A phase II study of capecitabine and lapatinib in advanced refractory colorectal adenocarcinoma: A Wisconsin Oncology Network study
Background
Colorectal adenocarcinoma is the second-leading cause of cancer-related death in the United States (1). Advances in care for patients with metastatic disease include the addition of irinotecan and oxaliplatin to 5-fluorouracil chemotherapy. These treatments have improved the tumor response rates, and in some studies they have increased overall survival (2-5). After progression of disease on first-line therapy, however, the prognosis is poor. The response rate of second-line chemotherapy is low regardless of the agents chosen. For example, oxaliplatin with fluorouracil and leucovorin is only 15%, while second line irinotecan with fluorouracil and leucovorin is only 4% (6). Clearly, novel therapies to treat patients with refractory colorectal cancers are needed.
Lapatinib is an oral dual tyrosine kinase inhibitor of the epidermal growth factor receptor (EGFR) and HER2/ErbB2 receptor (7). Activation of either EGFR or ErbB2 initiates a series of signaling cascades that includes mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), Akt, and p70S6K (8). Inhibition of EGFR with monoclonal antibodies (cetuximab, panitumumab) has been shown to generate stable disease and some objective responses; additionally, overall survival was improved in some studies (9,10). However, it has also been shown that only tumors with wild-type k-ras gene respond to EGFR directed therapy (11). HER-2 is over-expressed in a small percentage of patients but the clinical significance of HER-2 in colorectal cancers remains unknown (12). Oral tyrosine kinase inhibitors have not previously had a role in the treatment of colorectal cancer (13,14).
The combination of capecitabine and lapatinib is a well studied and effective regimen in metastatic breast cancer and may provide a novel approach for the treatment of colon cancer. Capecitabine is an oral fluoropyrimidine that has been shown to be effective in treating colorectal cancers, both in the metastatic (15) and adjuvant (16) settings. Capecitabine and 5-FU function, in part, by downregulating thymidylate synthetase (TS), an enzyme involved in DNA synthesis and repair. Downregulation of TS is thought to be a major mechanism of cytotoxicity (17). Although lapatinib may have limited single agent activity in this disease (see reference above), lapatinib may act synergistically with capecitabine by downregulating TS (18). In a large phase III study of women with metastatic breast cancer, the combination of capecitabine plus lapatinib improved progression free survival by 4 months when compared with capecitabine monotherapy. Treatment was well tolerated with the most common side effects including diarrhea, nausea, vomiting, rash and hand-foot syndrome (19). This combination has not been evaluated in patients with metastatic colorectal cancer.
To evaluate the effectiveness of capecitabine and lapatinib in advanced, refractory colorectal cancer, we conducted a multicenter, open-label, phase II study of this combination through the Wisconsin Oncology Network (WON).
Methods
Patients
Patients were eligible if they were over the age of 18 with an Eastern Cooperative Oncology Group performance status (ECOG PS) 0-1, and able to provide informed consent for the study. Patients had to have pathologically confirmed metastatic or locally advanced colon or rectal adenocarcinoma and documented progressive disease by Response Evaluation Criteria in Solid Tumors (RECIST) during prior treatment or within 6 months of their most recent dose of chemotherapeutic regimen containing a fluoropyrimidine, oxaliplatin or irinotecan. Patients may have previously received anti-EGFR monoclonal antibodies, bevacizumab, fluoropyrimidine, oxaliplatin or irinotecan-based treatments. However, prior EGFR testing including HER-2 analysis or k-ras mutational analysis was not considered in this study. At the time this study was initiated the significance of k-ras mutational status was not known. Patients had to have measureable disease (RECIST 1.0) (20), hemoglobin ≥9.0 g/dL, absolute neutrophil count ≥1,500/μL, platelet ≥100,000/μL, creatinine ≤2x the upper limit of normal or creatinine clearance ≥30 mg/mL, bilirubin ≤2 times the upper limit of normal, AST ≤2 times the upper limit of normal or ≤5 times the upper limit of normal if liver metastasis were present. Toxicities other than neuropathy and alopecia related to prior treatment had to be grade 1 or resolved prior to enrollment. All patients provided signed informed consent.
