Current status of immunotherapy and immune biomarkers in gastro-esophageal cancers
Introduction
Gastric and esophageal cancers are among the leading causes of mortality worldwide and are responsible for a combined total of 1,407,000 new cases and 1,123,000 deaths every year (1). The estimated new cases for esophageal and gastric cancers in United States are 16,910 and 26,370, respectively (2). The majority of these cancers are diagnosed at an advanced stage and outcomes remain poor for metastatic disease (3). Cytotoxic chemotherapy is active but it provides only modest benefit, with median overall survival (OS) reported in the range of 9–11 months (4,5). Recent advances have brought two targeted treatment options to daily clinical practice in first- and second-line settings, trastuzumab and ramucirumab respectively (6-8). Despite these advances, overall options remain limited and there is still a dire need for more effective and less toxic treatment options for patients with advanced gastroesophageal (GE) cancer.
In recent years, advent of immunotherapy has revolutionized the management of several malignancies especially melanoma, renal cell carcinoma, non-small cell lung cancer and more recently bladder cancer. Immune check point inhibition through antibodies that block cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) have led to meaningful improvements in survival (9,10). A significant global effort continues to explore how and when to integrate these agents in treatment of GE cancer. Recent results, which are summarized below, are encouraging in treatment-refractory patients and bring optimism for meaningful change in treatment algorithms and for better outcomes in this fatal disease.
Current treatment options for advanced GE cancers
The majority of patients with GE cancer are diagnosed at a locally advanced or advanced stage when systemic chemotherapy becomes the primary mode of treatment. Platinum and fluoropyrimidine combination chemotherapy with or without an anthracycline or taxane is the standard first-line treatment for patients with human epidermal growth factor receptor-2 (HER2) negative advanced gastric or esophageal cancer (4,5). Currently, HER-2 is the only target that guides clinicians in first-line treatment of advanced GE cancer. Integration of trastuzumab into first-line systemic treatment has brought improvement in survival and response rates in patients who have HER2 overexpression and/or amplification (6). While trastuzumab provided longest survival we had seen in advanced GE cancer trials and improved outcomes in standard practice, only 10–20% of patients are “HER-2 positive” (11,12).
Second-line treatment options include irinotecan, docetaxel and paclitaxel, based on randomized clinical trials demonstrating a survival advantage over best supportive care alone (13,14). Ramucirumab, a monoclonal antibody targeting vascular endothelial growth factor receptor-2 (VEGFR-2) has demonstrated improved survival both as monotherapy and in combination with paclitaxel in the second-line setting (7,8). However, despite these treatment options, advanced GE cancers remain fatal and there is unmet need for new therapies.
Checkpoint inhibitor trials in GE cancers
The immune system is regulated in such a way that there is an effective response to fight against the infection or cancer but there is no excessive over-activation to prevent tissue damage to healthy cells. Several checkpoints are involved to maintain the balance of this process. CTLA-4 and PD-1 are among the many inhibitory receptors expressed by regulatory T cells (Tregs, formerly known as suppressor T cells) that downregulate immune responses (15). Targeting these receptors blocks their inhibitory potential and restores immune system activity against tumor cells. Inhibitory antibodies modulating these immune checkpoints have been most frequently used in immune-oncology trials in GE cancers (Table 1).
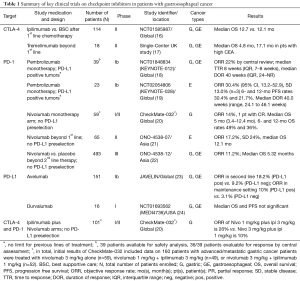
Full table
Anti-CTLA-4 trials
CTLA-4 (CD152) is a T-cell receptor that shares similarities with the co-stimulatory protein CD28, and gets activated when it binds to CD80 (B7-1) or CD86 (B7-2) on antigen-presenting cells. T-helper cells activated by CTLA-4 inhibit T cell activity, whereas in Tregs, increases T cell activity. Thus, the net effect is immune tolerance (9,25). Two main anti-CTLA-4 antibodies ipilimumab and tremelimumab are being extensively investigated in clinical trials that involves gastric and gastroesophageal junction (GEJ) malignancies (Table 2).
