increasing life appreciation, improving relationships with family and friends, changing the life priorities, increasing spirituality, and feeling stronger and more compassionate (17). Although the global health score is not different between rectal cancer patients and general population, there are other QoL domains that are worse in patients with rectal cancer. Thus, these patients have lower social-, physical-, role- and cognitive functioning, and more diarrhea, constipation, insomnia and fatigue (17,24).
According to Choy et al. (25) QoL suffers an important reduction in the immediate postoperative period after pelvic exenteration, with a rapid improvement in the first 3 months after hospital discharge and later a slower improvement during the first year after surgery. This study describes that patients reach, a year after surgery, QoL scores similar to the preoperative ones. However, despite the general tendency to improvement with follow-up, a small reduction in overall functioning is still present even 14 years after treatment for rectal cancer (26).
On the other hand, several studies found that patients feel uncertain after surgery and would like to receive more information (27-29). Furthermore, some patients have excessive concern in a normal postoperative period. Nevertheless, other patients suffer many complaints that they consider normal. Both situations can cause an unnecessary stress that deteriorates QoL (29). A Swedish study confirmed that a large proportion of patients experienced negative intrusive thoughts and this produces a decrease in overall QoL (30). Also, Wrenn et al. (31) showed that QoL factors traditionally considered most important to surgeons, such as incision length, hospital length of stay, and use of laparoscopy, were not the most valued from the patient´s perspective. The aspects that mattered most to patients were whether they would be cured of cancer and avoiding a permanent stoma. For these reasons, preoperative and postoperative counseling is very important and providing proper information and details can reduce the patient’s anxiety, which will result in a better perception of QoL.
Regarding gender, Schmidt et al. (32) found differences between men and women. Specifically, women had worse pre- and postoperative functional status and more fatigue and sleep disturbance than men. Insomnia is a common problem among patients surviving cancer surgery because they have a constant fear of tumor recurrence (33). This aspect can be reduced by providing detailed information and adequate support to patients (34).
It is important to mention that preoperative radio(chemo)therapy negatively influence on functional outcomes and the adverse effects may occur with a latency of several months or even years. Accordingly, preoperative radio(chemo)therapy sequelae may not be displayed in studies with short follow-ups (35). Additionally, adjuvant chemotherapy also affects the QoL scores. Van der Valk et al. (36) described worse overall QoL, worse physical functioning, more fatigue and dyspnea for patients treated with adjuvant chemotherapy compared with those not receiving chemotherapy treatment. However, all these differences disappeared 12 months after surgery.
Bowel dysfunction
In rectal surgery, loss of the normal rectal reservoir function can lead to a postoperative defecation disorder named anterior resection syndrome (ARS), and may seriously affect QoL (37-39). Physical activities, work, hobbies, family and social activities can be affected by the symptoms of ARS (17). ARS has a negative impact on patient QoL in up to 80% and is frequently underestimated by surgeons (39). Symptoms of this syndrome include incontinence (3–79%), urgency (0–69%), pad usage (6–65%), clustering of bowel movement (6–88%), inability to differentiate gas from stool (2–62%), incomplete evacuation (2–85%), nocturnal bowel movement (14–27%) and 4+ bowel movement/day (10–60%) (40). ARS is reported in 50–90% of patients (41). Usually, bowel dysfunction stabilizes within the first 1–2 years after surgery (42). Long term follow-up studies showed that the symptoms present 1 year after surgery remain the same in the following years. It has been reported that 47.5% of patients still experience ARS symptoms at a follow-up period of 13.7 years (43,44). There are validated scales to measure the severity of ARS and with them it is possible to differentiate between major ARS, identified in 36.9% of patients, and minor ARS in 33.9% of patients (41). Table 1 shows the incidence of ARS. Some studies have found an association between bowel intestinal dysfunction and QoL. In them, there were differences between the major ARS and no ARS groups, and between the major ARS and minor ARS groups, but not between the no ARS and minor ARS groups (45).

Full table
Kornmann et al. (47) concluded that elderly females had worse QoL scores in terms of coping/behavior and depression/self-perception compared with males and younger females. Similarly, Sturiale et al. (43) analysis established that age is a risk factor for ARS in patients older than 65. Fecal incontinence is more frequent in females (48). This may be due to traumatic injuries to the anal sphincter complex and pelvic floor in vaginal deliveries. Also, the female gender has a predisposition for fecal incontinence, due to age-related changes in the function of the anal sphincter (47).