Exclusion criteria included prior use of oral tyrosine kinase inhibitor (gefitinib or erlotinib), active inflammatory bowel disease, significant bowel obstruction as defined by the investigator, chronic diarrhea (≥ grade 2), known HIV/AIDS, central nervous system metastasis, current hepatic or biliary disease with the exception of Gilbert's disease, gallstones, metastatic disease to the liver or stable chronic liver disease (at the investigator's assessement) or uncontrolled cardiovascular disease including an abnormal ejection fraction. Patients could not have an active second malignancy except for adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, or other cancer for which the patient has been disease-free for at least 3 years. All women of child-bearing age had to either be surgically sterile or on oral contraceptives and were required to have a negative urine pregnancy test within 7 days of enrollment in the study.
Study design
This was a single arm, open-label phase II study. Lapatinib was administered at 1,250 mg by mouth daily one hour before or after breakfast on a continuous basis and not by weight or body surface area (BSA). Lapatinib was taken daily without planned breaks in treatment. Capecitabine was given at 2,000 mg/m2 of BSA, by mouth, divided into twice daily dosing on days 1 though 14. Each cycle was defined as 21 days. Doses were based on current body weight.
Study assessments
All patients had measureable disease at enrollment and disease response was defined by RECIST 1.0. Toxicity was determined by the National Cancer Institute's Common Terminology Criteria for Adverse Reactions (NCI-CTCAE) version 3.0. Patients had repeat history and physical examinations every 3 weeks, lab work every 3 weeks and a radiologic examination every 9 weeks to determine tumor response.
Toxicity
Toxicity grades were assigned using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3 (2006). Dose reductions for both lapatinib and capecitabine were allowed for toxicities grades 2 and 3. For grade 2 or 3 hematologic toxicity, bilirubin elevated less than or equal to two times the upper limit of normal, and grade 2 cardiac events both capecitabine and lapatinib were held until the toxicity was grade 0 or 1. Thereafter, lapatinib could be resumed at full dose; if the event appeared 3 or more times lapatinib could be dose reduced to 1,000 mg and required dose reduction with 4 episodes of grade 2 cardiac toxicity. Capecitabine required a dose reduction of 25% with 1-2 events, 50% with 3 events and discontinuation of therapy with 4 hematologic events. Dose reductions were required for capecitabine in patients with renal dysfunction with a creatinine clearance less than 51 mL/min. If the creatinine clearance was 30-50 mL/min, capecitabine was reduced by 25%. For creatinine clearance <30 mL/min, capecitabine was to be discontinued. If AST elevation >3 times the upper limit of normal and total bilirubin >2 times the upper limit of normal (35% direct) then study drugs were to be discontinued. If AST was >3 but <5 times the upper limit of normal and total bilirubin was ≤2 times the upper limit of normal without symptoms of hepatitis then study drug was held until lab values normalized. If the liver function tests stayed abnormal for 4 or more weeks, the patient was to be taken off study. Any grade 3 or 4 heart failure event or interstitial pneumonitis required withdrawal from the study or permission from the study chair to remain on study. Grade 4 toxicity of any kind required consultation with study chair to determine dose reductions and consideration for withdrawal from study. Supportive medications were allowed at the discretion of the investigator including antiemetics, anti-anxiolytics and anti-diarrheals.
Statistical analysis
The statistical design for this study is based on the primary endpoint of tumor response (RECIST) within the first 18 weeks of treatment with this regimen. Patients whose tumors showed complete response (CR) or partial response (PR) were classified as a response to treatment. All patients meeting the eligibility criteria who signed a consent form and began treatment were followed for one year or until death. The study used a two-stage Simon-Optimal study design permitting early termination for poor results. The design assumed that 0.05 success rate would be considered as unacceptably low and that a success rate of at least 0.2 would be considered promising. The design (with the null hypothesis that the true success rate is at most 5%) had a one-sided significance level of 5% and 85% power to detect a success probability of 20%. The maximum sample size was thus 39 [with an additional 4 (~10%) patients accrued to protect against ineligiblilities, cancellations, major violations, etc.].
The first stage enrolled 18 patients and required 2 or more objective responses to continue on to the second stage. Accrual was not suspended after the first 18 patients to evaluate for disease progression. The study was designed to be terminated if there were 0 or 1 responses in the first stage. If >1 response was seen, Stage 2 would enroll 21 additional patients. If there were 4 or less responses of the 39 patients then no further studies would be recommended.
Primary endpoint was overall response rate (ORR). Secondary outcomes included overall survival (OS), progression-free survival (PFS) and toxicity. The survival function for OS was estimated using Kaplan-Meier method. Though not planned in the original protocol, subgroup analysis was performed with respect to prior use of EGFR monoclonal antibody (cetuximab and panitumumab) and k-ras mutational status. Comparison of overall survival between those with and without prior EGFR monoclonal antibody use was performed with the log-rank test.