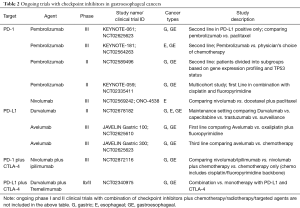
Full table
In a phase II trial, Tremelimumab, a fully humanized anti-CTLA4 monoclonal antibody, was tested in the second-line setting (17). It showed a response rate of 5% in total 18 enrolled patients, with a median OS of 4.8 months. The one patient who responded was continued on treatment for 32.7 months suggesting a durable response. The study also showed that patients with high carcinoembryonic antigen (CEA) levels had better OS (median OS 17.1 vs. 4.7 months) than those who did not. Although this trial reported suboptimal results, it resulted in a growing interest in combining CTLA4 and PD-1 inhibition. Another phase II study (NCT01585987) looked at the efficacy of ipilimumab as sequential or maintenance treatment immediately after first line chemotherapy in unresectable or metastatic gastric and GE cancer compared to best supportive care (16). Patients in the treatment group received 4 doses of ipilimumab followed by three monthly doses as maintenance until disease progression, after completion of their first line of chemotherapy. Fifty seven patients were treated in each arm of the study. Preliminary results showed no difference in median OS between ipilimumab and best supportive care arms (12.1 vs. 12.7 months).
Anti PD-1 trials
Gastric and GEJ cancers
In a multi-cohort phase Ib study (KEYNOTE 012), patients with at least 1% PD-L1 expression were treated with anti-PD1 monoclonal antibody pembrolizumab (18). 39 patients with recurrent or metastatic gastric and GEJ adenocarcinoma were treated with pembrolizumab with an objective response rate (ORR) of 22% (8 patients) and four patients showing no disease progression at the time of study publication. The majority of these patients were heavily pretreated. Median time to response was 8 weeks with inter quartile range (IQR) of 7–8 weeks and median duration of response was 40 weeks (IQR, 24–NR). Treatment was fairly well tolerated with grade 3 or 4 adverse events seen in 5 patients (consisting of grade 3 fatigue in two, grade 3 pemphigoid in one, grade 3 hypothyroidism in one, grade 3 peripheral neuropathy in one, and grade 4 pneumonitis in one). No patient discontinued therapy as a result of pembrolizumab related toxicity.
In another early-phase multi-cohort anti-PD-1 trial, the CheckMate-032, nivolumab was utilized in a PD-L1 biomarker unselected advanced gastric cancer patients (20). Updated results on 59 patients that were treated with nivolumab monotherapy showed an OR rate of 14% (1 complete response). Median OS was 5 months (95% CI, 3.4–12.4) and the 12-month OS rate was 36% (95% CI, 21–51). The toxicity profile for nivolumab was comparable to that seen in other tumor types with adverse events of any grade seen in 66% of patients. Grade 3 or 4 toxicity was seen in 17% and the most common grade 3 event seen was elevated transaminases. Other toxicities included pneumonitis, fatigue, diarrhea and hypothyroidism.
Recently, efficacy and safety of nivolumab as a salvage treatment after standard chemotherapy has been evaluated in a large randomized phase III study in Asian population (ONO-4538-12) (22). The preliminary results of the study were presented at the 2017 Gastrointestinal Cancers Symposium. Four hundred and ninety three patients who failed 1 or 2 lines of standard cytotoxic chemotherapy for unresectable or advanced gastric and GEJ cancers were randomized in a 2:1 ratio to receive nivolumab or placebo every 2 weeks. OR rate was 11.2% in nivolumab arm vs. 0% in placebo arm. Median OS was 5.32 vs. 4.14 months with hazard ratio (HR) of 0.63 (P<0.0001). OS rates at 6 and 12 months were 46.4% vs. 34.7% and 26.6% vs. 10.9% respectively. Grade 3 or 4 adverse events were seen in 11.5% vs. 5.5% of the patients, and treatment discontinuation due to adverse events occurred in 2.7% vs. 2.5% respectively. With these results, became the first immuno-oncology agent to demonstrate a survival benefit for treatment refractory gastric and GEJ cancers in a phase III study.
Esophageal cancer
In KEYNOTE-028, a phase Ib study, PD-L1 positive (with PD-L1 expression in ≥1% tumor cells) squamous cell or adenocarcinomas of esophagus were treated with pembrolizumab (19). Twenty-three patients were treated on this trial and 87% of them received ≥2 prior therapies. ORR was 30.4% (95% CI, 13.2–52.9) with stable disease in 3 patients (13%). Treatment was fairly well tolerated with no grade 4 adverse events and 17.4% (4 patients) grade 3 adverse events.
Promising activity with checkpoint inhibition in esophageal squamous cell carcinoma was also noted in recently published results from Japan (21). Sixty-five patients were enrolled in this single-arm phase II study which utilized single-agent nivolumab. Eleven (17%, 95% CI, 10–28) of 64 patients had a centrally assessed objective response. Serious adverse events included lung infection [4 (6%) patients], dehydration [2 (3%) patients], interstitial lung disease [2 (3%) patients], and hyponatremia. There were no treatment-related deaths. Overall the safety profile was manageable and comparable to similar trials, and the results contributed to the increasing optimism that checkpoint inhibition could be a reasonable treatment option for patients with chemotherapy-refractor esophageal cancer.
