Predictors of overall survival after surgery in gastric cancer patients from a Latin-American country
Introduction
More than 70% of gastric cancer cases occur in developing countries. In the Latin American Region this malignancy is the sixth cause of death due to cancer in both sexes (1). In Costa Rica, gastric cancer is the first cause of death due to cancer in men and the second one in women (2). Despite the high prevalence and mortality of this malignancy in Latin America few epidemiological data exist to adequately characterize the clinical outcomes of these patients. Previous reports have acknowledged that overall survival (OS) can vary according to ethnicity, health care access and genetic background (3,4).
In the non-metastatic setting, the surgical removal of the tumour and related lymph nodes remains as the best curative option for some patients. However, the rate of recurrence reaches up to 60% (5,6). For these patients, the use of neoadjuvant (7) or adjuvant treatment with chemotherapy (8,9), radiotherapy (10) or both (11) has shown to improve OS and disease-free survival (DFS) in several randomized clinical trials. Other prognostic variables have also been recognized to categorize patients according to their risk of poor outcomes, including age, histology, tumour differentiation, type of lymph node dissection, and surgical margin status (5). However, the majority of these trials have been carried out in Asian or Caucasian populations with conflicting results. The aim of this study was to identify potential predictors of OS in a cohort of Hispanic patients from Costa Rica after surgery for gastric cancer.
Methods
We retrospectively reviewed the clinical records of all consecutive patients who underwent curative intention surgery for gastric adenocarcinoma from January 2009 to January 2012 in the four major public hospitals of Costa Rica (Hospital San Juan de Dios, Hospital Calderón Guardia, Hospital Max Peralta and Hospital México). Baseline clinical and tumour characteristics, as well as treatment data were manually retrieved from the clinical records. All cases were reclassified according to the TNM criteria as described by AJCC 7th edition. Patient performance status was evaluated according to the Eastern Cooperative Oncology Group (ECOG) criteria. We excluded patients with other primary malignant tumour or ECOG performance status equal or greater than 2.
The surgical procedure consisted of total or subtotal gastrectomy depending on tumour location. Total gastrectomy was performed for patients with cancer of the gastroesophageal junction (GEJ) or upper gastric body, and subtotal gastrectomy was done for patients with cancer in the lower body, antrum or pylorus. The type of lymph node dissection (D1 or D2) was chosen at the discretion of the surgical oncologist or general surgeon. Margin status was defined as microscopically negative (R0), microscopically positive (R1), or macroscopically positive (R2). R1 margin status was defined as the presence of tumour cells in the resection margins on standard microscopic examination. Histological subtype according to Lauren’s classification was documented for all tumours.
Adjuvant treatment, either chemotherapy or chemoradiotherapy (CRT), was chosen by the Tumour Board Committee composed by medical oncologists, radiation oncologists and surgeons. The treatment plan in case of cytotoxic systemic adjuvant therapy was chosen at the discretion of the medical oncologist. Selected regimens included 6 months of one of the following regimens: capecitabine alone (2,000 mg/m2/d from day 1 to day 14 every 21 days) (CAP monotherapy), the combination of a fluoropyrimidine (5FU 400 mg/m2 day 1 followed by 2,400 mg/m2/48 hours infusion every 15 days and leucovorin 400 mg/m2 day 1, or capecitabine 2,000 mg/m2/d from day 1 to 14 every 21 days) and oxaliplatin (85 mg/m2 every 15 days or 130 mg/m2 every 21 days) or cisplatin (50 mg/m2 every 15 days) (FOLFOX or CAPEOX regimens). Other selected regimen was the combination of epirubicin (50 mg/m2 day 1), cisplatin (60 mg/m2 day 1) and capecitabine (1,250 mg/m2 daily for 21 days) every 3 weeks (EPX regimen). The CRT regimen consisted in 5FU 425 mg/m2/d and leucovorin 20 mg/m2/d for 5 days, followed by radiation to a total of 45 Gy (1.8 Gy per day, given 5 days per week for 5 weeks) beginning on day 28, with FU 400 mg/m2/d and leucovorin 20 mg/m2/d on the first four and the last 3 days of radiotherapy. Thirty days after radiotherapy, two additional cycles of 5FU and leucovorin were given for 5 days every 28 days.
Patients were followed up at least at 3-month intervals during the first 2 years, then at 6-month intervals for 3 years, and yearly thereafter. Follow-up consisted of physical examination and computer tomography or ultrasound images as clinically indicated. Patients underwent an esophagogastroduodenoscopic examination within 6 to 12 months after operation and yearly thereafter, or when clinically indicated.
