Survival differences among patients with hepatocellular carcinoma based on the stage of disease and therapy received: pre and post sorafenib era
Introduction
The cancer death rate has dropped by 23% since 1991. Despite this progress, death rates are increasing for liver cancers (1). Hepatocellular carcinoma (HCC) remains the leading cause of cancer death worldwide (1). For last three decades, the incidence of HCC has continued to increase (1.9 per 100,000 vs. 3.1 per 100,000 vs. 4.9 per 100,000 during 1983–1992, 1993–2002 and 2003–2012 respectively) (2). It develops mostly in the cirrhotic liver and the common risk factors known to be responsible includes, hepatitis B and C infection, alcoholic liver disease, metabolic syndrome, and non-alcoholic fatty liver disease (NAFLD) (3). While survival from HCC seemed to have been improving [relative survival rate (RSR) at 5 years; 4.7% vs. 10.6% vs. 18.2% during 1983–1992, 1993–2002 and 2003–2012 respectively] (4), mortality from HCC has continued to rise (5). Multiple factors such as demographic features, comorbidities, stage and extent of the disease, therapy options can play a vital role in the survival from HCC. The emergence of multiple newer treatment modalities has changed the paradigm of HCC treatment (6). Local tumor-directed therapies have been perfected, such as radio-frequency ablation, novel agents for transarterial chemoembolization (TACE) along with improvements in hepatic resection and liver transplantation. There is also an emergence of new molecular-targeted agents such as Sorafenib. Sorafenib inhibits molecular components of the RAF-MEK-ERK signaling pathway, which suppresses tumor growth and vascular endothelial growth factor receptors, affecting tumor angiogenesis (7). Sorafenib was approved by FDA in 2007 for the treatment of advanced stage HCC with vascular invasion and distant metastasis, based on the results of the pivotal SHARP clinical trial (7). Sorafenib has become the standard first-line therapy for advanced stage HCC (6,8). The American Association for the Study of Liver Diseases (AASLD), National Comprehensive Cancer Network (NCCN) and European Association for the Study of the Liver (EASL) endorsed Sorafenib as the first-line therapy for Barcelona Clinic Liver Cancer (BCLC) stage C patients (those with unresectable portal vein invasion, extrahepatic spread), with reasonable performance status (ECOG 0–2), and good liver function (Child-Pugh class A or B) (9,10).
The aim of our study is to analyze the impact of these treatment modalities into the survival of HCC patients, based on the extent of HCC in real life practice. We accessed and analyzed data on HCC patients from 2001–2013, from the Surveillance, Epidemiology, and End results (SEER) database (11). To evaluate the difference in survival since the approval of sorafenib, we have analyzed the data dividing into two groups; patient diagnosed between 2001–2007 and 2008–2013.
Methods
The SEER database is derived from cancer registries representing approximately 28% of the U.S. population and is maintained by the National Cancer Institute (www.seer.cancer.gov) (11). The SEER population-based cancer registries contain information on cancer incidence and survival in selected geographic areas. Selection of the included geographic areas was based on the quality of their cancer reporting systems and population diversity.
A retrospective cohort study was performed using data from the SEER database, based on the November 2015 submission, which was released in April 2016. Data was examined from 2001 through 2013 from eighteen SEER registries. The SEER data set includes information on patient demographics, tumor characteristics, cancer-associated treatments, use of cancer-directed surgery, and survival for individuals with cancer. Surgical intervention is coded in the SEER database as a separate variable, whenever it is performed. The actual surgical procedure directed at the primary site is coded as a separate variable. No record of chemotherapy is available in this database. BCLC classification is most widely accepted currently, they have categorized HCC patients into five categories based on the extent of disease, Child-Pugh score, and performance status. SEER does not provide details to calculate Child-Pugh score and information on performance status, hence, accurate BCLC staging information could not be obtained.