Combination of anti-CTLA4 and anti-PD-1
Nivolumab in combination with ipilimumab at two separate dose levels was assessed in gastric cancer in separate arms of the CheckMate-032 study (20). Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every three weeks for four cycles followed by nivolumab as a single agent was associated with a response rate of 26%, whereas nivolumab 3 mg/kg plus ipilimumab 1 mg/kg every 3 weeks for four cycles followed by nivolumab as a single agent was associated with a response rate of 10%. Higher rates of grade 3 or greater adverse events were seen (21–45%) with combination therapy when compared to the rate associated with nivolumab therapy alone. This combination strategy is now under investigation in a randomized controlled trial (CheckMate 649, NCT02872116).
Anti PD-L1 trials
Atezolizumab, avelumab and durvalumab are among anti-PD-L1 agents that are under investigation in gastric cancer. In a phase 1b study (JAVELIN Study, NCT01772004), avelumab was evaluated in gastric and GEJ adenocarcinoma patients as a first line maintenance or second line therapy (23). One hundred fifty one patients were treated (second line 62 pts, maintenance 89 pts). Results showed modest benefit with median PFS of 6 weeks in second line setting and 11 weeks in maintenance setting. ORR in second line patients was 18.2% in PD-L1 positive and 9.1% in PD-L1 negative patients, and in maintenance patients was 10% in PD-L1 positive and 3% in PD-L1 negative patients. Adverse events of any grade occurred in 59% patients with grade 3 or greater toxicity seen in 10%. One treatment related death occurred due to hepatic failure. There are ongoing phase III trials with avelumab in gastric cancer (JAVELIN gastric 100, NCT02625610 and JAVELIN gastric 300, NCT02625623) (Table 2).
Similarly, phase I trial of durvalumab (MEDI4736) showed modest activity in gastric cancer (24) and there are ongoing trials investigating durvalumab as single agent and in combination with tremelimumab (NCT02340975). Similar approaches using dual checkpoint inhibition is among combination strategies aiming for better efficacy.
Vaccine trials
Cancer vaccines enhance the ability of the human immune system to seek and destroy tumor cells by boosting tumor-specific T lymphocytes. Tumor antigens must be presented to T cells by dedicated antigen-presenting cells like dendritic cells. Usually, these antigens are delivered as small intracellular peptides or proteins and are presented by major histocompatibility complex (MHC) molecules on the surface of tumor cells to cytotoxic T cells. In order to enhance the immune response, antigens are commonly delivered in combination with adjuvants (26).
MAGE (melanoma antigen encoding gene) was first identified in melanoma, and later was found to be expressed in different solid tumors (27). 38% of gastric cancers express MAGE (28). MAGE-3 expression can be induced by Helicobacter pylori (29). In a mouse model of gastric cancer, a nanovaccine with a MAGE-3 peptide was used, and enhancement of the immune response with resultant tumor regression was noted (30). NY-ESO-1, a member of the cancer/testis antigen (CTA) family, elicits humoral and cellular immune responses in patients with advanced cancer. Use of NY-ESO-1 vaccines in esophageal cancer patients led to CD4 and CD8 T-cell immune responses in few studies, with one of them reporting tumor regression (31-34).
The gastrin peptide has also been targeted in a randomized phase II clinical trial in combination with cytotoxic chemotherapy with prolonged median survival in responders than non-responders (10.3 vs. 3.8 months; P<0.0001) (35). Targeting the angiogenic pathway has also been associated with some activity in gastric cancer. A phase I/II study from Japan used peptides derived from VEGFR1 and VEGFR2 in association with S-1 and cisplatin in advanced gastric cancer (36). Twenty two patients were treated on this study with response rate of 55% with a median overall survival of 14.2 months and immune response against VEGFR1 and VEGFR2 seen in 82% of patients; however, only those with an immunological response to the VEGFR2-169 peptides showed statistically significantly improved survival.
Immune microenvironment and molecular correlates in GE cancers
Many cancers are characterized by an inflammatory tumor microenvironment (TME). Tumor cells undergo several genetic alterations, which allow the host immune system to recognize them as foreign. Cytotoxic T-cells are then activated in response to tumor antigen stimulation and infiltrate TME to destroy cancer cells (15). The presence of tumor infiltrating lymphocytes (TIL) has been associated with improved survival rates in melanoma, breast and colorectal cancers (16,17,25).