The primary outcome of this observational study was OS, as defined from the date of primary surgery to the date of death according to the Costa Rican National Registry. Cases were censored on January 1st, 2016. DFS was defined from the date of primary surgery to the date of clinically confirmed recurrence or death. Recurrence was defined as the presence of a biopsy-proven tumour with adenocarcinoma features or the presence of imaging highly suspicious of tumour recurrence. Recurrences were classified as locoregional (regional nodes or gastric) or advanced (peritoneal or hematogenous spread).
The study was approved by the Ethical Scientific Committee of the University of Costa Rica (# 817-B2-371) and the Institutional Scientific Ethics Committee of the Caja Costarricense del Seguro Social (R013-SABI-00048).
Statistical analysis
Categorical variables are presented as percentages and continuous variables as means and standard deviations (SD) or medians and interquartile ranges (IQR), depending on their parametric or non-parametric distribution, respectively. The Chi-square test or Fisher’s exact test were used for comparison of frequencies, while the ANOVA test was used for comparison of quantitative variables. An OS and DFS analysis was performed using the Kaplan-Meier method. The Log-rank test was used to compare survival curves. Univariate and multivariate COX regression analyses were used to calculate the crude and adjusted hazard ratios (HR) with their 95% confidence interval (95% CI). Variables with a P value less than 0.10 by univariate analysis were included in the multivariate analysis using the backward stepwise technique.
A P value less than 0.05 was considered statistically significant. Statistical analysis was carried out using SPSS version 21.0 for Mac (Chicago, IL, USA). All statistical tests were two-tailed. A P value less than 0.05 was considered statistically significant.
Results
Between January 2009 and January 2012, a total of 236 patients fulfilled the inclusion criteria. Median follow-up time was 46.5 months (IQR: 13.3–60.7 months). Demographic and clinical-pathological features are summarized in Table 1. Overall, there was a predominance of male cases (1.4:1). A total of 57 cases (24.2%) were less than 50 years old, and 77 patients (32.6%) were older than 70 years. Young patients were more likely to have diffuse histology (55.3% vs. 15.4%; P=0.006) and poor or undifferentiated tumours (64.7% vs. 28.8%; P<0.001) than patients older than 70 years. The tumour lesions were predominantly located in the gastric body, and a quarter of patients had distal tumors. In total, 110 patients (46.6%) had poor or undifferentiated tumours. Of note, patients who underwent total gastrectomy were more likely to have undifferentiated tumours than patients having partial gastrectomy (48.6% vs. 23.6%; P=0.013). Regardless the type of adjuvant therapy, the most frequent type of lymphadenectomy was D2 dissection and the extent of lymph node yield was comparable between the three groups.
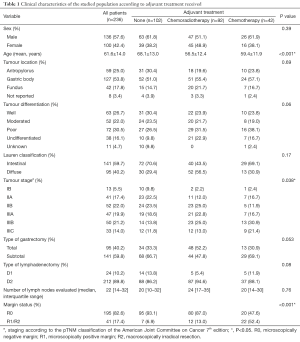
Full table
Patients who did not receive any further treatment after surgery were more likely to be older than their counterparts. Similarly, the vast majority of patients who underwent observation after the surgical procedure had a negative surgical margin. On the opposite, 52.4% of patients who received adjuvant chemotherapy had a positive margin, either microscopically or macroscopically. Patients receiving any type of adjuvant therapy had more advanced disease than those patients undergoing surgery alone (stage III: 63.4% vs. 44.1%; P=0.038). Most patients were given combination chemotherapy. In detail, 22 patients received CAPEOX (52.4%), ten patients received EPX (23.8%), eight subjects FOLFOX (19.0%) and capecitabine alone was used in two cases (4.8%).
During the follow-up time 145 patients (55.1%) experienced a recurrent event. Recurrences were more common at distant location (n=130, 89.7%), while 15 patients (10.3%) had locoregional recurrences. Median DFS was 40.1 months (95% CI: 28.3–51.9 months). Median survival after recurrence was 10.0 months (95% CI: 6.2–13.8 months).
Overall, a total of 131 patients died during follow-up. Median OS was 47.6 months (95% CI: 37.7–60.4 months), and the OS rate at 5 years was 45.1% (95% CI: 43.6–46.7%). Postoperative mortality was 1.7% for all patients. Figure 1 shows the probabilities of OS according to adjuvant treatment (either adjuvant chemotherapy, CRT or none), margin status, type of lymphadenectomy, and clinical stage. There was no difference in OS in patients receiving surgery alone or adjuvant therapy (Figure 1A).
