Treatment disparities affect outcomes for patients with stage I esophageal cancer: a national cancer data base analysis
Introduction
The incidence of esophageal cancer (EC) continues to rise in the Western world because of several prevalent risk factors including obesity, tobacco smoking, and Barrett’s esophagus (1). Although overall long-term prognosis is poor at 18%, high cure rates can be achieved when the disease is diagnosed early (2). Historically, esophagectomy (Eso) was considered the classic treatment for stage I EC. However, advancements in endoscopic therapies including ablation and local excision (LE) have resulted in comparable to superior EC-related mortality and overall survival (OS), making LE an acceptable alternative to esophagectomy (Eso)for select clinical T1 EC (3,4). For patients who cannot or choose not to undergo surgery, definitive chemoradiation (CRT) is the recommended treatment (5).
Despite these national guidelines, sociodemographic disparities influencing treatment selection are an unfortunate reality and pose challenges at all levels of cancer-directed treatment. From potential underutilization of cancer screening among ethnic minorities to the underutilization of surgical management for elderly patients with EC, cancer outcomes can be negatively affected in subsets of patients because of ongoing discrepancies in healthcare (6-9).
In the current literature on stage I EC, most observational studies provide comparisons between surgical methods or highlight treatment disparities among elderly patients, with most studies being restricted to Medicare-eligible patients (10-13). Information is limited on comprehensive treatment patterns and outcomes for all patients with stage I EC, particularly in the modern era. By using the National Cancer Data Base (NCDB), an extensive dataset that captures more than 70% of all newly diagnosed cancer cases nationwide (14), we examined the use of Eso, LE, CRT, and observation (Obs) for stage I EC, with a particular focus on disparities in treatment selection and the potential effect of such disparities on survival.
Methods
The NCDB is a nationwide clinical surveillance resource originally established in 1989 by the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons. De-identified oncologic data are acquired annually from more than 1,500 CoC-approved centers and are standardized by rules similar to those used for the Surveillance, Epidemiology, and End Results (SEER) registry. Data include patient demographics, socioeconomic status, tumor characteristics, initial course of therapy, and OS in addition to radiation therapy specifics, making the NCDB a valuable investigative tool (15). This study was exempt from Institutional Review Board evaluation as the information utilized was de-identified.
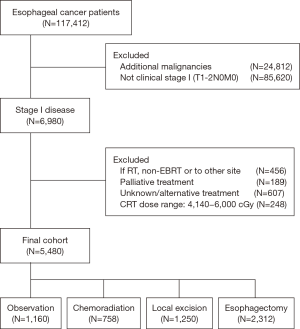
We first identified 6,980 patients as having been diagnosed with clinical T1–2N0 EC in 2004–2012; 5,480 were included in this study (Figure 1). Patients with cT2 disease were included only if they had been diagnosed in 2010–2012 to reflect the implementation of the 7th American Joint Commission on Cancer staging system. Patients were grouped according to treatment: Eso, LE, CRT, or Obs. CRT consisted of concurrent CRT (radiation dose range, 41.4–60 Gy) with chemotherapy begun within 14 days of starting radiation therapy. Those in the Obs group did not receive any form of therapy.
Type of treatment facility was dichotomized into academic/research (AR) or non-academic (non-AR) facilities, the latter consisting of community cancer programs or comprehensive community cancer programs. Hospital volume for a given surgery was defined as the mean annual caseload, which was calculated by dividing the total number of surgical procedures performed at a given institution by the number of years in the study period. Hospital volume was then stratified into quintiles, with the highest 20% considered “high volume” facilities, the middle 60% “medium volume”, and the remaining 20% “low volume”. Distance from treating facility was categorized as either local (0–25 miles) or distant (>25 miles).
Baseline patient sociodemographic, clinical, and facility characteristics were compared among the treatment groups with Pearson χ2 tests. Multinomial logistic regression was used to examine correlations between covariates and treatment selection. Covariates in the model included year of diagnosis, age group, sex, race, comorbidity score, income quartiles, type of insurance, tumor location, facility type, and distance from facility. Thirty- and 90-day postoperative mortality rates (among surgical patients), 5-year OS estimates, and hazard ratios (HR) were analyzed. To reduce selection bias, outcomes other than postoperative mortality were adjusted by using the inverse probability of treatment weighting (IPTW) method. To obtain the IPTW reflecting the differences in sample sizes for the three treatment groups, a generalized logit function was used to contrast a reference group to the other three groups, in which propensity scores to conditionally predict a patient’s probability of receiving a particular treatment were obtained after adjustment for the covariates. The IPTW method, which is based on propensity scores, calculates a weight for each subject that equals the inverse of the probability of receiving the treatment actually received (16-18). These weights, in turn, are incorporated into the survival analysis to enhance the robustness of the analysis. The IPTW-adjusted patient, tumor, and treatment characteristics are shown in Table S1. Statistical analyses were done with SAS v9.4 (Cary, NC, USA).