Gastric cancer
In gastric cancer, presence of higher numbers of TILs was shown to be associated with improved outcomes, and higher number of myeloid-derived suppressor cells (MDSCs) with poorer outcomes (37,38). Role of regulatory T-cells (Tregs), however, is controversial as some studies have shown their association with advanced stage and poor survival, and few others have found association with improved survival (39,40).
In terms of PD-1 and its ligands, several studies have shown that PD-L1 expression is seen in up to 50% of gastric cancer cases, and correlate with advanced stage, lymph node involvement and poor survival (41-44). Correlation between PD-L1 expression and response to checkpoint inhibitors are discussed separately in next section.
Molecular subsets—the cancer genome atlas classification
The Cancer Genome Atlas (TCGA) Network performed a comprehensive molecular characterization of gastric cancer, leading to classification of gastric cancer into four subclasses—those associated with (I) Epstein-Barr virus (EBV) infection, (II) microsatellite instability (MSI), (III) chromosomal instability (CIN) and (IV) low rates of gene mutation and amplification, thus genomic stability (GS) (45). EBV-associated gastric cancers exhibit recurrent amplifications of 9p24.1 locus which contains PD-L1 and PD-L2. This upregulation of PD-L1 and PD-L2 makes this subtype an attractive option for evaluation of checkpoint inhibitors (45,46). In MSI subclass, the DNA mismatch repair deficiency leads to accumulation of somatic mutations resulting in increased neoantigen burden, which is another emerging biomarker for immunotherapy response, as discussed later in this review. Helicobacter pylori induced damage is found to cause genomic alterations in many cases of CIN tumors (47). An upregulation of PD-L1 expression was found on gastric epithelial cells due to H. pylori exposure in preclinical studies (48). Further efforts in implementing such genomic classification systems in both retrospective biomarker analysis of completed large trials and in new trial design will clarify the role of such molecular subsets in predicting outcomes.
Esophageal cancer
Esophageal cancer is characterized by the presence of tumor-induced chronic inflammation. Infiltration of tumors by lymphocytes has been associated with improved survival in both squamous cell and adenocarcinoma histologies (49,50). The presence of tumor-infiltrating CD8+ T cells is inversely associated with grade and stage of tumor, and lymph node metastasis. Also, TILs is found to be an independent prognostic factor of prolonged progression-free survival (PFS) and OS (51). In addition to CD8+ T-cells, infiltration of Tregs and MDSCs in the TME is associated with worse outcomes in esophageal cancer, while infiltration by natural killer (NK) cells confers an OS benefit (52-54).
A study of 41 cases of squamous cell esophageal cancer by real-time quantitative PCR revealed that around 44% of patients had PD-L1 or PD-L2 expression, and that PD-L1 and PD-L2 positivity correlated with poor prognosis in univariate and multivariate analysis (55). A large study of 354 esophageal adenocarcinomas showed that PD-L2 positivity of tumor cells is more predominant (in around 52% patients) than PD-L1 positivity (in <2% cases). It was also found that PD-L1 and PD-L2 positive tumors have higher PD-1 positive TILs compared to those in PD-L1 and PD-L2 negative tumors. PD-L2 positivity was associated with low grade, early stage tumors with a trend towards improved survival. On the other hand, PD-L1 expression did not correlate with clinical outcomes. In addition, presence of PD-1 positive TILs was inversely associated with tumor grade, stage and mortality rates (56).
Molecular subsets
Recently, a new integrated genomic study by TCGA Research Network identified genetic alterations that distinguish the two most common subtypes of esophageal cancer—squamous cell and adenocarcinoma (57). These two pathologic subtypes were noted to carry distinct set of alterations. In addition, the authors noted molecular similarities between esophageal adenocarcinoma and CIN subtype of gastric adenocarcinoma and proposed this as indirect proof that gastro-esophageal adenocarcinomas can be considered a single entity in this regard. More data is needed to better understand the role of molecular classification of esophageal cancers.
Role of biomarkers in predicting response to immunotherapy in GE cancers
PD-L1 expression
PD-L1 expression as a predictive biomarker of anti-PD-1 therapy in patients with advanced carcinoma has been evaluated in multiple tumor types particularly non-small cell lung cancer and renal cell cancer (58,59). But in advanced gastric/GEJ cancer, data is limited regarding the predictive value of PD-L1 expression. In addition, the issue of heterogeneity of biomarker expression in gastric cancer makes it even more complicated (60). In KEYNOTE-012 study discussed above where pembrolizumab monotherapy was administered only in patients with PD-L1 expression, the study reported a trend towards an association between higher levels of PDL1 expression and outcomes (ORR, PFS and OS) (18). Since the study sample was small, it is difficult to draw conclusions from this data if PD-L1 expression correlates with pembrolizumab efficacy. However, the ongoing trials (KEYNOTE-059, KEYNOTE-061 and KEYNOTE-062) may help us fully understand the role of PD-L1 expression as predictive biomarker for pembrolizumab efficacy. Similar effort is ongoing to uncover the predictive role of PD-L1 expression in response to other checkpoint inhibitors.