Study population
SEER*Stat version 8.3.2 was used for all data collection and survival analysis. Patient inclusion criteria based on the site recode International Classification of Diseases for Oncology, third Edition (ICD-O-3) [2008] for HCC (C22.0), and year of diagnosis (2001 through 2013). HCC are histologically defined by the following ICD-O-3 histology codes for malignant cases: HCC (8170/3, 8172/3, 8173/3, 8174/3, 8175/3). Eighteen years or older patients were included. Only actively followed or treated cases were included. Cases identified on autopsy or reported only on a death certificate were excluded. Patients with the fibrolamellar variant of HCC were excluded as they differ in clinical course and prognosis, compared to conventional HCC (12). Diagnosis made based on only clinical suspicion without radiological, laboratory or microscopic confirmation were excluded. Patients with death reported within the first month of diagnosis were excluded, as SEER data reports their survival as zero months. Step by step patient selection process is shown in Figure 1.
Patient race was categorized as white, black, Asian (Asian/ Pacific Islander), and native American (American Indian/Alaska native) based on SEER coding scheme (11). Based on SEER Summary staging manual [2000], the extent of the HCC at diagnosis was defined as localized, regional disease, distant disease, or missing. AJCC (American Joint Committee on Cancer) stage of HCC was obtained based on Collaborative Stage Data Set Type (CS extension codes) and divided into single nodule without vascular invasion, multiple nodules in different lobes without vascular invasion, any number of nodules with vascular invasion. Presence or absence of metastasis was categorized using CS metastasis codes. Therapy received was categorized using SEER site-specific therapy of primary site codes and divided into four groups: no surgery received, tumor-directed therapy, hepatic resection/lobectomy, and hepatectomy with a transplant. Tumor-directed therapy included heat radiofrequency ablation, photodynamic therapy, electrocautery, fulguration, cryosurgery, laser, or alcohol and acetic acid ablation.
Statistical analysis
Statistical methods used were non-parametric Kaplan-Meier survival estimates and Cox proportional hazards models. Equality of survival curves was tested using Cox-regression based tests and log-rank tests. Akaike and Bayesian information criterion in addition to graphical exploration of residuals using the Cox-Snell and Martingale residual fit were used as model diagnostics for selecting best models that fitted the data. Proportionality of hazards assumptions was also assessed to avoid gross violations of these model assumptions. Descriptive statistics of the patient’s demographics were analyzed using proportions, and Chi-square test of independence for categorical variables whereas Medians, Means, and standard errors were estimated if variables were continuous. Non-parametric Kaplan-Meier survival estimates together with semi-parametric Cox proportional hazards models were fitted to a continuous-time varying data, where the survival time was recorded in months from the SEER database. Stata statistical software, release 14 was used for analysis (13). Models examined the different rates of survival (or hazards) by mainly focusing on HCC staging, summary staging, therapy received while controlling for age at diagnosis and sex. In addition to the overall survival from the complete data, the analysis was also performed by stratifying the data into two groups; patients diagnosed between 2001–2007 and 2008–2013. Missing observations were considered to be missing at random and therefore dropped during the analysis.
Results
We identified a total of 50,088 HCC cases that met the inclusion criteria from SEER database. Demographic features are mentioned in the Table 1.
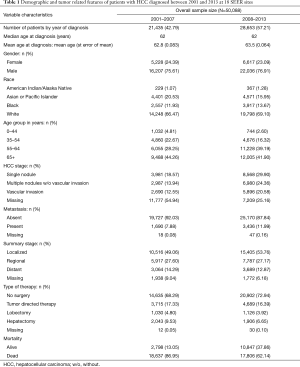
Full table
Out of the 50,088 patients, 36,443 (73%) died. The median relative survival for the entire population was 14 months with 5-year RSR of 21.20%; 11 months for those diagnosed in 2001–2007 with 5-year RSR 19.30% and 17 months for those diagnosed in 2008–2013 with 5-year RSR 22.40%, which was statistically significant, P<0.01. Survival rates were significantly lower for patients with age 65 years or older when compared to the groups with age less than 65 years (5-year RSR was 13% for 65+ years old vs. 24.8% for age group 18–44 years, 21.1% for 45–54 years, 20.7% for 55–64 years; P<0.001). Although survival remained poor, the 65+ years group had the highest increase in RSR since 2008, when compared to other age groups (5-year RSR was 11.10% vs. 14.20% for patients diagnosed in 2001–2007 and 2008–2013 respectively; P value <0.001).