Full table
Results
Patients
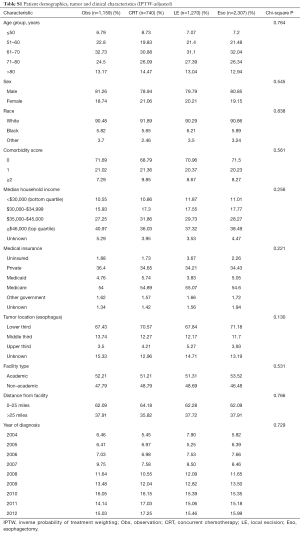
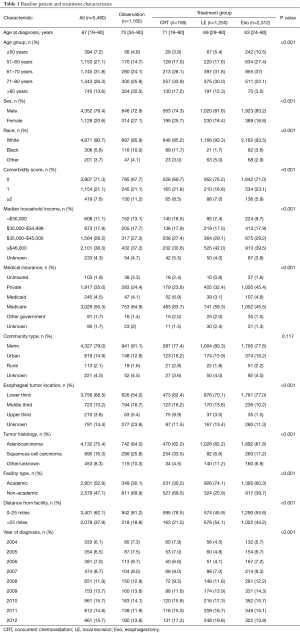
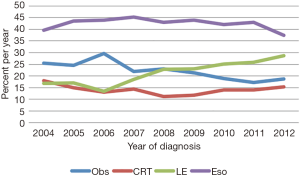
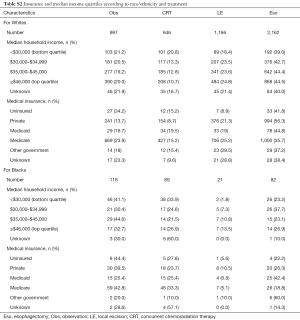
Baseline characteristics of all patients are shown in Table 1. Of 5,480 patients, 2,312 (42%) were treated with Eso, 1,250 (23%) LE, 758 (14%) CRT, and 1,160 (21%) Obs. Over time, the use of LE increased from 17% to 29%, while use of Obs declined from 26% to 19% (Figure 2). Surgical management was more often performed in ARs (n=2,321, 65% of all surgeries) than in non-ARs (n=1,241, 35%). Median age at diagnosis was 67 years (range, 19–90 years) for the entire group and was highest in the Obs group at 73 years (range, 34–90 years). A treatment-selection pattern by age was evident. Specifically, the proportion of patients undergoing LE, CRT, and Obs increased with age, whereas use of Eso decreased with age. A higher proportion of men than women received Eso (44% vs. 34%) or LE (23% vs. 20%). However, age was again a likely driving factor, because twice as many women were ≥80 years old at diagnosis (24%) as were men (11%). Additional pertinent characteristics included 71% of patients having a comorbidity score of 0 and 69% having a tumor in the lower third of the esophagus.
Full table
Analysis by race revealed that 308 patients (5.6%) were Black and 4,971 (90.7%) were White. On average, Blacks were younger at diagnosis than Whites [age 63 years (range, 28–90 years) vs. 68 years (range, 19–90 years)]. The rate of any surgery for Black patients was half that for White patients (33% vs. 67%), and the rate of Obs was disproportionately higher among Black patients (38% vs. 20%). Differences in treatment selection may have been influenced by the greater proportion of Blacks having comorbidity scores ≥2 (11% vs. 7% of Whites). However, other socioeconomic factors such as type of insurance and median household income may have influenced the observed discrepancies. Compared with Whites, Blacks were more often uninsured (5.8% vs. 1.6%) or on Medicaid (19% vs. 3.5%), and belonged to lower median income quartiles (<$30,000: 36% Blacks vs. 10% Whites). Yet even within the highest income quartile (≥$46,000), the rate of nonsurgical management was disproportionately higher among Blacks vs. Whites: CRT, 27% vs. 11%; and Obs, 33% vs. 20% (Table S2).