Immune signature
An immune-related gene expression signature composed of genes associated with T cell cytotoxic function, antigen presentation machinery, and IFN-γ signaling was identified in melanoma patients in KEYNOTE-001 study, that was associated with response to pembrolizumab (61). This immune signature was interestingly reproducible and was associated with a trend towards better outcomes to the anti-PD-1 treatment, when applied to gastric cancer patients in KEYNOTE-012 study (61). If validated, such gene expression profiling could serve as a sensitive tool that defines common features of the immune microenvironment associated with response to pembrolizumab across multiple tumor types.
Another study on gene expression profiling showed that Asian and non-Asian gastric cancers exhibit distinct tumor immunity signatures related to T-cell function (62). Specifically, there was higher expression of T- cell markers (CD3, CD45R0, CD8) and lower expression of FOXP3 (immunosuppressive T-regulatory cell marker) in non-Asians compared to their Asian counterparts. Non-Asians tended to have significantly higher CD68/CD3 ratios compared with Asians, and increased CD68/CD3 ratios were significantly and independently associated with worse survival outcomes (P=9×10−3).
Tumor mutational burden (TMB)
The overall quantity of mutations in a cancer genome, termed TMB, is among the promising potential biomarkers for response to immunotherapy in different tumor types. Across different cancer types, cancers that have a higher TMB, thus a higher neoantigen exposure to the immune system, seem more likely to respond to checkpoint inhibitors (63-65). While MSI is one common mechanism by which cancer cells can acquire high TMB, the role TMB in predicting response to checkpoint inhibition does not seem to be limited to MSI-H. In metastatic colorectal cancer (mCRC), it has been reported that TMB, when compared to assessment of microsatellite status alone, significantly increased the number of mCRC patients who may benefit from checkpoint inhibitors (66). There are ongoing international investigations to validate this biomarker in different tumor types, including GE cancers. At 2017 Gastrointestinal Cancers Symposium, the results of TMB analysis in different types of gastrointestinal cancers were presented (67). Comprehensive genomic profiling was performed on 1,375 tumors using a 592-gene panel. Overall, among 113 gastric adenocarcinoma specimens, high TMB was seen in 11% with mean TMB of 9.0 mutations/megabase, whereas only 2.4% of all esophageal adenocarcinoma (n=82) and 0% of esophageal SCC (n=17) tumors had high TMB. There was no correlation between PD-L1 and TMB in GE tumors. TMB and MSI were found to be highly correlated in colorectal and gastric tumors.
MSI
MSI is a result of defective mismatch repair. When mutations occur in mismatch repair genes, such as MLH1, MSH2, MSH6, and PMS2; or when they are silenced epigenetically, replication errors within the microsatellite sequences of DNA cannot be repaired, producing a hypermutated phenotype (68). Twenty two percent of gastric cancers are associated with MSI-H, and these patients are usually of female sex, older in age and with distal, well-differentiated adenocarcinoma and with an early stage at presentation (69). MSI status has both prognostic and predictive value. Patients with MSI-H cancers have better OS following surgical resection compared to those with microsatellite stable (MSS) cancers, and the benefit of adjuvant chemotherapy might be less in patients with MSI cancers (70,71).
MSI patients have a high mutational burden which is associated with high levels of neo-antigen presentation. This in turn has been known to be associated with better response to anti-PD-1 therapy across non-GE tumor types (72). Therefore, PD-1 inhibition may be an attractive potential therapy for gastric cancer patients with mismatch repair deficiency or MSI status. However, in KEYNOTE-012 study, only 2 out of 4 gastric cancers with MSI showed a response.
Helicobacter pylori and EBV
H. pylori infection is believed to account for nearly 50% of gastric cancers. Infection with H. pylori causes a T-cell inflammatory response in gastric mucosa, but also induces increased PD-L1 expression, leading to T-cell anergy (47,48). Given the increased PD-L1 expression and presence of TILs, it is hypothesized that gastric cancers might likely respond to checkpoint inhibitor therapy. However, this theory is yet to be proven.
EBV-mediated gastric cancers constitute a molecular subclass under the TCGA classification. In early stage gastric cancer, EBV status is predictive of a low risk of developing metastatic disease after a surgical resection. EBV-mediated gastric cancers are characterized by high levels of PD-L1 expression and presence of TILs (46). Thus, it is hypothesized that it could be another subclass of gastric cancers which may respond well to checkpoint inhibitors. However, clinical trial data on the interaction between EBV status and checkpoint inhibitor therapy in gastric cancer is limited, perhaps owing to lower number of advanced gastric cancers associated with EBV.