The study was approved by the institutional review board at each institution that participated and conducted with adherence to good clinical practices (GCP).
Results
Demographics
The first patient was enrolled in June 2008, with the last patient enrolled in April 2009, for an average enrollment rate of 2.6 patients per month. The patient population was primarily Caucasian (97%) and 55% male (see Table 1). The majority of patients did not have K-ras mutational analysis done. Most patients (72%) were ECOG PS 1. Twenty patients had prior EGFR monoclonal antibody use. The enrolled patients were heavily pretreated, with a mean of 3.3 prior chemotherapy regimens (range, 1-9). Of the 4 patients with known wild type K-ras status, 2 had not received a prior EGFR monoclonal antibody. Enrollment in the study was terminated after 29 patients when the stopping criterion was met with no objective responses among the 18 patients evaluable for response.
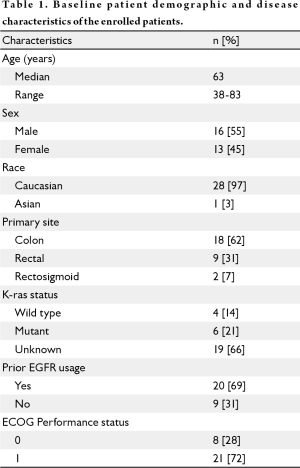
Full table
Efficacy
The overall response rate was 0% with no partial or complete responses. Twelve patients had stable disease for an overall disease control rate of 41.4% (95% confidence interval 23.5-61.1%). The disease control rate was not significantly different between those with and without prior EGFR usage (data not shown).
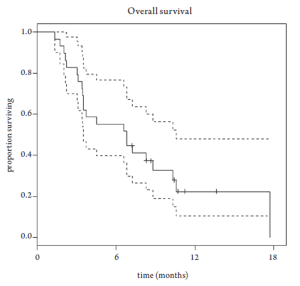
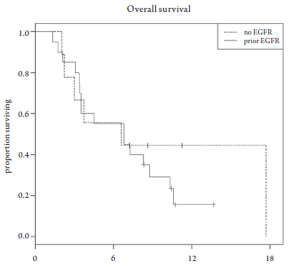
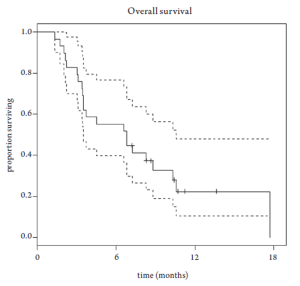
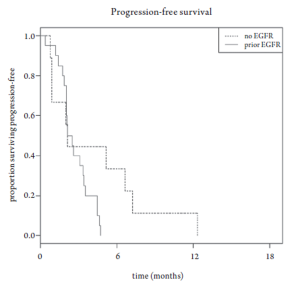
Median overall survival was 6.8 months (95% CI 3.5-10.6 months, Figure 1). Overall survival did not differ based upon prior EGFR monoclonal antibody usage (Figure 2). One-year survival rate was 22% (95% CI 11-48%). At the time of the final analysis, there were 4 patients still alive. Median progression-free survival was 2.1 months (95% CI 2.0-3.5 months, Figure 3) and did not differ based upon prior EGFR monoclonal antibody usage (Figure 4).
Safety analysis
Toxicities are listed in table 2. Toxicities were generally mild (grade 1 and 2) and comparable with previous published studies of capecitabine and lapatinib. The most common toxicities were fatigue (83% any grade), hand-foot syndrome (69% any grade) and diarrhea (59% any grade). The most severe toxicities were hand-foot syndrome (3 patients, or 10%, with grade 3 severity) and diarrhea, nausea, and fatigue, each affecting 2 patients (7%) with grade 3 severity. There were no grade 4 or 5 adverse events.
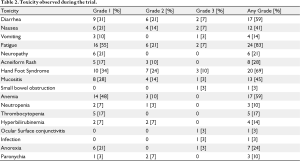
Full table
Conclusions
In this open-label, phase II study of capecitabine and lapatinib in metastatic colorectal adenocarcinoma activity of the combination for refractory colorectal cancer was limited. Though this regimen was well tolerated in general, there were some grade 2 adverse events noted.