Full table
Factors affecting treatment selection
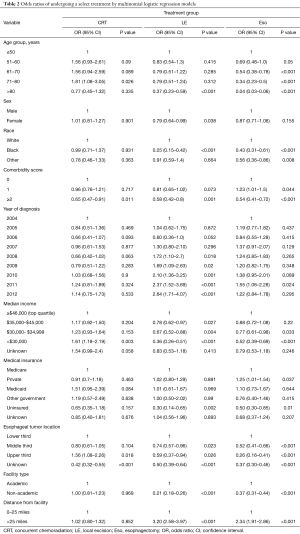
Racial disparities were again evident in our multinomial logistic regression model, as Blacks were less likely to undergo LE [odds ratio (OR) =0.25, 95% confidence interval (CI): 0.15–0.42, P<0.001] or Eso (OR =0.43, 95% CI: 0.31–0.61, P<0.001) than White patients (Table 2). Patients belonging to the lowest median income quartile were less likely to undergo LE (OR =0.36, 95% CI: 0.26–0.51, P<0.001) or Eso (OR =0.52, 95% CI: 0.39–0.69, P<0.001) than patients in the top income quartile. Reduced odds of having surgery were also seen for uninsured patients and treatment at non-ARs compared with ARs (LE OR =0.21, 95% CI: 0.18–0.26, P<0.001; Eso OR =0.37, 95% CI: 0.31–0.44, P<0.001). Other noteworthy factors that reduced the probability of undergoing Eso or LE included having a comorbidity score of ≥2, a tumor located outside the lower third of the esophagus, and living 0–25 miles from the treating facility. Only the odds of undergoing LE were significantly influenced by year of diagnosis, with an interval increase in those odds with each year after 2007.
Full table
Outcomes
Among the facilities performing surgical procedures, the mean annual hospital volume was 22 Eso cases and 34 LE cases (range, 1–159 cases). Overall 30-day postoperative mortality rates were 3.1% at low-volume facilities, 2.1% at medium-volume facilities, and 1% at high-volume facilities (P=0.022), and corresponding 90-day postoperative mortality rates were 6.7%, 3.7%, and 2.1% (P<0.001). When stratified by type of procedure, postoperative mortality was worse after Eso than after LE, both at 30 days (2.9% vs. 0.5%, P<0.001) and at 90 days (5.5% vs. 1.4%, P<0.001).
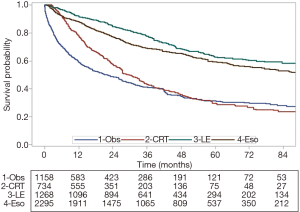
To best mimic a well-balanced cohort, we adjusted the treatment groups by using propensity score-weighted measurements (IPTW) to obtain balanced comparison groups, in which no significant differences were found in any of the covariates to be modeled in the analysis (Table S1). Median survival times, in months, by treatment group were: 90 for Eso, 116 for LE, 29 for CRT, and 22 for Obs. The IPTW-adjusted Kaplan-Meier curves are shown in Figure 3, with the highest 5-year OS estimates belonging to the LE group at 63% followed by 59% for Eso, 31% for Obs, and 29% for CRT (P<0.001).
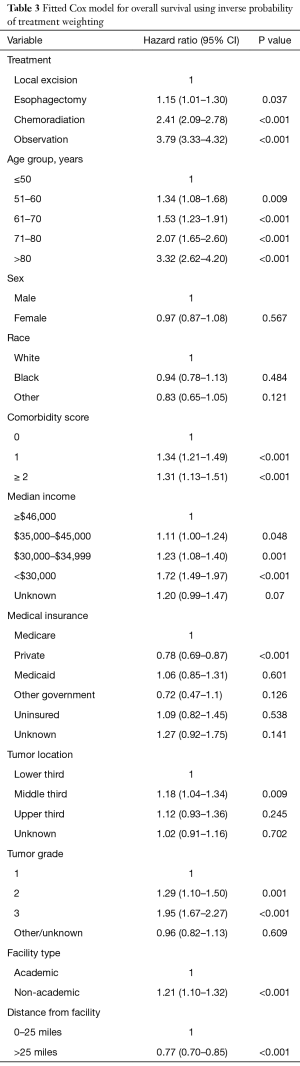
In our IPTW-adjusted Cox modeling analysis (Table 3), all treatments were associated with worse outcomes relative to LE: Eso HR =1.15, 95% CI: 1.01–1.30, P=0.037; CRT HR =2.41, 95% CI: 2.09–2.78, P<0.001; and Obs HR =3.79, 95% CI: 3.33–4.32, P<0.001. When Obs was set as the reference, all treatments were again associated with significantly improved survival (data not shown). Factors found to negatively affect outcomes were older age, higher comorbidity index scores, higher tumor grade, lower income, and treatment at non-ARs (HR =1.21, 95% CI: 1.10–1.32, P<0.001).