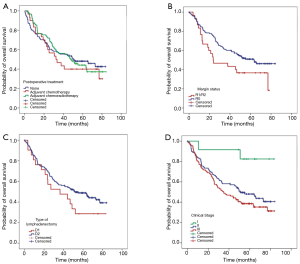
A negative margin (R0) was associated with better OS than R1/2 resections (HR: 0.61; 95% CI: 0.37–0.99; P=0.049). Similarly, OS was significantly better in patients with stage I with a median OS not reached vs. 51.1 months (95% CI: 34.2–67.9) for stage II and 34.8 months (95% CI: 22.3–47.4) for stage III patients. Patients who underwent total gastrectomy experienced worse OS than those having subtotal, with a median OS of 28.5 months (95% CI: 18.7–38.4 months) vs. 55.1 months (95% CI: 34.5–75.7 months), however, after adjusting for potential confounders this association was no longer significant (Table 2).
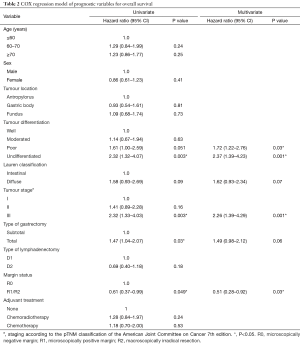
Full table
The results of the univariate and multivariate analysis are shown in Table 2. The variables independently associated with OS were: margin status, tumour differentiation, and pathological stage III.
Discussion
In this study we retrospectively identified prognostic variables of OS in a multi-institutional cohort of Hispanic patients from Costa Rica after curative-intent surgery for gastric cancer. Although the prevalence and mortality of gastric cancer is high in some Latin-American countries, scarce data exist in the literature with regards to the long-term outcomes of the affected patients from this particular region. Our study, therefore, is among the few being conducted in Latin America for addressing this particular issue, which is relevant in order to inform clinical decision-making in this part of the world. This is especially important when considering that previous studies show that the outcome of patients with gastric cancer varies according to geographic regions (3,4).
As described above, our population in this study was mainly composed of male patients with a relatively high proportion of patients under the age of 50 years (24.7%). Our results are in accordance to recent epidemiological studies carried out in the US, where Hispanic patients are more frequently diagnosed with gastric cancer at younger ages than any other ethnic group (21% vs. 7–16%) (3,4). This percentage of young patients with stomach cancer is also very similar with the rate reported by Sierra et al. for patients living in Central and South America (12). As described by other authors (13), we found that patients at younger age were more likely to have malignant tumours with diffuse histology (according to Lauren’s classification) and poor or undifferentiated tumours than older patients.
In agreement with previous reports (7,14), we observed that the majority of recurrences (89.7%) were at a distant location and had short OS after recurrence. Of note, we observed that the DFS after surgery was longer in our cohort than that reported for Asian (29.0 months) or American (27.2 months) patients. Differences in the characteristics of the studied populations, such as tumor location, clinical stage and surgical procedures can explain the aforementioned disparities (15).
In our study, the univariate analysis of prognostic variables for OS did not reveal any significant association between adjuvant therapy after surgery and improved survival, compared to those experiencing surgery alone. Although it has been demonstrated that adjuvant chemotherapy confers a survival advantage compared to surgery alone, the available evidence derives mainly from Asian populations (8,9) with conflicting results in Western countries (16-18). It is also likely that a number of clinico-pathological variables may be highly connected to the disparities associated to the effectiveness of chemotherapy in different parts of the world. However, we cannot preclude a lack of effectiveness of this approach due to the retrospective design of this study, some imbalances in patient’s characteristics, and differences in treatment protocols, as shown in Table 1.
Our study shows no survival benefit from patients receiving adjuvant CRT vs. surgery alone. Although the INT 0116 Trial provided evidence for this approach, the efficacy of CRT after D2 dissection remains controversial because only 10% of the patients included in this trial went through formal D2 dissection (10). In fact, retrospective analysis of patients who underwent CRT after D2 dissection in the INT 0116 Trial did not show any survival benefit in comparison to patients who underwent surgery alone in this Western population (19). Despite that, a retrospective study carried out in Asian patients did reveal a survival advantage of CRT versus surgery alone (20). Finally, recent results from the ARTIST trial have determined the efficacy of CRT after D2 dissection, particularly in patients with node-positive disease and intestinal Laurent’s classification (21). Based on our results and those of previous studies, it is likely that the survival benefit associated to the administration of adjuvant CRT in gastric cancer is highly dependent on several clinico-pathological parameters, including type of gastrectomy, type of lymphadenectomy and margin status, similar to what may happen with the use of chemotherapy. Thus, it is important to identify potential clinico-pathological factors that may be of relevance in any particular population, or geographical location, in order to identify the gastric cancer patients that may benefit the most from adjuvant CRT, or even chemotherapy.