Conclusions and future directions
Emerging evidence suggests that checkpoint inhibitors hold potential to change treatment approach for advanced cancers of upper gastrointestinal tract. However, the following key issues are still under rigorous investigation internationally: the biomarkers of predictive of response; the correct time point for evaluating the biomarkers and the immune response; the potential benefit of combining treatment approaches, such as combination with chemotherapy, targeted agents (e.g., targeting the stroma, or tumor vasculature), radiotherapy and other locoregional therapies [e.g., the phase II trial studying the abscopal effect of using pembrolizumab with palliative radiotherapy in patients with advanced GE cancer, NCT02830594 (73)]. As for the most commonly evaluated biomarker PD-L1, it should be remembered that across different trials utilizing checkpoint inhibitors in different tumor types, PD-L1 positivity rates and its correlation with outcomes have been reported to be different. The varying results may be due to different tumor types, different methods of evaluation and different cut-off. Nonetheless, there seems to a strong signal that PD-L1 is among the important biomarkers for GE cancers. Ongoing studies to validate the promising biomarkers such as PD-L1 expression, MSI status, TMB, TILs and gene-expression signature might bring the much anticipated information we need to better guide our patients. In addition, the correct algorithm to combine these different methods still needs to be studied on a large scale. It should also be noted that there are numerous other biomarkers under investigation across different tumors. Among emerging biomarker discussions in other tumor types, recent reports (74) on the association between gut microbiome and response to PD-1 based therapy in melanoma might increase interest in studying “bacterial signatures” in new GE cancer trials, while by nature this concept is more elusive in these gastrointestinal cancers.
As we eagerly wait for the results of biomarker validation efforts and while we are yet to understand how we should best incorporate checkpoint inhibitors in treatment of GE cancer, off-label use of checkpoint inhibitors gradually has become a more commonly utilized option for refractory patients in daily clinical practice. While this option is providing us cautious hope of durable responses in some patients, it is important to educate the patients well about the need for further data on predictors of benefit and the potential side-effects and to stay vigilant about monitoring for toxicity, including the rare but serious events that have been encountered in clinical trials across all tumor types.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-E86. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Shah MA. Update on Metastatic Gastric and Esophageal Cancers. J Clin Oncol 2015;33:1760-9. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and Oxaliplatin for Advanced Esophagogastric Cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Cutsem EV, Moiseyenko VM, Tjulandin S, et al. Phase III Study of Docetaxel and Cisplatin Plus Fluorouracil Compared With Cisplatin and Fluorouracil As First-Line Therapy for Advanced Gastric Cancer: A Report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687-97. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC Med 2016;14:20. [Crossref] [PubMed]
- Carlo MI, Voss MH, Motzer RJ. Checkpoint inhibitors and other novel immunotherapies for advanced renal cell carcinoma. Nat Rev Urol 2016;13:420-31. [Crossref] [PubMed]
- Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19:1523-9. [Crossref] [PubMed]
- Boku N. HER2-positive gastric cancer. Gastric Cancer 2014;17:1-12. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage Chemotherapy for Pretreated Gastric Cancer: A Randomized Phase III Trial Comparing Chemotherapy Plus Best Supportive Care With Best Supportive Care Alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Harris TJ, Drake CG. Primer on tumor immunology and cancer immunotherapy. J Immunother Cancer 2013;1:12. [Crossref] [PubMed]
- Lee WS, Park S, Lee WY, et al. Clinical impact of tumor-infiltrating lymphocytes for survival in stage II colon cancer. Cancer 2010;116:5188-99. [Crossref] [PubMed]
- Bogunovic D, O'Neill DW, Belitskaya-Levy I, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A 2009;106:20429-34. [Crossref] [PubMed]
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Doi T, Piha-Paul SA, Jalal SI, et al. Updated results for the advanced esophageal carcinoma cohort of the phase Ib KEYNOTE-028 study of pembrolizumab (MK-3475). J Clin Oncol 2016.34. abstract 7.
- Janjigian YY, Bendell JC, Calvo E, et al. CheckMate-032: Phase I/II, open-label study of safety and activity of nivolumab (nivo) alone or with ipilimumab (ipi) in advanced and metastatic (A/M) gastric cancer (GC). J Clin Oncol 2016.34. abstract 4010.
- Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol 2017;18:631-9. [Crossref] [PubMed]
- Kang YK, Satoh T, Ryu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. J Clin Oncol 2017.35. abstract 2.
