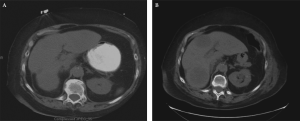
Neuroendocrine tumors (NETs) are rare neoplasms with wide spectrum of clinical presentations that are
classified according to differentiation, grade, and stage.
Differentiation refers to the degree in which the neoplastic
cells resemble their non-neoplastic equivalent (
1). The
term well-differentiated refers to neoplastic cells that
closely resemble their non-neoplastic counter equivalent
having organoid and nesting appearances; while poorlydifferentiated
is reserved for neoplasms that bear less
resemblance to their cells of origin, and have diffuse
architecture and irregular nuclei (
1). Histologic grade refers
to the aggressiveness of the neoplasm with high-grade
having a more aggressive and less predictive course; poorlydifferentiated
NETs are traditionally considered high grade
(
1). Tumor stage refers to the extend of tumor spread.
Majority of NETs are carcinoid tumors, which are welldifferentiated
and have a better prognosis than the usual
adenocarcinoma. Large-cell neuroendocrine carcinoma
(LCNEC) is a rare subtype of NETs with an aggressive
nature and a poor prognosis due to its tendency for early
metastasis (
2). While NETs can arise in different organs,
colonic NETs are exceptionally rare (
2,
3). A study by
Bernick et al showed that 0.6% of patients with colorectal
cancer had neuroendocrine carcinoma and only 0.2% of
those were large cell neuroendocrine carcinomas (
4,
5).
While the colonic LCNET are rare tumors, they share
histological features with the more well described large cell
neuroendocrine carcinomas of the lung. The histological
classification of LCNETs of the lung was initially proposed
by Travis et al (
6) and subsequently adopted by the World
Health Organization. These tumors are characterized by
(i) neuroendocrine appearance under light microscopy
(including an organoid, nesting, trabecular, rosette, and
palisading pattern) (
7), (ii) large cells with a polygonal
shape, ample cytoplasm, coarse chromatin and frequent
nucleoli, (iii) very high mitotic rate (greater than 10/10 highpower
fields) along with frequent necrosis, and evidence
of neuroendocrine features by immunohistochemistry or
electron microscopy (
4,
6).
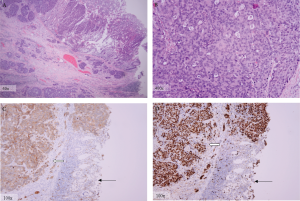
The tumor in this case met the morphological criteria
proposed by Travis et al (
6), and had neuroendocrine
immunohistochemical features including diffuse
cytoplasmic staining for synaptophysin . Unlike
adenocarcinomas, most poorly differentiated LCNETs, like
the one in this case, are negative for CK-20 (a tumor marker
traditionally confined to the intestinal epithelial, urothelial,
and Merkel cells) (
8). Nonetheless, several case reports of
CK-20 positive LCNEC have been reported in the literature,
suggesting a potentially common precursor for these tumors
and the conventional colonic adenocarcinomas (
2,
3,
9).
Ki-67 antige is a surrogate marker for cell proliferation
and is detected in the nucleus of actively cycling cells. Since Ki-67 is strictly related to cell replication and not to
DNA repair, it can serve as an excellent marker for tumor
growth (
10). In the case presented, 90% of the tumor cell
nuclei stained positive for Ki-67, demonstrating its highly
aggressive nature with rapid metastases to the liver. While
tumor aggressiveness of the tumor has been associated
to the extent of Ki-67 expression in some studies (
11,
12),
others have argued that the prognostic value of Ki-67
is dependent on the tissue type and that it may not be
generalized to all tumors (
13). Similarly, prognostic value
of Ki-67 in LCNECs is unclear at this time.
Similar to the adenocarcinomas of the colon, liver is the
most common site of metastasis for the NETs of the colon
(
14). Given the aggressive nature of colonic NET, patients
most often present with metastatic disease at time of initial
diagnosis (
4). The aggressive nature of colonic large cell
neuroendocrine tumors is evident in this case by the rapid
progression of the tumor and near replacement of the liver
by tumor in a period spanning approximately 10 days
from the time of initial presentation to the time of second
operative exploration.
In conclusion, colonic large-cell neuroendocrine
carcinomas are rare and aggressive tumors. Most are located
in the cecum or the rectum, are metastatic at presentation,
and have a poor prognosis with median overall survival
reported to be 10.4 months (range of 0 to 263.7 months)
(
4). While surgical resection is the primary treatment
modality, the benefit of chemo- or radiation therapy, as used
for conventional colorectal adenocarcinomas, has not been
established for colonic LCNET (
3,
4,
15,
16). Interestingly a
recent case report indicated clinical benefit to post-operative
chemoradiation in a patient with LCNET (17). Thus further
studies are needed to determine the molecular genetics of
these rare tumors and define the optimal systemic and local
therapies.