Another risk factor of anal dysfunctions is radiation therapy, administered pre- or postoperatively (46,49). Moreover, neoadjuvant treatment is a risk factor for ARS after long-term follow-up (43,49). These disturbances are due to fibrosis in the anal sphincters and possible adverse effects of radiotherapy on sacral nerves (49). Nevertheless, Pietrzak et al. (50) reported no significant difference between short-course radiotherapy and long-course chemoradiotherapy with regard to function after a median follow-up of 13 months.
The location of the rectal lesion determines a total (TME) or partial mesorectal excision (PME). After rectal resection, high or low anastomosis can be performed. TME has been shown to be a risk factor for ARS, which has consequences on the patient’s QoL (46). In the same way, a worse functional outcome in low anastomosis have been reported (51). The construction of neorectal reservoir during reconstruction in rectal surgery can improve functional outcomes (52-55). There are different reconstructive techniques such as colonic J pouch, side-to-end coloanal anastomosis or transverse coloplasty. However, recent studies showed that colonic J-pouch and side-to-end coloanal anastomosis or transverse coloplasty lead to a better functional outcome than straight coloanal anastomosis for the first year after surgery, but after 24 months the function is similar regardless of the type of reconstruction (49,56,57). On the other hand, anastomotic leakage is associated with increased morbidity and mortality, but the relationship of this complication with the presence of bowel dysfunction is controversial (46). Some studies have reported an increased risk dysfunction after anastomotic leakage (58), while others have not confirmed this fact (59,60).
Diverting stoma?
Performing a diverting stoma reduces the rate of reoperation after anastomotic leakage, but does not reduce its incidence (60). We must also take into account that a loop ileostomy can produce alteration of body anatomy, peristomal dermatitis, diarrhea, dehydration or psychological impact (61). According to the literature, a diverting stoma produces a reduction in QoL before reversal, with decreased social and physical function (61-63). Sometimes the ileostomy cannot be reversed, so it becomes a permanent stoma. Näsvall et al. (64) found a decrease in overall QoL, in physical role functioning and lower perception of body image in patients operated for rectal cancer with permanent stoma compared to patients without permanent stoma. Also, they reported more fatigue and loss of appetite in the stoma group.
Permanent stoma or sphincter preserving surgery?
Classically, the standard surgical technique for rectal cancer was the APR or the anterior resection with terminal colostomy. As a result, patients required permanent stomas. Nevertheless, in the last decades, the development of surgical techniques has allowed us to perform sphincter-preserving resection for rectal cancer, such as LAR or ISR. With these, rates of permanent stoma have decreased. Many studies have compared the QoL between APR and sphincter-preserving resection. Surprisingly, in most studies no differences in global QoL have been found between the two groups (20,65-68). However, Grumann et al. (69) and Feddern et al. (70) showed that LAR patients experienced a worse QoL than APR patients. In contrast, Engel et al. (71) and Du et al. (72) observed that APR patients had a lower QoL. Furthermore, Monastyrska et al. (73) described a significant difference in QoL assessment prior to and after surgery in each group, but 6 months after the procedure, no differences were found between both groups. Table 2 shows the QoL scores in patient groups (LAR and APR) one year after treatment.
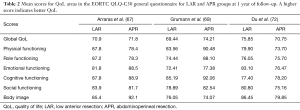
Full table
Some studies confirm worse physical function of patients after APR (65,73,74). On the other hand, there are studies found that patients after LAR scored higher in emotional and cognitive functioning (18,73), but other studies reported that patients undergoing LAR presented significantly worse cognitive and social function (75). We must take into account that patients after stoma formation may experience difficulties accepting their own bodies. There are studies that found a worse perception of body image in patients after APR compared with patients undergoing to sphincter-preserving procedures (20,66). Conversely, in other studies, the perception of body image of APR patients was similar to those of LAR patients (67,73).
Within sphincter-preserving procedures, one severe adverse effect is the intestinal disorders. Generally, a higher fecal incontinence scoring, frequent defecation and urgency have been described in the literature in patients undergoing LAR or ISR compared to patients after APR (65,67,73). Trenti et al. (66) reported that after sphincter saving procedure, 62% of the patients presented a major ARS that impaired global QoL. Moreover, patients with high anastomosis have a higher risk of developing ARS than patients with low anastomosis (65). These problems can be the explanation that the global QoL is not different between APR and LAR.
Open, laparoscopic, transanal or robotic?
