
Hepatoid adenocarcinoma of the peritoneal cavity: Prolonged survival after debulking surgery and 5-fluorouracil, leucovorin and oxaliplatin (FOLFOX) therapy
Introduction
Hepatoid adenocarcinoma is an alpha-fetoprotein-producing carcinoma arising in extra-hepatic organs, with morphologic similarity to hepatocellular carcinoma. In this report, we describe the case of a young female with a peritoneal mass and the diagnostic and management dilemma associated with hepatoid adenocarcinoma.
Case report
The patient is a 44-year-old female who presented at the age of 42 years in April 2008 with right lower quadrant pain, gradually increasing over a few months prior to evaluation. Work up included a CT scan of the abdomen and pelvis, which revealed a 6.4 cm × 7.0 cm heterogeneous mass in the right lower quadrant (RLQ) interposed between the right lateral abdominal wall and the wall of the ascending colon (Figure 1A). The colon itself and the liver appeared unremarkable, and minimal free fluid in the cul-de-sac was noted. A CT guided biopsy of the mass showed a moderately differentiated adenocarcinoma of unknown origin. She underwent an extensive gynecologic work up, colonoscopy and upper endoscopy, all of which were negative. The patient was taken for an exploratory laparotomy in June 2008 that showed a 7 cm well-encapsulated heterogenous tumor in the right paracolic gutter invading into the retroperitoneum and involving the abdominal wall. The liver and the gallbladder appeared normal with the exception of a 1.5 cm × 1.0 cm calcified lymph node seen on the undersurface of the liver, at the base of the gallbladder. This was removed and pathology showed non-small cell carcinoma with hepatoid features. Intraoperative gynecologic and urologic consults were done, which ruled out involvement of these organ systems.
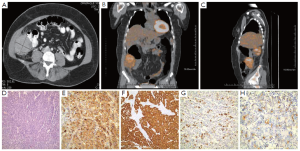
She subsequently underwent a PET scan in July 2008, which revealed mild uptake in the mass, and no metastatic lesions (Figure 1B, C). The patient still had significant right lower quadrant discomfort, anorexia and weight loss. Decision was made to go ahead with surgical resection of the RLQ mass, mostly to obtain more pathologic information, but also to provide some therapeutic benefit. Pre-operative blood work revealed a high alpha-fetoprotein (AFP) of 62,409 ng/mL and normal CEA of 0.8 ng/mL, CA-125 of 19 U/mL, CA 19-9 of 8 and β-HCG less than 5 mIU/mL. In August 2008, she underwent repeat laparotomy exposing the tumor that extended into the right abdominal wall. It appeared to be intimately attached to the ascending colon and the cecum although not directly invading the colon. A right hemicolectomy was done and the posterior extension of the mass was identified. It did not involve the ureter or the kidney, and the mass was dissected off the retroperitoneum. Pathology showed a 7.5 cm high-grade adenocarcinoma with hepatoid differentiation. Additional smaller satellite foci were identified microscopically. The tumor was composed of medium-sized polygonal cells with granular eosinophilic cytoplasm and frequent mitosis, arranged in a trabecular pattern resembling hepatocellular carcinoma type (hepatoid) morphology (Figure 1D). The right colon showed some adhesions on the serosal surface but no evidence of tumor. The appendix was negative for tumor. The tumor invaded the posterior fibroadipose tissue but the excised skeletal muscle was negative. The morphological differential diagnosis included metastatic hepatocellular carcinoma, hepatoid adenocarcinoma or hepatoid germ cell tumor. Immunohistochemical stains were performed and showed diffuse 3+ AFP staining (Figure 1E), diffuse 3+ CAM 5.2 staining (Figure 1F), patchy 3+ HepPar staining (Figure 1G) and luminal/focal canalicular polyclonal CEA staining (Figure 1H) along with positive CK20 and CK7. Germ cell markers CD30, OCT 3/4 and PLAP (Placental alkaline phosphate) were negative. Ten mesenteric lymph nodes were negative for cancer. The gallbladder fossa nodule showed similar histopathological features.
Post-operatively, serum AFP level decreased but was still elevated at 755 ng/mL at 1 month. Given the presence of two sites of disease, high grade of the tumor and persistence of elevated AFP post surgery, she appeared to be at the high risk of recurrence. Acknowledging the paucity of data on adjuvant systemic therapy for this tumor, chemotherapy with 5-Fluorouracil, leucovorin and oxaliplatin (FOLFOX) was considered based on the close relationship of the tumor with the colon. Adjuvant therapy with FOLFOX was given every 2 weeks for 12 cycles for 6 months (October 2008-April 2009). The AFP level showed a gradual decline from 92 ng/mL in September 2008, 51 ng/mL in October 2008, 10 ng/mL in November 2008, 2.8 ng/mL in January 2009 and is still within normal limits until her last visit in November 2011 (6 ng/mL). Repeat annual CT scans have shown no evidence of disease recurrence.