There were coincident limitations to this study. First, this study was designed prior to routine K-ras testing. Patients with K-ras mutations are unlikely to benefit from EGFR inhibition. Though the only approved treatments that target the EGFR in colorectal adenocarcinoma are monoclonal antibodies cetuximab and panitumumab, oral tyrosine kinase inhibitors such as lapatinib could potentially provide a therapeutic alternative in the K-ras wild type population. In our study, only a minority of patients had K-ras mutational analysis. Of the 4 patients with known K-ras wild type, only 2 of these patients had not been pre-treated with a monoclonal antibody. Although neither of these patients had a response, they both had stable disease (5, 7 mo). It is unclear if there is any benefit in K-ras wild type patients as there were too few patients to analyze in this subset analysis.
There are no published clinical trials assessing the utility of sequential therapy with panitumumab after progression on cetuximab or vice versa. There has been one published study of lapatinib use after monoclonal antibody failure and this study failed to show any clinical benefit with lapatinib monotherapy (21). The optimal arena to test this combination therefore may be prior to EGFR antibody administration in the treatment of k-ras wild type tumors.
There are pre-clinical data suggesting that lapatinib, a tyrosine kinase inhibitor that inhibits the EGFR pathway along with HER-2, may act synergistically with capecitabine through the down regulation of resistance factors such as TS. Our study did not show activity with this regimen, suggesting that lapatinib was unable to overcome fluoropyrimidine resistance.
Our study was designed using the two-stage Simon-Optimal study design. Although the study was designed to be terminated if there were 0 or 1 responses in the first 18 patients, the study was also designed not to be delayed while the first 18 patients were evaluated for a response. This led to an additional 11 patients enrolled in our study, for 0 responses in a total of 29 patients. While eliminating suspension of accrual pending an interim analysis can lead to faster accrual, it can also unnecessarily enroll additional patients in studies where the efficacy is in question. We would advocate for halting studies for interim analysis to reduce the number of patients unnecessarily treated with ineffective investigational therapies in clinical studies. The relatively rapid accrual rate, however, highlights the ongoing need for more therapeutic options in this patient population.
In summary, the combination of capecitabine and lapatinib failed to show any clinical activity in heavily pretreated patients with colorectal adenocarcinoma. Further studies could be considered to evaluate this combination as an oral alternative therapy to an intravenous monoclonal antibody in patients with K-ras wild type tumors without prior monoclonal antibody therapy.
Funding
This research was supported in part by P30 CA14520.
Acknowledgements
The investigators appreciate the participation of the members of the Wisconsin Oncology Network in the design conduct of the study.
Footnote
No potential conflict of interest.
References
- American Cancer Society. Cancer Facts & Figures 2003. Atlanta, GA: American Cancer Society, 2003.
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-2947. [PubMed]
- Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 2000;18:136-147. [PubMed]
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905-914. [PubMed]
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355:1041-1047. [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-237. [PubMed]
- Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther 2001;1:85-94. [PubMed]
- Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 2002;21:6255-6263. [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-345. [PubMed]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-1664. [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-1765. [PubMed]
- Ochs AM, Wong L, Kakani V, et al. Expression of vascular endothelial growth factor and HER2/neu in stage II colon cancer and correlation with survival. Clin Colorectal Cancer 2004;4:262-267. [PubMed]
- Townsley CA, Major P, Siu LL, et al. Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer 2006;94:1136-1143. [PubMed]
- Kozuch P, Malamud S, Wasserman C, Homel P, Mirzoyev T, Grossbard M. Phase II trial of erlotinib and capecitabine for patients with previously untreated metastatic colorectal cancer. Clin Colorectal Cancer 2009;8:38-42. [PubMed]
- Hoff PM, Ansari R, Batist G, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol 2001;19:2282-2292. [PubMed]
- Scheithauer W, McKendrick J, Begbie S, et al. Oral capecitabine as an alternative to i.v. 5-fluorouracil-based adjuvant therapy for colon cancer: safety results of a randomized, phase III trial. Ann Oncol 2003;14:1735-1743. [PubMed]
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 2003;3:330-338. [PubMed]
- Budman DR, Soong R, Calabro A, Tai J, Diasio R. Identification of potentially useful combinations of epidermal growth factor receptor tyrosine kinase antagonists with conventional cytotoxic agents using median effect analysis. Anticancer Drugs 2006;17:921-928. [PubMed]
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-2743. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-216. [PubMed]
- Fields AL, Rinaldi DA, Henderson CA, et al. An Open-Label Multicenter Phase II Study of Oral Lapatinib (GW572016) as Single Agent, Second-Line Therapy in Patients with Metastatic Colorectal Cancer. ASCO Annual Meeting Proceedings 2005;23:s3583.