Open surgery has been the conventional technique for the treatment of rectal cancer until the appearance of laparoscopic surgery. In comparison with open approach, minimally invasive techniques are associated with favorable short-term outcomes, such as reduced blood loss, reduced pain and shorter hospital stay (76). However, there are no differences regarding oncological outcomes or effects on QoL between either approach (77-79). Some studies showed that patients operated for rectal cancer with laparoscopic sphincter preservation were associated with a better QoL, fewer male sexual problems, better physical functioning, less micturition and gastrointestinal problems, when compared to the open approach in the first months after surgery, but the benefit disappeared after one year. In addition, transient benefits of QoL were reported, in terms of global health status, pain, and body imaging (80-85). In contrast, Jayne et al. (86) showed a higher rate of sexual dysfunction in laparoscopic surgery. On the other hand, the CLASICC trial (87) and Scarpa et al. (88) reported similar QoL results between laparoscopic and open surgery, particularly in the long-term follow-up. Some differences in QoL scores between laparoscopic and open approaches are presented in Table 3.
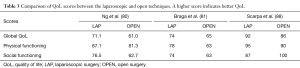
Full table
An innovative technique developed to reduce the unwanted effects of open surgery as well as improve the technical advantages of laparoscopic surgery is the robot, based on the use of instruments that allow 360º movement, tremor elimination and a three-dimensional vision. For these reasons, using this approach in the narrow pelvis could be beneficial, since a precise TME reduces local recurrence rates (89,90). Kamali et al. (33) showed less postoperative pain after robotic surgery compared to the laparoscopic approach despite having a shorter follow-up. A systematic review and meta-analysis by Broholm et al. (91) demonstrated a lower incidence of sexual dysfunction in-the robotic approach compared to laparoscopy. In the same way, Kim et al. (92) concluded that the robotic approach was associated with less impairment of urinary and sexual function, but the QoL was comparable in both groups.
Recently, transanal total mesorectal excision technique (TaTME) has appeared and some published studies have showed that it is a safe alternative to laparoscopic TME for middle and low rectal cancer. TaTME allows a precise dissection of the mesorectal plane, due to the improved vision by transanal approach, which entails potential short-term clinical advantages, such as lower conversion rate, lower leak rate, and slightly lower short-term morbidity (93-95). Literature regarding QoL in TaTME procedures is scarce. A comparative study between laparoscopic surgery and transanal approach, showed a significant difference between the two groups in terms of fecal incontinence scored by a single item regarding leakage of stools, favoring the laparoscopic group. Moreover, there were significant differences in QoL favoring the laparoscopic approach to TME in terms of role functioning, fatigue, and financial difficulties (96). However, Pontallier et al. (97) showed better erectile function in the transanal group compared to conventional laparoscopy. Similarly, in the study by Bjoern et al. (98), TaTME had better scores on the reported QoL, related to urinary symptoms. We need further prospective studies to establish the potential impact of TaTME procedures in QoL.
Urinary dysfunction
Usually, urinary problems after rectal resection are less frequent and less severe than bowel dysfunction, but its presence can deteriorate patient’s QoL. Wani et al. (20) reported that 19% of patients suffered from post-operative urinary dysfunction and were more frequently observed after APR than after LAR. Nevertheless, other studies did not find differences between surgical techniques (63,65,99). Radiotherapy, tumor size, intra-abdominal sepsis, and age older than 65 years have been associated with voiding dysfunction disorders after rectal cancer excision (100-102). Moreover, in the surgical procedure the pelvic floor innervation can be injured, which can produce micturition dysfunction. An increasing risk has also been detected with preoperative blood loss, preoperative difficulty in bladder emptying and autonomic nerve damage (103,104).
In some studies, a higher incidence of micturition problems was observed after APR than LAR (20). In contrast, other reports observed that urinary disturbances were comparable in the two groups (65).
Sexual dysfunction
The term sexual dysfunction refers to a set of symptoms, among which are included impotency, inability to ejaculate, erectile dysfunction, lack of sexual desire or dyspareunia, and are frequently reported in rectal cancer patients (24,63,105). These problems appear after rectal resection in 11% to 27% of patients. Young patients are more affected by sexual problems than the elderly (105,106). Thyø et al. (105) have found differences in overall QoL between patients with sexual dysfunction and patients without sexual dysfunction, comparing the score with EORTC QoL data. The sexual impairment may be due to autonomic pelvic nerve injury or, indirectly, by vascular damage, produced by radiotherapy or by surgery. Further, psychological factors can also influence in these disorders (17,24,63). In the same way, it has been reported that female sexual function and capability to become sexually aroused is complex and can easily be inhibited by negative influences such as pain (105). Konanz et al. (65) have found a markedly worse sexual function in patients after APR, compared to after ISR or LAR. For this reason, a good strategy could be reversing a diverting stoma as soon as possible. A study published in 2017 indicated that a low coloanal anastomosis might cause more sexual problems with negative impact on sexual functioning according to the EORTC quality-of-life questionnaire CR38 scores in the group without a stoma, but also the appearance of a parastomal hernia or bulge around the stoma significantly impaired sexual functioning and enjoyment (64).