Discussion
Hepatoid adenocarcinoma (HAC) is a rare variant of adenocarcinoma. The first case of HAC was an alpha-fetoprotein (AFP)-producing gastric carcinoma reported in 1985. It was described as having foci of both adenocarcinomatous and hepatocellular differentiation (1). HAC arises from the mucosa of endodermal and urogenital organs. Thus, numerous cases of carcinomas with hepatoid differentiation have been reported in a variety of primary organs including the gastrointestinal tract, ovary, pancreas, lung, kidney, uterus, and urinary bladder; the stomach being the most common site (2).
Review of literature revealed that this may be the 6th case of hepatoid adenocarcinoma involving the peritoneum (3-7). It is interesting to note that the peritoneum is of a different embryologic origin from the liver. Three cases presented as large masses (3-5) and 2 cases presented as diffuse peritoneal nodules (6,7). The former patients complained of abdominal pain (3,4) and thigh pain (5), while the diffuse peritoneal HAC cases had massive ascites on admission (6,7). Actually, in one case of diffuse peritoneal HAC, the authors were undecided if the diagnosis was primary peritoneal HAC or hepatoid yolk sac tumor (7).
The diagnosis based on clinical and histologic characteristics can be difficult as in our patient whose initial results revealed undifferentiated adenocarcinoma. A careful search for a primary malignancy is necessary, as the peritoneum is a common site of metastases for abdominal and pelvic tumors. A thorough work-up in our patient did not reveal any primary malignancy that could account for the peritoneal mass. Our patient appeared to have an intraperitoneal hepatoid adenocarcinoma with a retroperitoneal component and metastasis in the gallbladder fossa (an uncommon site for metastasis), or that there were two distinct hepatoid adenocarcinomas.
Hepatoid adenocarcinoma morphologically resembles hepatocellular carcinoma (HCC), hence the name, in terms of their expansive tumor growth that is composed of large eosinophilic or clear cells, in a sheet-like or trabecular pattern with sinusoidal vascular channels (5). Differentiating HAC from HCC can be particularly challenging especially as many of them can present with liver metastases. Like HCC, they are immunoreactive with alpha-fetoprotein (AFP), polyclonal CEA (canalicular pattern), CK8 and CK18 (8).
Serum alpha-fetoprotein (AFP) was markedly elevated in our patient and AFP was also detected in the cytoplasm by immunohistochemical staining. However, AFP is not unique to HAC and is more commonly found in hepatocellular carcinoma, cholangiocarcinoma and teratomatous germ cell tumors (2). Therefore, other immunohistochemical stains are necessary.
Monoclonal antibody HepPar1 expression seems to be restricted to normal and neoplastic liver cells and is more sensitive than AFP. HepPar1 reactivity has been demonstrated consistently in hepatocellular carcinomas, hepatoblastomas and hepatoid adenocarcinoma, but only rarely in cholangiocarcinomas and metastatic tumors to the liver. Maitra, et al. studied 7 hepatoid adenocarcinomas of the gastrointestinal tract (6 gastric and 1 from the gallbladder) for HepPar1 immunoreactivity. Focal HepPar 1 expression was seen in 6 of 7 tumors (9). On the other hand, Terracciano, et al. reviewed the immunohistochemical characteristics of 8 cases of HAC with liver metastases compared to hepatocellular carcinoma. They found that HepPar1 was negative in all but one HAC case, concluding that diffuse positivity for HepPar1 is more consistent with HCC than HAC and may be related to incomplete hepatocellular differentiation of HAC (8).
Immunostaining for cytokeratins are helpful in defining HAC. CK8 (CAM 5.2) and CK18, markers of simple parenchyma, are positive in hepatocytes in HCC and HAC (5). CK19 and CK20 are positive in 94% and 47% of HAC, respectively (8) while these were negative in HCC in a study by Maeda, et al. (9) and positive in 8.2% and 1.6% in Terraciano, et al.'s report (8). Staining for CK7 can be positive (4,10) or negative (8,11).
Since HAC are a very heterogeneous group of tumors, no standard treatment exists. HAC are treated like adenocarcinomas of the common type descending from the involved organ system. Patients with localized tumors underwent surgery (12). In two cases of primary peritoneal HAC, excision or debulking of the tumor was not done because the tumor was unresectable (4) and the patient refused (5). Both patients received sorafenib and had an initial partial response. One patient died 6 months after diagnosis from progression of disease (4) and the other patient was lost to follow up after 7 months (5).
Sorafenib, the current reference standard systemic treatment of HCC, inhibits multiple signaling kinases including Raf family members, platelet-derived growth factor receptor, vascular endothelial growth factor receptors 1 and 2, c-Kit, and Fms-like tyrosine kinase 3. Clinical studies involving large numbers of patients demonstrated clear improvements in the overall survival of patients with unresectable HCC following treatment with sorafenib (13). It has been used with some success in HAC. Karayiannakis, et al. reported an overall survival of 20 months in a 60-year old female with HAC of the gallbladder treated with surgery followed by sorafenib for 15 months until her disease progressed (11). Petrelli, et al. reported a 37 year old male with metastatic pancreatic hepatoid adenocarcinoma who had more than 7 months of progression-free survival on sorafenib. Treatment was discontinued after 8 months when patient developed severe hyperbilirubinemia. The patient died 1 year after diagnosis (14).