Analysis based on AJCC HCC stage
To further categorize and understand the improvement in survival for patients diagnosed after 2007, we performed Kaplan-Meier analysis based on the stage of the disease, as shown in Figures 2 and 3. As shown in Table 2, the survival significantly improved for the group of patients with single nodule (30 vs. 36 months) or multiple nodules without vascular invasion (14 vs. 19 months), respectively for 2001–2007 and 2008–2013 in each category (P<0.05). Survival for advanced cases remained significantly poor without any real improvement since 2008, 8 months for patients with vascular invasion and only 4 months for patients with distant metastasis. Moreover, as shown in Table 3, when compared with patients diagnosed before 2008, hazards of having vascular invasion or presence of distant metastasis was significantly higher for patients diagnosed after 2008, P<0.05.
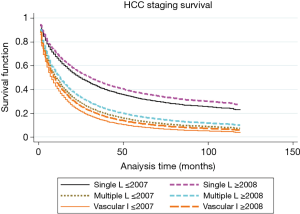
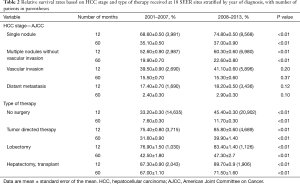
Full table

Full table
Analysis based on summary stage
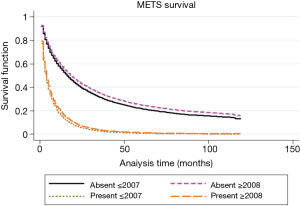
Median relative survival for patients diagnosed in 2001–2007 was 23, 8, and 4 months, respectively for localized, regional and distant stages. While patients diagnosed in 2008–2013 had better survival; 31, 11, and 5 months respectively for localized, regional and distant stages. As shown in Table 3, regional and distant stages showed poorer prognosis after 2008 with higher hazard ratios (HRs).
Analysis based on therapy received
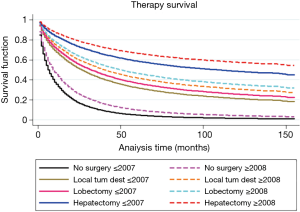
Approximately 70% of patients from our study population did not receive any HCC directed surgical intervention and among this more than 40% of patients were with early stage HCC with single nodule only. Median relative survival for those who did not receive any surgery was significantly lower when compared with patients who received any sort of surgical procedure (9 months for patients with no surgery vs. 38 months for patient received tumor-directed therapy vs. 49 months who received lobectomy; P<0.001). Even though survival for those who did not receive any surgery remained extremely low, it improved significantly after 2007 (as shown in Table 2). This survival improvement in the group of no surgery was limited only to patients with the single nodule, the median survival of 9 months for patients diagnosed in 2001–2007 with 5 years RSR 14.3% vs. 17 months for patients diagnosed in 2008–2013 with 5 years RSR 20.10%, P<0.05. As shown in Figure 4, hepatectomy with transplant showed the best survival compared to any other mode of therapy (5 years RSR was 69%; P<0.01 when compared to any other groups).
Discussion
The incidence of HCC has continued to rise over last few decades (2), and this could be attributed to the rising prevalence of obesity and type 2 diabetes mellitus (T2DM), the two major risk factors for Non-alcoholic fatty liver disease (NAFLD) (3). NAFLD is rapidly becoming the most common liver disease worldwide (14). The prevalence of NAFLD in the general population of western countries is 20–30%, with about 2–3% of the general population is estimated to have non-alcoholic steatohepatitis (NASH) which can progress to liver cirrhosis and HCC (14). Furthermore, increasing incidence of childhood obesity is quite alarming. Childhood obesity has more than doubled in children and quadrupled in adolescents in past 30 years (15), raises the possibility of even higher incidence of NAFLD and thus HCC in future.
HCC remains one of the leading causes of cancer morbidity and mortality worldwide. The advances in therapeutic modalities have changed the paradigm of HCC treatment in recent years, particularly tumor-directed therapy and molecular targeted therapy. However, to best of our knowledge, there is limited information on the impact of these on the survival trend of HCC patients in the population. So we present the analysis of HCC patients from SEER database based on the extent of disease and therapy received.