Conclusions and future perspectives
Patients undergoing rectal resection, particularly those with neoadjuvant treatment, may see their QoL affected. For this reason, patients should be informed of the treatment benefits and risk of postoperative dysfunctions. In the same way, treatment decisions must be based on both the patient preferences and clinical judgment. The management of these patients should be multidisciplinary to ensure that after treatment they should have an optimal QoL.
In the next years, this field of study would benefit from: an increase in the number of methodologically studies comparing patients with the general population at multiple assessment times; the development of instruments that are able to seize the specific symptomatology of rectal cancer and to assess its impact of patients’ QoL; and widening the collection of reference data for generic questionnaires as well as starting to collect normative data for specific questionnaires, reporting details about the sample drawn from the general population.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. [Crossref] [PubMed]
- Ferlay J, Bray F, Pisani P. GLOBOCAN 2000: Cancer incidence, mortality and prevalence worldwide. version 1.0. IARC CancerBase No. 5. Lyon: IARC Press, 2001.
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [Crossref] [PubMed]
- Abraham NS, Davila JA, Rabeneck L, et al. Increased use of low anterior resection for veterans with rectal cancer. Aliment Pharmacol Ther 2005;21:35-41. [Crossref] [PubMed]
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 2014;64:252-71. [Crossref] [PubMed]
- Bryant CLC, Lunniss PJ, Knowles CH, et al. Anterior resection syndrome. Lancet Oncol 2012;13:e403-8. [Crossref] [PubMed]
- Hendren SK, O'Connor BI, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg 2005;242:212-23. [Crossref] [PubMed]
- Denlinger CS, Barsevick AM. The challenges of colorectal cancer survivorship. J Natl Compr Canc Netw 2009;7:883-93. [Crossref] [PubMed]
- Ho VP, Lee Y, Stein SL, et al. Sexual function after treatment for rectal cancer: a review. Dis Colon Rectum 2011;54:113-25. [Crossref] [PubMed]
- Borstlap WAA, van Oostendorp SE, Klaver CEL, et al. Organ preservation in rectal cancer: a synopsis of current guidelines. Colorectal Dis 2017;20:201-10. [Crossref] [PubMed]
- The WHOQOL Group. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 1995;41:1403-9. [Crossref] [PubMed]
- Koller M, Kussman J, Lorenz W, et al. Symptom reporting in cancer patients: the role of negative affect and experienced social stigma. Cancer 1996;77:983-95. [Crossref] [PubMed]
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. [Crossref] [PubMed]
- Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer 2009;45:3017-26. [Crossref] [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [Crossref] [PubMed]
- Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 1999;35:238-47. [Crossref] [PubMed]
- Giandomenico F, Gavaruzzi T, Lotto L, et al. Quality of life after surgery for rectal cancer: a systematic review of comparisons with the general population. Expert Rev Gastroenterol Hepatol 2015;9:1227-42. [Crossref] [PubMed]
- Orsini RG, Thong MS, van de Poll-Franse LV, et al. Quality of life of older rectal cancer patients is not impaired by a permanent stoma. Eur J Surg Oncol 2013;39:164-70. [Crossref] [PubMed]
- Serpentini S, Del Bianco P, Alducci E, et al. Psychological well-being outcomes in disease-free survivors of mid-low rectal cancer following curative surgery. Psychooncology 2011;20:706-14. [Crossref] [PubMed]
- Wani RA, Bhat IU, Parray FQ, et al. Quality of Life After "Total Mesorectal Excision (TME)" for Rectal Carcinoma: a Study from a Tertiary Care Hospital in Northern India. Indian J Surg Oncol 2017;8:499-505. [Crossref] [PubMed]
- Gosselink MP, Busschbach JJ, Dijkhuis CM, et al. Quality of life after total mesorectal excision for rectal cancer. Colorectal Dis 2006;8:15-22. [Crossref] [PubMed]
- Nord E. The significance of contextual factors in valuing health states. Health Policy 1989;13:189-98. [Crossref] [PubMed]
- Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med 1999;48:1531-48. [Crossref] [PubMed]
- Couwenberg AM, Burbach JPM, van Grevenstein WMU, et al. Effect of Neoadjuvant Therapy and Rectal Surgery on Health-related Quality of Life in Patients With Rectal Cancer During the First 2 Years After Diagnosis. Clin Colorectal Cancer 2018;17:e499-e512. [Crossref] [PubMed]
- Choy I, Young JM, Badgery-Parker T, et al. Baseline quality of life predicts pelvic exenteration outcome. ANZ J Surg 2017;87:935-9. [Crossref] [PubMed]
- Wiltink LM, Chen TY, Nout RA, et al. Health-related quality of life 14 years after preoperative short-term radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomised trial. Eur J Cancer 2014;50:2390-8. [Crossref] [PubMed]
- Lamers RE, Cuypers M, Husson O, et al. Patients are dissatisfied with information provision: perceived information provision and quality of life in prostate cancer patients. Psychooncology 2016;25:633-40. [Crossref] [PubMed]
- Galloway SC, Graydon JE. Uncertainty, symptom distress, and information needs after surgery for cancer of the colon. Cancer Nurs 1996;19:112-7. [Crossref] [PubMed]
- van der Heijden JAG, Thomas G, Caers F, et al. What you should know about the low anterior resection syndrome-Clinical recommendations from a patient perspective. Eur J Surg Oncol 2018;44:1331-7. [Crossref] [PubMed]
- Prytz M, Ledebo A, Angenete E, et al. Association between operative technique and intrusive thoughts on health-related Quality of Life 3 years after APE/ELAPE for rectal cancer: results from a national Swedish cohort with comparison with normative Swedish data. Cancer Med 2018;7:2727-35. [Crossref] [PubMed]
- Wrenn SM, Cepeda-Benito A, Ramos-Valadez DI, et al. Patient Perceptions and Quality of Life After Colon and Rectal Surgery: What Do Patients Really Want? Dis Colon Rectum 2018;61:971-8. [PubMed]
- Schmidt C, Daun A, Malchow B, et al. Sexual Impairment and its Effect on Quality of Life in Patients with Rectal cancer. Dtsch Arztebl Int 2010;107:123-30. [PubMed]
- Kamali D, Omar K, Imam SZ, et al. Patient quality of life and short-term surgical outcomes between robotic and laparoscopic anterior resection for adenocarcinoma of the rectum. Tech Coloproctol 2017;21:355-61. [Crossref] [PubMed]
- Hartford K, Wong C, Zakaria D. Randomized controlled trial of a telephone intervention by nurses to provide information and support to patients and their partners after elective coronary artery bypass graft surgery: effects of anxiety. Heart Lung 2002;31:199-206. [Crossref] [PubMed]
- Loos M, Quentmeier P, Schuster T, et al. Effect of preoperative radio(chemo)therapy on long-term functional outcome in rectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:1816-28. [Crossref] [PubMed]
- van der Valk MJM, Hilling DE, Meershoek-Klein Kranenbarg E, et al. Quality of Life After Curative Resection for Rectal Cancer in Patients Treated With Adjuvant Chemotherapy Compared With Observation: Results of the Randomized Phase III SCRIPT Trial. Dis Colon Rectum 2019;62:711-20. [Crossref] [PubMed]
- Pucciani F. A review on functional results of sphincter-saving surgery for rectal cancer: the anterior resection syndrome. Updates Surg 2013;65:257-63. [Crossref] [PubMed]
- Chapman SJ, Bolton WS, Corrigan N, et al. A Cross-Sectional Review of Reporting Variation in Postoperative Bowel Dysfunction After Rectal Cancer Surgery. Dis Colon Rectum 2017;60:240-7. [Crossref] [PubMed]
- Bohlok A, Mercier C, Bouazza F, et al. The burden of low anterior resection syndrome on quality of life in patients with mid or low rectal cancer. Support Care Cancer 2020;28:1199-206. [PubMed]
- Scheer AS, Boushey RP, Liang S, et al. The Long-term Gastrointestinal Functional Outcomes Following Curative Anterior Resection in Adults With Rectal Cancer: A Systematic Review and Meta-analysis. Dis Colon Rectum 2011;54:1589-97. [Crossref] [PubMed]
- Ihnát P, Vávra P, Prokop J, et al. Functional outcome of low rectal resection evaluated by anorectal manometry. ANZ J Surg 2018;88:E512-E516. [Crossref] [PubMed]