Full table
Discussion
Patients diagnosed with stage I EC in the United States represent a heterogeneous population subject to various clinical treatment patterns. As expected per national guidelines, most patients (65%) were treated surgically with either Eso or LE. Interestingly, use of LE rose over time, and LE was associated with improved postoperative mortality rates and improved OS compared with Eso. Although esophagectomy has historically been the standard of care, endoscopic therapies have gained popularity for several reasons. Partial or total esophagectomy is considered aggressive surgery, with high rates of major morbidities as well as 30-day postoperative mortality rates ranging from 1.4% to 9.8% (19-22). Our 30-day Eso postoperative mortality rate of 2.9% is in accordance with modern-day reports and likely reflects the adoption of minimally invasive techniques (23). LE has the advantages of esophageal preservation, potentially greater quality-adjusted life years with similar long-term survival, and salvage therapy options such as re-excision or esophagectomy (4,24-26). Possible explanations for the observed survival benefit with LE over Eso in our cohort include well-selected cases with low risk of nodal metastasis, T1a disease, or higher rates of negative margin status, all of which could not be confirmed or evaluated in our analysis. Overall, the choice of surgical procedure should be made individually, with Eso the preferred option for select patients with cT1b disease or high-risk features (27,28).
Discrepancies in healthcare provided to minorities has been well documented at all stages of disease and cancer care (29,30). In our study, multiple disparities were evident with regard to ethnicity, age, and type of treatment facility. For example, Black patients were less likely to undergo any form of surgery than were White patients, regardless of median income or medical insurance. These findings are similar to other reports for Blacks and Hispanics in alternative registries such as the California Cancer registry or SEER (31,32). One particular SEER study investigating racial disparities in use of surgery for locoregional EC found a significantly lower rate of cancer-directed surgery among Blacks than Whites (40% vs. 53%; P<0.001), whereas rates of receiving only radiation therapy were higher among Blacks (35% vs. 45%) (32). We were able to provide a more thorough analysis for all group stratifications, as radiation dose restrictions and the use of concurrent chemotherapy were included in our models. Although we did not observe a significant difference in mortality based on ethnicity, other studies estimate an increase in mortality risk as high as 33% for Black patients relative to White patients with any type of cancer or all stages of EC (29).
With a median age of 67 years, elderly patients constituted a substantial portion of our population with stage I EC, which makes treatment selection more challenging as age has been considered a predictor of negative outcomes after surgery (22,33). A notable trend of decreasing odds in undergoing any surgery was found with increasing age in our group. Aside from proper assessment of patient or tumor characteristics, one explanation for this observation may be underutilization of referrals to surgical consults in this age group. Using the SEER database, Steyerberg et al. analyzed referral patterns for patients ≥65 years with locoregional EC and discovered that older patients were less likely to be referred to a surgeon (61% for age ≥85 years vs. 80% for age 65–75), which in turn led to lower rates of surgery (23% for age ≥85 years vs. 55% for age 65–70 yuears) (10). Elderly patients instead tend to be treated conservatively with CRT or Obs (7,34). In a previous study of patients ≥80 years old, we found that 43% received no treatment, 22% CRT, 25% LE, and 10% LE. However, surgical management, particularly LE, was associated with low postoperative mortality rates and superior outcomes relative to Obs: LE HR =0.3, 95% CI: 0.24–0.38, P<0.001; and Eso HR =0.32, 95% CI: 0.23–0.44, P<0.001 (8). Therefore, all attempts should be made to provide surgical options if possible, including a thorough risk assessment based on performance status rather than by age alone.