- Chung HC, Arkenau HT, Wyrwicz L, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: Analysis of safety and clinical activity. J Clin Oncol 2016.34. abstract 4009.
- Lutzky J, Antonia SJ, Blake-Haskins A, et al. A phase 1 study of MEDI4736, an anti-PD-L1 antibody, in patients with advanced solid tumors. J Clin Oncol 2014.32. abstract 3001.
- Jiang D, Gao Z, Cai Z, et al. Clinicopathological and prognostic significance of FOXP3+ tumor infiltrating lymphocytes in patients with breast cancer: a meta-analysis. BMC Cancer 2015;15:727. [Crossref] [PubMed]
- Butterfield LH. Cancer vaccines. BMJ 2015;350:h988. [Crossref] [PubMed]
- van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991;254:1643-7. [Crossref] [PubMed]
- Inoue H, Mori M, Honda M, et al. The expression of tumor-rejection antigen "MAGE" genes in human gastric carcinoma. Gastroenterology 1995;109:1522-5. [Crossref] [PubMed]
- Fukuyama T, Yamazaki T, Fujita T, et al. Helicobacter pylori, a carcinogen, induces the expression of melanoma antigen-encoding gene (Mage)-A3, a cancer/testis antigen. Tumour Biol 2012;33:1881-7. [Crossref] [PubMed]
- Yang J, Li ZH, Zhou JJ, et al. Preparation and antitumor effects of nanovaccines with MAGE-3 peptides in transplanted gastric cancer in mice. Chin J Cancer 2010;29:359-64. [Crossref] [PubMed]
- Kageyama S, Wada H, Muro K, et al. Dose-dependent effects of NY-ESO-1 protein vaccine complexed with cholesteryl pullulan (CHP-NY-ESO-1) on immune responses and survival benefits of esophageal cancer patients. J Transl Med 2013;11:246. [Crossref] [PubMed]
- Kawada J, Wada H, Isobe M, et al. Heteroclitic serological response in esophageal and prostate cancer patients after NY-ESO-1 protein vaccination. Int J Cancer 2012;130:584-92. [Crossref] [PubMed]
- Wada H, Sato E, Uenaka A, et al. Analysis of peripheral and local anti-tumor immune response in esophageal cancer patients after NY-ESO-1 protein vaccination. Int J Cancer 2008;123:2362-9. [Crossref] [PubMed]
- Uenaka A, Wada H, Isobe M, et al. T cell immunomonitoring and tumor responses in patients immunized with a complex of cholesterol-bearing hydrophobized pullulan (CHP) and NY-ESO-1 protein. Cancer Immun 2007;7:9. [PubMed]
- Ajani JA, Randolph Hecht J, Ho L, et al. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: The GC4 study. Cancer 2006;106:1908-16. [Crossref] [PubMed]
- Masuzawa T, Fujiwara Y, Okada K, et al. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int J Oncol 2012;41:1297-304. [Crossref] [PubMed]
- Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 2008;99:1704-11. [Crossref] [PubMed]
- Choi HS, Ha SY, Kim HM, et al. The prognostic effects of tumor infiltrating regulatory T cells and myeloid derived suppressor cells assessed by multicolor flow cytometry in gastric cancer patients. Oncotarget 2016;7:7940-51. [Crossref] [PubMed]
- Perrone G, Ruffini PA, Catalano V, et al. Intratumoural FOXP3-positive regulatory T cells are associated with adverse prognosis in radically resected gastric cancer. Eur J Cancer 2008;44:1875-82. [Crossref] [PubMed]
- Haas M, Dimmler A, Hohenberger W, et al. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 2009;9:65. [Crossref] [PubMed]
- Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochemica 2006;108:19-24. [Crossref] [PubMed]
- Yuan J, Zhang J, Zhu Y, et al. Programmed death-ligand-1 expression in advanced gastric cancer detected with RNA in situ hybridization and its clinical significance. Oncotarget 2016;7:39671-9. [Crossref] [PubMed]
- Zhang L, Qiu M, Jin Y, et al. Programmed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factors. Int J Clin Exp Pathol 2015;8:11084-91. [PubMed]
- Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017;66:794-801. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Derks S, Liao X, Chiaravalli AM, et al. Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers. Oncotarget 2016;7:32925-32. [Crossref] [PubMed]
- Koeppel M, Garcia-Alcalde F, Glowinski F, et al. Helicobacter pylori Infection Causes Characteristic DNA Damage Patterns in Human Cells. Cell Rep 2015;11:1703-13. [Crossref] [PubMed]