- Ribas Y, Aguilar F, Jovell-Fernández E, et al. Clinical application of the LARS score: results from a pilot study. Int J Colorectal Dis 2017;32:409-18. [Crossref] [PubMed]
- Sturiale A, Martellucci J, Zurli L, et al. Long-term functional follow-up after anterior rectal resection for cancer. Int J Colorectal Dis 2017;32:83-8. [Crossref] [PubMed]
- Pieniowski EHA, Palmer GJ, Juul T, et al. Low Anterior Resection Syndrome and Quality of Life After Sphincter-Sparing Rectal Cancer Surgery: A Long-term Longitudinal Follow-up. Dis Colon Rectum 2019;62:14-20. [Crossref] [PubMed]
- Chen TY, Wiltink LM, Nout RA, et al. Bowel Function 14 Years After Preoperative Short-Course Radiotherapy and Total Mesorectal Excision for Rectal Cancer: Report of a Multicenter Randomized Trial. Clin Colorectal Cancer 2015;14:106-14. [Crossref] [PubMed]
- Bregendahl S, Emmertsen KJ, Lous J, et al. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis 2013;15:1130-9. [PubMed]
- Kornmann VN, Walma MS, de Roos MA, et al. Quality of Life After a Low Anterior Resection for Rectal Cancer in Elderly Patients. Ann Coloproctol 2016;32:27-32. [Crossref] [PubMed]
- Andromanakos N, Filippou D, Skandalakis P, et al. Anorectal incontinence. Pathogenesis and choice of treatment. J Gastrointestin Liver Dis 2006;15:41-9. [PubMed]
- Badic B, Joumond A, Thereaux J, et al. Long-term functional and oncological results after sphincter-saving resection for rectal cancer - Cohort study. Int J Surg 2018;52:1-6. [Crossref] [PubMed]
- Pietrzak L, Bujko K, Nowacki MP, et al. Quality of life, anorectal and sexual functions after preoperative radiotherapy for rectal cancer: report of a randomised trial. Radiother Oncol 2007;84:217-25. [Crossref] [PubMed]
- Bretagnol F, Troubat H, Laurent C, et al. Long-term functional results after sphincter-saving resection for rectal cancer. Gastroenterol Clin Biol 2004;28:155-9. [Crossref] [PubMed]
- Tsang WW, Chung CC, Li MK. Prospective evaluation of laparoscopic total mesorectal excision with colonic J-pouch reconstruction for mid and low rectal cancers. Br J Surg 2003;90:867-71. [Crossref] [PubMed]
- Hüttner FJ, Tenckhoff S, Jensen K, et al. Meta-analysis of reconstruction techniques after low anterior resection for rectal cancer. Br J Surg 2015;102:735-45. [Crossref] [PubMed]
- Doeksen A, Bakx R, Vincent A, et al. J-pouch vs side-to-end coloanal anastomosis after preoperative radiotherapy and total mesorectal excision for rectal cancer: a multicentre randomized trial. Colorectal Dis 2012;14:705-13. [Crossref] [PubMed]
- Okkabaz N, Haksal M, Atici AE, et al. J-pouch vs. side-to-end anastomosis after hand-assisted laparoscopic low anterior resection for rectal cancer: A prospective randomized trial on short and long term outcomes including life quality and functional results. Int J Surg 2017;47:4-12. [Crossref] [PubMed]
- Keane C, Wells C, O’Grady G, et al. Defining low anterior resection syndrome: a systematic review of the literature. Colorectal Dis 2017;19:713-22. [Crossref] [PubMed]
- Mege D, Meurette G, Vitton V, et al. Sacral nerve stimulation can alleviate symptoms of bowel dysfunction after colorectal resections. Colorectal Dis 2017;19:756-63. [Crossref] [PubMed]
- Nesbakken A, Nygaard K, Lunde OC. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br J Surg 2001;88:400-4. [Crossref] [PubMed]
- Bittorf B, Stadelmaier U, Merkel S, et al. Does anastomotic leakage affect functional outcome after rectal resection for cancer? Langenbecks Arch Surg 2003;387:406-10. [Crossref] [PubMed]
- Miura T, Sakamoto Y, Morohashi H, et al. Risk factor for permanent stoma and incontinence quality of life after sphincter-preserving surgery for low rectal cancer without a diverting stoma. Ann Gastroenterol Surg 2017;2:79-86. [Crossref] [PubMed]
- De Palma GD, Luglio G. Quality of life in rectal cancer surgery: What do the patient ask? World J Gastrointest Surg 2015;7:349-55. [Crossref] [PubMed]
- O’Leary DP, Fide CJ, Foy C, et al. Quality of life after low anterior resection with total mesorectal excision and temporary loop ileostomy for rectal carcinoma. Br J Surg 2001;88:1216-20. [Crossref] [PubMed]