Another relevant discrepancy in treatment selection was the type of treating facility. Nearly two-thirds of all surgical procedures were performed in an AR institution; indeed, surgeries constituted 80% of all therapies provided in ARs. In contrast, surgeries constituted only 48% of treatments in non-ARs, and treatment at non-ARs was independently associated with worse outcomes compared with ARs (HR =1.21, 95% CI: 1.10–1.32, P<0.001). Several studies have shown a strong correlation between type of treating facility and survival, with ARs being associated with superior outcomes for patients with different types of cancer (8,35). Lower mortality rates in ARs may be attributable to having more in-house experience and resources to handle perioperative complications or treatment-related toxicities. By NCDB definition, ARs must assess more than 500 newly diagnosed cancer cases each year and provide postgraduate medical education in at least four programs (14). Therefore, the lower 30- and 90-day postoperative mortality rates at high-volume centers versus low-volume centers is logical and warrants further evaluation when aggressive treatments are being considered at non-ARs.
We acknowledge several limitations to this study. As with any retrospective analysis, specific details regarding patient performance status, perioperative complications, and non-cancer-specific causes of death could not be assessed. Information about recurrence rates, surveillance management, and salvage therapies would also have been valuable, particularly for the surgical groups, to evaluate potential differences in outcomes after Eso versus LE. On the other hand, our study had noteworthy strengths, including the source of our data. The NCDB, now recognized as the largest clinical registry in the world with more than 34 million cancer patient records (14), is an excellent resource for comparative effectiveness research and for exploring trends in cancer care. Our cohort is relatively large and inclusive, with no age restrictions as in SEER-Medicare studies. We were also able to perform a robust analysis of health care disparities given the substantial information available on sociodemographic and facility characteristics. Finally, the IPTW method, a form of propensity score matching, was also used with the aim of reducing the effects of confounding compared with unadjusted observational studies (36).
In conclusion, our analysis suggests that discrepancies in treatment selection for patients with clinical stage I EC can lead to significant differences in long-term outcomes. Endoscopic therapy is becoming an increasingly popular alternative to esophagectomy and has been associated with lower postoperative mortality rates and the greatest survival benefit. Persistent disparities among Blacks and elderly patients were evident in our study and should continue to be addressed to provide optimal care to all patients. Finally, external validation of our findings is recommended to establish appropriate selection criteria for endoscopic therapy in the modern era.
Acknowledgements
None.
Footnote
Conflicts of Interest: SH Lin has research funding from Elekta, STCube Pharmaceuticals, Peregrine, Hitachi Chemical Inc., and Roche/Genentech, has served as consultant for AstraZeneca, and received honoraria from US Oncology and ProCure.
Ethical Statement: Research using the National Cancer Database was reviewed by the Institutional Review Board and granted an exemption.
References
- Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol Clin North Am 2015;44:203-31. [Crossref] [PubMed]
- SEER Stat Fact Sheets: Esophageal Cancer. 2016.
- Prasad GA, Wu TT, Wigle DA, et al. Endoscopic and Surgical Treatment of Mucosal (T1a) Esophageal Adenocarcinoma in Barrett’s Esophagus. Gastroenterology 2009;137:815-23. [Crossref] [PubMed]
- Pech O, Bollschweiler E, Manner H, et al. Comparison Between Endoscopic and Surgical Resection of Mucosal Esophageal Adenocarcinoma in Barrettʼs Esophagus At Two High-Volume Centers. Ann Surg 2011;254:67-72. [Crossref] [PubMed]
- Esophageal and Esophagogastric Junction Cancers (Version 2.2016). Natl Compr Cancer Netw, 2016.
- Suarez L, Pulley L. Comparing acculturation scales and their relationship to cancer screening among older Mexican-American women. J Natl Cancer Inst Monogr 1995.41-7. [PubMed]
- Molena D, Stem M, Blackford AL, et al. Esophageal Cancer Treatment Is Underutilized Among Elderly Patients in the USA. J Gastrointest Surg 2017;21:126-36. [Crossref] [PubMed]
- Moreno AC, Verma V, Hofstetter WL, et al. Patterns of Care and Treatment Outcomes of Elderly Patients with Stage I Esophageal Cancer: Analysis of the National Cancer Data Base. J Thorac Oncol 2017;12:1152-60. [Crossref] [PubMed]
- Berry MF, Zeyer-Brunner J, Castleberry AW, et al. Treatment modalities for T1N0 esophageal cancers: a comparative analysis of local therapy versus surgical resection. J Thorac Oncol 2013;8:796-802. [Crossref] [PubMed]
- Steyerberg EW, Neville B, Weeks JC, et al. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol 2007;25:2389-96. [Crossref] [PubMed]
- Lin SH, Zhang N, Godby J, et al. Radiation modality use and cardiopulmonary mortality risk in elderly patients with esophageal cancer. Cancer 2016;122:917-28. [Crossref] [PubMed]
- Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg 2007;133:1186-92. [Crossref] [PubMed]
- Wani S, Drahos J, Cook MB, et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: A population-based study. Gastrointest Endosc 2014;79:224-32.e1. [Crossref] [PubMed]
- American College of Surgery. National Cancer Data Base.