- Wu YY, Lin CW, Cheng KS, et al. Increased programmed death-ligand-1 expression in human gastric epithelial cells in Helicobacter pylori infection. Clin Exp Immunol 2010;161:551-9. [Crossref] [PubMed]
- Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003;63:1555-9. [PubMed]
- Schumacher K, Haensch W, Roefzaad C, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 2001;61:3932-6. [PubMed]
- Rauser S, Langer R, Tschernitz S, et al. High number of CD45RO+ tumor infiltrating lymphocytes is an independent prognostic factor in non-metastasized (stage I-IIA) esophageal adenocarcinoma. BMC Cancer 2010;10:608. [Crossref] [PubMed]
- Kono K, Kawaida H, Takahashi A, et al. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 2006;55:1064-71. [Crossref] [PubMed]
- Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011;60:1419. [Crossref] [PubMed]
- Hsia JY, Chen JT, Chen CY, et al. Prognostic significance of intratumoral natural killer cells in primary resected esophageal squamous cell carcinoma. Chang Gung Med J 2005;28:335-40. [PubMed]
- Ohigashi Y, Sho M, Yamada Y, et al. Clinical Significance of Programmed Death-1 Ligand-1 and Programmed Death-1 Ligand-2 Expression in Human Esophageal Cancer. Clin Cancer Res 2005;11:2947-53. [Crossref] [PubMed]
- Derks S, Nason KS, Liao X, et al. Epithelial PD-L2 Expression Marks Barrett's Esophagus and Esophageal Adenocarcinoma. Cancer Immunol Res 2015;3:1123-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Spencer KR, Wang J, Silk AW, et al. Biomarkers for Immunotherapy: Current Developments and Challenges. Am Soc Clin Oncol Educ Book 2016;35:e493-503. [Crossref] [PubMed]
- Kim MA, Lee HJ, Yang HK, et al. Heterogeneous amplification of ERBB2 in primary lesions is responsible for the discordant ERBB2 status of primary and metastatic lesions in gastric carcinoma. Histopathology 2011;59:822-31. [Crossref] [PubMed]
- Ayers M, Lunceford J, Nebozhyn M, et al. Relationship between immune gene signatures and clinical response to PD-1 blockade with pembrolizumab (MK-3475) in patients with advanced solid tumors. J Immunother Cancer 2015;3:80. [Crossref]
- Lin SJ, Gagnon-Bartsch JA, Tan IB, et al. Signatures of tumour immunity distinguish Asian and non-Asian gastric adenocarcinomas. Gut 2015;64:1721-31. [Crossref] [PubMed]
- Rizvi NA, Hellmann MD, Snyder A, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015;348:124-8. [Crossref] [PubMed]
- Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med 2014;371:2189-99. [Crossref] [PubMed]
- Johnson DB, Frampton GM, Rioth MJ, et al. Targeted Next Generation Sequencing Identifies Markers of Response to PD-1 Blockade. Cancer Immunol Res 2016;4:959-67. [Crossref] [PubMed]
- George TJ, Frampton GM, Sun J, et al. Tumor mutational burden as a potential biomarker for PD1/PD-L1 therapy in colorectal cancer. J Clin Oncol 2016.34. abstract 3587.
- Salem ME, Xiu J, Weinberg BA, et al. Characterization of tumor mutation burden (TMB) in gastrointestinal (GI) cancers. J Clin Oncol 2017.35. abstract 530.
- Parsons R, Li G, Longley M, et al. Mismatch repair deficiency in phenotypically normal human cells. Science 1995;268:738-40. [Crossref] [PubMed]
- Falchetti M, Saieva C, Lupi R, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol 2008;39:925-32. [Crossref] [PubMed]
- Kim H, An JY, Noh SH, et al. High microsatellite instability predicts good prognosis in intestinal-type gastric cancers. J Gastroenterol Hepatol 2011;26:585-92. [Crossref] [PubMed]
- Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197-203. [Crossref] [PubMed]
- Le DT, Andre T, Kim TW, et al. KEYNOTE-164: Phase 2 study of pembrolizumab for patients with previously treated, microsatellite instability-high advanced colorectal carcinoma. J Clin Oncol 2016;34:TPS3631. -TPS. [Crossref]
- Chao J, Chen YJ, Frankel PH, et al. Combining pembrolizumab and palliative radiotherapy in gastroesophageal cancer to enhance anti-tumor T-cell response and augment the abscopal effect. J Clin Oncol 2017;35:TPS220. -TPS. [Crossref]
- Gopalakrishnan V, Spencer C, Reuben A, et al. Association of diversity and composition of the gut microbiome with differential responses to PD-1 based therapy in patients with metastatic melanoma. 2017 ASCO-SITC Clinical Immuno-Oncology Symposium. J Clin Oncol 2017;35:abstract 2.