In the case of diffuse peritoneal HAC, cisplatin was administered intraperitoneally to relieve the patient's abdominal pain and bloating. The patient died 6 months after diagnosis (6). In the more complicated case of peritoneal HAC versus hepatoid yolk sac tumor, debulking was done and the patient improved clinically after receiving 6 cycles of carboplatin and etoposide (7).
Our patient underwent chemotherapy with FOLFOX after resection of the tumor. FOLFOX was chosen because, although the tumor was peritoneal, it was closely related to the colon, suggesting possible colonic origin. However, we have to admit that the absence of mucosal abnormalities on colonoscopy makes this theory less probable. FOLFOX has been reported to result in an overall survival of 2 years as adjuvant treatment in a case of HAC of the colon (12). In the case of peritoneal HAC colliding with liposarcoma, neoadjuvant FOLFOX resulted in stable disease after 6 cycles and enabled debulking surgery (3).
HAC is known to have a poor prognosis. Based on a review of 83 cases with available survival data, the estimated 1-year survival rate is 55%. Forty-three patients died within the first 12 months and 40 patients were alive for more than 12 months (4). The poor prognosis is related to the extensive venous permeation and locally advanced or metastatic presentation (9). Also implicated for the poor prognosis of HAC is the production of AFP, alpha1-antitrypsin and alpha1-antichemotrypsin, which have immunosuppressive properties (15). Our patient's favorable outcome is likely a combination of different factors: she was relatively young and healthy with no comorbidities, her tumor was resected completely, and chemotherapy prevented recurrence of disease for more than 3 years now.
In summary, the heterogeneity of hepatoid adenocarcinoma makes the diagnosis difficult. The associated poor prognosis emphasizes the need for accurate and early diagnosis with immunohistochemistry. Optimal management is still not well defined. However, our case shows that aggressive surgery followed by adjuvant chemotherapy resulted in a favorable outcome. FOLFOX needs further investigation in the treatment of HAC.
Footnote
No potential conflict of interest.
References
- Ishikura H, Fukasawa Y, Ogasawara K, Natori T, Tsukada Y, Aizawa M. An AFP-producing gastric carcinoma with features of hepatic differentiation. A case report. Cancer 1985;56:840-848. [PubMed]
- Kishimoto T, Nagai Y, Kato K, Ozaki D, Ishikura H. Hepatoid adenocarcinoma: a new clinicopathological entity and the hypotheses on carcinogenesis. Med Electron Microsc 2000;33:57-63. [PubMed]
- Orditura M, Lieto E, Ferraraccio F, et al. Hepatoid carcinoma colliding with a liposarcoma of the left colon serosa presenting as an abdominal mass. World J Surg Oncol 2007;5:42. [PubMed]
- Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie 2010;33:263-269. [PubMed]
- Nandipati KC, Allamaneni S, Farjado M, Sung KJ, Azmat I, Chandoke I. Primary hepatoid adenocarcinoma of retroperitoneum. Am Surg 2009;75:523-525. [PubMed]
- Kitamura H, Ikeda K, Honda T, Ogino T, Nakase H, Chiba T. Diffuse hepatoid adenocarcinoma in the peritoneal cavity. Intern Med 2006;45:1087-1091. [PubMed]
- Gopaldas R, Kunasani R, Plymyer MR, Bloch RS. Hepatoid malignancy of unknown origin--a diagnostic conundrum: review of literature and case report of collision with adenocarcinoma. Surg Oncol 2005;14:11-25. [PubMed]
- Terracciano LM, Glatz K, Mhawech P, et al. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol 2003;27:1302-1312. [PubMed]
- Maitra A, Murakata LA, Albores-Saavedra J. Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol 2001;115:689-694. [PubMed]
- Tsung JS, Yang PS. Hepatoid carcinoma of the ovary: characteristics of its immunoreactivity. A case report. Eur J Gynaecol Oncol 2004;25:745-748. [PubMed]
- Karayiannakis AJ, Kakolyris S, Giatromanolaki A, et al. Hepatoid Adenocarcinoma of the Gallbladder: Case Report and Literature Review. J Gastrointest Cancer 2011. [Epub ahead of print].
- Slotta JE, Jüngling B, Kim YJ, Wagner M, Igna D, Schilling MK. Hepatoid adenocarcinoma of the transverse colon. Int J Colorectal Dis 2011; Epub ahead of print. [PubMed]
- Matsuda Y, Fukumoto M. Sorafenib: complexities of Raf-dependent and Raf-independent signaling are now unveiled. Med Mol Morphol 2011;44:183-189. [PubMed]
- Petrelli F, Ghilardi M, Colombo S, et al. A Rare Case of Metastatic Pancreatic Hepatoid Carcinoma Treated with Sorafenib. J Gastrointest Cancer 2011. [Epub ahead of print].
- Inagawa S, Shimazaki J, Hori M, et al. Hepatoid adenocarcinoma of the stomach. Gastric Cancer 2001;4:43-52. [PubMed]