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55. [Crossref]
- Sugihara M. Survival analysis using inverse probability of treatment weighted methods based on the generalized propensity score. Pharm Stat 2010;9:21-34. [Crossref] [PubMed]
- Estimating Causal Effects from Large Data Sets Using Propensity Scores. Ann Intern Med 1997;127:757-63. [Crossref] [PubMed]
- Bailey SH, Bull DA, Harpole DH, et al. Outcomes after esophagectomy: a ten-year prospective cohort. Ann Thorac Surg 2003;75:217-22; discussion 222. [Crossref] [PubMed]
- Whooley BP, Law S, Alexandrou A, et al. Critical appraisal of the significance of intrathoracic anastomotic leakage after esophagectomy for cancer. Am J Surg 2001;181:198-203. [Crossref] [PubMed]
- Raymond DP, Seder CW, Wright CD, et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann Thorac Surg 2016;102:207-14. [Crossref] [PubMed]
- Law S, Wong KH, Kwok KF, et al. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg 2004;240:791-800. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Zehetner J, Demeester SR, Hagen JA, et al. Endoscopic resection and ablation versus esophagectomy for high-grade dysplasia and intramucosal adenocarcinoma. J Thorac Cardiovasc Surg 2011;141:39-47. [Crossref] [PubMed]
- Chu JN, Choi J, Tramontano A, et al. Surgical vs Endoscopic Management of T1 Esophageal Adenocarcinoma: a Modeling Decision Analysis. Clin Gastroenterol Hepatol 2018;16:392-400.e7. [Crossref] [PubMed]
- Wang S, Huang Y, Xie J, et al. Does delayed esophagectomy after endoscopic resection affect outcomes in patients with stage T1 esophageal cancer? A propensity score-based analysis. Surg Endosc 2018;32:1441-8. [Crossref] [PubMed]
- Worrell S, DeMeester SR. Endoscopic Resection and Ablation for Early-Stage Esophageal Cancer. Thorac Surg Clin 2016;26:173-6. [Crossref] [PubMed]
- Newton AD, Predina JD, Xia L, et al. Surgical Management of Early-Stage Esophageal Adenocarcinoma Based on Lymph Node Metastasis Risk. Ann Surg Oncol 2018;25:318-25. [Crossref] [PubMed]
- Shavers VL. Racial and Ethnic Disparities in the Receipt of Cancer Treatment. J Natl Cancer Inst 2002;94:334-57. [Crossref] [PubMed]
- Taylor JS, Rajan SS, Zhang N, et al. End-of-Life Racial and Ethnic Disparities Among Patients With Ovarian Cancer. J Clin Oncol 2017;35:1829-35. [Crossref] [PubMed]
- Tran PN, Taylor TH, Klempner SJ, et al. The impact of gender, race, socioeconomic status, and treatment on outcomes in esophageal cancer: A population-based analysis. J Carcinog 2017;16:3. [PubMed]
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Racial disparities in esophageal cancer survival after surgery. J Surg Oncol 2016;113:659-64. [Crossref] [PubMed]
- Ferguson MK, Celauro AD, Prachand V. Prediction of major pulmonary complications after esophagectomy. Ann Thorac Surg 2011;91:1494-1500; discussion 1500-1. [Crossref] [PubMed]
- Abrams JA, Buono DL, Strauss J, et al. Esophagectomy compared with chemoradiation for early stage esophageal cancer in the elderly. Cancer 2009;115:4924-33. [Crossref] [PubMed]
- Chu QD, Zhou M, Peddi P, et al. Influence of facility type on survival outcomes after pancreatectomy for pancreatic adenocarcinoma. HPB 2017;19:1046-57. [Crossref] [PubMed]
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]