- Tsunoda A, Tsunoda Y, Narita K, et al. Quality of life after low anterior resection and temporary loop ileostomy. Dis Colon Rectum 2008;51:218-22. [Crossref] [PubMed]
- Näsvall P, Dahlstrand U, Löwenmark T, et al. Quality of life in patients with a permanent stoma after rectal cancer surgery. Qual Life Res 2017;26:55-64. [Crossref] [PubMed]
- Konanz J, Herrle F, Weiss C, et al. Quality of life of patients after low anterior, intersphincteric, and abdominoperineal resection for rectal cancer—a matched-pair analysis. Int J Colorectal Dis 2013;28:679-88. [Crossref] [PubMed]
- Trenti L, Galvez A, Biondo S, et al. Quality of life and anterior resection syndrome after surgery for mid to low rectal cancer: a cross-sectional study. Eur J Surg Oncol 2018;44:1031-9. [Crossref] [PubMed]
- Arraras JI, Suárez J, Arias-de-la-Vega F, et al. Quality of life assessment by applying EORTC questionnaires to rectal cancer patients after surgery and neoadjuvant and adjuvant treatment. Rev Esp Enferm Dig 2013;105:255-61. [Crossref] [PubMed]
- Bong JW, Lim SB, Lee JL, et al. Comparison of Anthropometric Parameters after Ultralow Anterior Resection and Abdominoperineal Resection in Very Low-Lying Rectal Cancers. Gastroenterol Res Pract 2018. [Crossref] [PubMed]
- Grumann MM, Noack EM, Hoffmann IA, et al. Comparison of quality of life in patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg 2001;233:149-56. [Crossref] [PubMed]
- Feddern ML, Emmertsen KJ, Laurberg S. Quality of life with or without sphincter preservation for rectal cancer. Colorectal Dis 2019;21:1051-7. [Crossref] [PubMed]
- Engel J, Kerr J, Schlesinger-Raab A, et al. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg 2003;238:203-13. [Crossref] [PubMed]
- Du P, Wang SY, Zheng P, et al. Comparison of overall survival and quality of life between patients undergoing analreconstruction and patients undergoing traditional lower abdominal stoma after radical resection. Clin Transl Oncol 2019;21:1390-7. [Crossref] [PubMed]
- Monastyrska E, Hagner W, Jankowski M, et al. Prospective assessment of the quality of life in patients treated surgically for rectal cancer with lower anterior resection and abdominoperineal resection. Eur J Surg Oncol 2016;42:1647-53. [Crossref] [PubMed]
- Kasparek MS, Hassan I, Cima RR, et al. Quality of life after coloanal anastomosis and abdominoperineal resection for distal rectal cancers: sphincter preservation vs quality of life. Colorectal Dis 2011;13:872-7. [Crossref] [PubMed]
- Reinwalds M, Blixter A, Carlsson E. Living with a resected rectum after rectal cancer surgery-Struggling not to let bowel function control life. J Clin Nurs 2018;27:e623-34. [Crossref] [PubMed]
- Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477-84. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: the ACOSOG Z6051 Randomised Clinical Trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: the ALaCaRT randomized clinical trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Bartels SA, Vlug MS, Ubbink DT, et al. Quality of life after laparoscopic and open colorectal surgery: a systematic review. World J Gastroenterol 2010;16:5035-41. [Crossref] [PubMed]
- Ng SS, Leung WW, Wong CY, et al. Quality of life after laparoscopic vs open sphincter-preserving resection for rectal cancer. World J Gastroenterol 2013;19:4764-73. [Crossref] [PubMed]
- Braga M, Frasson M, Vignali A, et al. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum 2007;50:464-71. [Crossref] [PubMed]
- Li J, Chen R, Xu YQ, et al. Impact of a laparoscopic resection on the quality of life in rectal cancer patients: results of 135 patients. Surg Today 2010;40:917-22. [Crossref] [PubMed]
- Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. [Crossref] [PubMed]
- Yang L, Yu YY, Zhou ZG, et al. Quality of life outcomes following laparoscopic total mesorectal excision for low rectal cancers: a clinical control study. Eur J Surg Oncol 2007;33:575-9. [Crossref] [PubMed]
- Asoglu O, Matlim T, Karanlik H, et al. Impact of laparoscopic surgery on bladder and sexual function after total mesorectal excision for rectal cancer. Surg Endosc 2009;23:296-303. [Crossref] [PubMed]