Early stage HCC
Patients with a single nodule or up to 3 nodules ≤3 cm in size without vascular invasion are considered as early stage HCC (BCLC staging) (8,9). Treatment also depends on the Child-Pugh score and Eastern Cooperative Oncology Group (ECOG) performance status of the patient. As per NCCN and BCLC guidelines (8,9), various treatment options for early stage HCC, includes tumor resection, transplant or ablation, all of which are potentially curative. Our analysis shows, the survival of patients with single nodule significantly improved since 2008, which was limited to patients who did not have surgical intervention and the patients who had tumor-directed therapy. Since the guidelines recommend essentially surgical options for early stage HCC, survival improvement in patients with single nodule with no surgical intervention can essentially be explained by lead time bias only. As seen in Table 1, a higher number of patients were diagnosed with single nodule or localized disease after 2007. This could be secondary to aggressive screening and clinical surveillance protocols of individuals with known risk factors for HCC, with better access to health care (16,17). Local tumor-directed therapies have emerged showing promising results recently (18). Our analysis confirms the survival improvement in early stage HCC patients, who received tumor-directed loco regional therapies. Unfortunately, our analysis revealed, approximately 70% of patients with HCC did not receive any surgical intervention and more than 40% of patients were with single nodule, at the stage which is potentially curable. Unfortunately, SEER data does not provide information on underlying reasons for not receiving the surgical intervention, the analysis suggests, the potentially curative therapies for early stage HCC are underutilized. This could partly be secondary to decreased access to these sophisticated procedures, which are often offered at the tertiary centers only. A similar trend was found in a recently published meta-analysis; early stage HCC patients, who are elderly, non-Caucasian and lower socioeconomic status received significantly lower rates of tumor-directed therapies (19). Further research in this area to identify various underlying reasons would be very vital to overcoming this challenge.
Intermediate stage HCC
Patient with large multinodular HCC with Child-Pugh A or B falls under intermediate stage HCC (8,9). As per guidelines, treatment option mainly includes tumor-directed therapies, mainly trans-arterial chemoembolization (TACE). Various studies have shown a survival benefit of TACE (20,21). Unfortunately, SEER data does not provide specific information on how many patients exactly received TACE.
Advanced stage HCC
Patients with any numbers of nodule with vascular invasion and/or distant metastasis are considered advanced stage HCC (8,9). Treatment would further depend upon the Child-Pugh score and ECOG performance status of the patient. Supportive care used to be the only treatment option for advanced-stage HCC, but sorafenib has become the standard of treatment ever since the FDA approval in 2007 (8,9). SHARP trial showed 3 months survival benefit of sorafenib in advanced stage HCC (7). Since then, sorafenib has been studied as a single agent or in combination with other modalities with inconsistent results (21-26). Our analysis of SEER database shows the survival of advanced stage HCC remains extremely poor with no significant difference after the approval of sorafenib. In an observational study for SEER data, Sanoff et al. (27) reported that in Medicare beneficiaries, there was no statistically significant difference in survival of advanced staged HCC patients, whether they received sorafenib or not. This discrepancy between the clinical trial results and real world results could be secondary to several factors including patient health status, socio-economic factors, comorbidities, disease burden, tolerability of the medication, treatment adherence and accessibility to medical care. Parikh et al. (28) also reported similar findings showing marginal benefit with no cost effectiveness of using sorafenib in advanced stage HCC Medicare beneficiary patients. Although, SEER does not provide details on chemotherapy rendered to patients, the higher HR of advanced stage HCC since 2008 suggests, efficacious treatment options for advanced stage HCC is still lacking.
Our study has few limitations. First and foremost is retrospective nature of the study. Secondly, while quite detailed, SEER database lacks in the information about the etiology of HCC (cause of cirrhosis), and other comorbidities. Furthermore, it lacks the information about the chemotherapy rendered to patients. Thirdly, this data also lacks in the information about the Child-Pugh score and functional status of the patient, which directly affects candidacy of patients to different therapies and thus the survival.