Our findings did not show a survival benefit for individuals who underwent D2 dissection versus D1 lymphadenectomy. This finding is supported by a recent meta-analysis suggesting no significant difference in OS between these two types of lymph node dissection (22). This, however, must be cautiously interpreted because our median follow-up was 46.5 months and some authors argue that the survival benefit is apparent only after a longer follow-up. For example, the Dutch D1D2 Trial reported a significant higher gastric-cancer-related death in the D1 group compared with the D2 group only after 15 years of follow-up (23). Of note, the vast majority of patients enrolled in our study were surgically treated with D2 dissection, which might be reflected on the high 5-year OS rate achieved by the whole population (45.1%). Given the very low percentage of patients with D1 dissection (10.2%), we cannot exclude the possibility of a type II error due to the small size of this particular subgroup.
It is well established that the effectiveness of surgery depends on the adequacy of the surgical procedure. Indeed, the majority of our patients underwent D2 dissections with negative margins and high number of lymph nodes retrieved. Previous studies have suggested that the number of lymph nodes examined is related to OS. For example, Smith and colleagues reported a linear trend for superior OS based on the number of lymph nodes dissected, with no clear cut-off point (24).
Our study has some limitations due to its retrospective design. For example, we noticed some imbalances in the patients’ characteristics according to the selection of adjuvant treatment received. We could not either evaluate the adherence or side effects of each treatment strategy. Therefore, caution must be exercised when interpreting our findings, as conclusions about the effectiveness of each adjuvant approach must be validated in a prospective manner. Despite these caveats, we described a cohort of Hispanic patients after surgery for gastric cancer with an appropriate follow-up time.
In summary, our findings identified prognostic variables of OS for this specific population, including clinical stage, negative margin status, and tumour differentiation. Importantly, the present study suggests that surgery alone confers a long-term survival benefit for gastric cancer patients, with comparable results to other series of previous reports. Prospective studies are needed to properly validate these and other potential prognostic variables.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethical Scientific Committee of the University of Costa Rica (# 817-B2-371) and the Institutional Scientific Ethics Committee of the Caja Costarricense del Seguro Social (R013-SABI-00048).
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Caja Costarricense del Seguro Social. Incidencia y mortalidad del Cáncer en Costa Rica. Costa Rica: 2015. Availiable online: http://www.ccss.sa.cr/cancer?v=41. Accesed 28 April 2017
- Kim J, Sun CL, Mailey B, et al. Race and ethnicity correlate with survival in patients with gastric adenocarcinoma. Ann Oncol 2010;21:152-60. [Crossref] [PubMed]
- Al-Refaie WB, Tseng JF, Gay G, et al. The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma. Results from the National Cancer Data Base. Cancer 2008;113:461-9. [Crossref] [PubMed]
- Chang JS, Kim KH, Yoon HI, et al. Locoregional relapse after gastrectomy with D2 lymphadenectomy for gastric cancer. Br J Surg 2017;104:877-84. [Crossref] [PubMed]
- Spolverato G, Ejaz A, Kim Y, et al. Rates and patterns of recurrence after curative intent resection for gastric cancer: a United States multi-institutional analysis. J Am Coll Surg 2014;219:664-75. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated Analysis of SWOG-Directed Intergroup Study 0116: A Phase III Trial of Adjuvant Radiochemotherapy Versus Observation After Curative Gastric Cancer Resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Sierra MS, Cueva P, Bravo LE, et al. Stomach cancer burden in Central and South America. Cancer Epidemiol 2016;44 Suppl 1:S62-73. [Crossref] [PubMed]
- Santoro R, Carboni F, Lepiane P, et al. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg 2007;94:737-42. [Crossref] [PubMed]
- Wu CW, Lo SS, Shen KH, et al. Incidence and factors associated with recurrence patterns after intended curative surgery for gastric cancer. World J Surg 2003;27:153-8. [PubMed]
- Strong VE, Song KY, Park CH, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg 2010;251:640-6. [Crossref] [PubMed]
- Di Constanzo F, Gasperoni S, Manzione L, et al. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst 2008;100:388-98. [Crossref] [PubMed]
- DeVita F, Giuliani F, Orditura M, et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: a randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann Oncol 2007;18:1354-8. [Crossref] [PubMed]
- Bouché O, Ychou M, Burtin P, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol 2005;16:1488-97. [Crossref] [PubMed]
- Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430-6. [Crossref] [PubMed]
- Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 2005;63:1279-85. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumours Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Mocellin S, McCulloch P, Kazi H, et al. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev 2015.CD001964. [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol 2005;23:7114-24. [Crossref] [PubMed]