- Jayne DG, Brown JM, Thorpe H, et al. Bladder and sexual function following resection for rectal cancer in a randomized clinical trial of laparoscopic versus open technique. Br J Surg 2005;92:1124-32. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomized controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Scarpa M, Erroi F, Ruffolo C, et al. Minimally invasive surgery for colorectal cancer: quality of life, body image, cosmesis, and functional results. Surg Endosc 2009;23:577-82. [Crossref] [PubMed]
- Ng KH, Lim YK, Ho KS, et al. Robotic-assisted surgery for low rectal dissection: from better views to better outcome. Singapore Med J 2009;50:763-7. [PubMed]
- Wibe A, Syse A, Andersen E, et al. Norwegian Rectal Cancer Group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs. abdominoperineal resection. Dis Colon Rectum 2004;47:48-58. [Crossref] [PubMed]
- Broholm M, Pommergaard HC, Gögenür I. Possible benefits of robot-assisted rectal cancer surgery regarding urological and sexual dysfunction: a systematic review and meta-analysis. Colorectal Dis 2015;17:375-81. [Crossref] [PubMed]
- Kim HJ, Choi GS, Park JS, et al. The impact of robotic surgery on quality of life, urinary and sexual function following total mesorectal excision for rectal cancer: a propensity score-matched analysis with laparoscopic surgery. Colorectal Dis 2018;20:O103-13. [Crossref] [PubMed]
- Velthuis S, Nieuwenhuis DH, Ruijter TEG, et al. Transanal versus traditional laparoscopic total mesorectal excision for rectal carcinoma. Surg Endosc 2014;28:3494-9. [Crossref] [PubMed]
- Veltcamp Helbach M, Deijen C, Velthuis S, et al. Transanal total mesorectal excision for rectal carcinoma. Short-term outcomes and experience after 80 cases. Surg Endosc 2016;30:464-70. [Crossref] [PubMed]
- Deijen CL, Tsai A, Koedam TWA, et al. Clinical outcomes and case volume effect of transanal total mesorectal excision for rectal cancer: a systematic review. Tech Coloproctol 2016;20:811-24. [Crossref] [PubMed]
- Veltcamp Helbach M, Koedam TWA, Knol JJ, et al. Quality of life after rectal cancer surgery: differences between laparoscopic and transanal total mesorectal excision. Surg Endosc 2019;33:79-87. [Crossref] [PubMed]
- Pontallier A, Denost Q, Van Geluwe B, et al. Potential sexual function improvement by using transanal mesorectal approach for laparoscopic low rectal cancer excision. Surg Endosc 2016;30:4924-33. [Crossref] [PubMed]
- Bjoern MX, Nielsen S, Perdawood SK. Quality of Life After Surgery for Rectal Cancer: a Comparison of Functional Outcomes After Transanal and Laparoscopic Approaches. J Gastrointest Surg 2019;23:1623-30. [Crossref] [PubMed]
- Ito N, Ishiguro M, Tanaka M, et al. Response shift in quality-of-life assessment in patients undergoing curative surgery with permanent colostomy: a preliminary study. Gastroenterol Nurs 2010;33:408-12. [Crossref] [PubMed]
- Bregendahl S, Emmertsen KJ, Lindegaard JC, et al. Urinary and sexual dysfunction in women after resection with and without preoperative radiotherapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis 2015;17:26-37. [Crossref] [PubMed]
- Kim NK, Aahn TW, Park JK, et al. Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum 2002;45:1178-85. [Crossref] [PubMed]
- Tekkis PP, Cornish JA, Remzi FH, et al. Measuring Sexual and Urinary Outcomes in Women after Rectal Cancer Excision. Dis Colon Rectum 2009;52:46-54. [Crossref] [PubMed]
- Lange MM, Van De Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment Cancer treatment. Nat Rev Urol 2011;8:51-7. [Crossref] [PubMed]
- Lange MM, Maas CP, Marijnen CAM, et al. Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br J Surg 2008;95:1020-8. [Crossref] [PubMed]
- Thyø A, Emmertsen KL, Laurberg S. The Rectal Cancer Female Sexuality Score: Development and Validation of a Scoring System for Female Sexual Function After Rectal Cancer Surgery. Dis Colon Rectum 2018;61:656-66. [Crossref] [PubMed]
- Scheele J, Lemke J, Meier M, et al. Quality of Life After Sphincter-Preserving Rectal Cancer Resection. Clin Colorectal Cancer 2015;14:e33-40. [Crossref] [PubMed]