In conclusion, the incidence of HCC is increasing and HCC remains one of the commonest cause of cancer deaths worldwide, which is quite concerning. In spite of all the advances in the treatment of the etiology of HCC such as hepatitis B and hepatitis C (16,17) along with the advances in HCC treatment itself, survival from HCC remains extremely poor. This can partially be explained by the poor utility of some of the efficacious treatment options in early stage HCC, and also due to lack of any effective treatment options for advanced stage HCC cases. Not utilizing effective treatment options in early stage HCC, while it is still curable, can impose a significant financial burden. As these patients would progress on to the advanced stage where treatment options are very limited and not as cost-effective. Our study emphasizes the urgent need for efficacious and cost-effective treatment options for advanced stage HCC and also highlights the need for further research to identify various barriers and the possible need for healthcare policy changes to better utilize the existing treatment modalities particularly for early stages of HCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: No formal approval of IRB is required as data were collected from a source that was publicly available and did not contain unique patient identifiers. We obtained permission to access research data files of SEER database with the reference number 13253-Nov2014. Given that these data are de-identified and ethics approval is waived, the study did not require informed consent.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191-9. [Crossref] [PubMed]
- Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci 2016;61:1234-45. [Crossref] [PubMed]
- Wang S, Sun H, Xie Z, et al. Improved survival of patients with hepatocellular carcinoma and disparities by age, race, and socioeconomic status by decade, 1983-2012. Oncotarget 2016;7:59820-33. [Crossref] [PubMed]
- Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542-53. [Crossref] [PubMed]
- de Lope CR, Tremosini S, Forner A, et al. Management of HCC. J Hepatol 2012;56 Suppl 1:S75-87. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Benson AB, Abrams TA, Ben-Josef E, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw 2009;7:350-91. [Crossref] [PubMed]
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program () SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (1973-2013 varying) - Linked To County Attributes - Total U.S., 1969-2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission.www.seer.cancer.gov
- Mavros MN, Mayo SC, Hyder O, et al. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. J Am Coll Surg 2012;215:820-30. [Crossref] [PubMed]
- StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
- Bellentani S, Scaglioni F, Marino M, et al. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010;28:155-61. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. (2011). Prevalence of Childhood Obesity in the United States, 2011-2014. Available online: https://www.cdc.gov/nchs/data/databriefs/db219.pdf
- AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:932-54. [Crossref] [PubMed]
- Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261-83. [Crossref] [PubMed]
- Shi Y, Zhai B. A Recent Advance in Image-Guided Locoregional Therapy for Hepatocellular Carcinoma. Gastrointest Tumors 2016;3:90-102. [Crossref] [PubMed]
- Tan D, Yopp A, Beg MS, et al. Meta-analysis: under utilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther 2013;38:703-12. [Crossref] [PubMed]
- Xing M, Kokabi N, Prajapati HJ, et al. Survival in unresectable AJCC stage I and II HCC and the effect of DEB-TACE: SEER versus tertiary cancer center cohort study. J Comp Eff Res 2016;5:141-54. [Crossref] [PubMed]
- Cosgrove DP, Reyes DK, Pawlik TM, et al. Open-Label Single-Arm Phase II Trial of Sorafenib Therapy with Drug-eluting Bead Transarterial Chemoembolization in Patients with Unresectable Hepatocellular Carcinoma: Clinical Results. Radiology 2015;277:594-603. [Crossref] [PubMed]
- Imedio ER, Beveridge RD, Urtasun JA, et al. Safety and efficacy of sorafenib in the treatment of advanced hepatocellular carcinoma: a single center experience. Med Oncol 2014;31:948. [Crossref] [PubMed]
- Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol 2016;65:1140-7. [Crossref] [PubMed]
- Merchante N, Ibarra S, Revollo B, et al. Real-life experience with sorafenib for the treatment of hepatocellular carcinoma in HIV-infected patients. AIDS 2017;31:89-95. [Crossref] [PubMed]
- Brade AM, Ng S, Brierley J, et al. Phase 1 Trial of Sorafenib and Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2016;94:580-7. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Sanoff HK, Chang Y, Lund JL, et al. Sorafenib Effectiveness in Advanced Hepatocellular Carcinoma. Oncologist 2016;21:1113-20. [Crossref] [PubMed]
- Parikh ND, Marshall VD, Singal AG, et al. Survival and cost-effectiveness of sorafenib therapy in advanced hepatocellular carcinoma: An analysis of the SEER-Medicare database. Hepatology 2017;65:122-33. [Crossref] [PubMed]



