The status of targeted agents in the setting of neoadjuvant radiation therapy in locally advanced rectal cancers
Introduction
Radiotherapy is routinely used in rectal cancer as an adjuvant treatment (prior to or following surgery) in an attempt to eradicate microscopic (or occasionally macroscopic) residual disease and reduce the risk of local recurrence. Preoperative chemoradiation can also facilitate the achievement of a curative resection, where clinical staging suggests tumour extends to or beyond the mesorectal fascia (MRF). Finally radiotherapy is used as a palliative treatment to relieve cancer-related symptoms such as pain and rectal bleeding. Radiotherapy in early-stage rectal cancer as a definitive radical treatment in its own right can also substitute for surgery. Historically, a high local recurrence rate in rectal cancer has been observed when patients are treated with surgery, and between 10-40% of patients still require a permanent stoma. In resectable cancers, both short course preoperative radiotherapy (SCPRT) and long-course preoperative chemoradiation (CRT) have been shown to be effective in reducing the risk of local recurrence. High quality surgery using total mesorectal excision (TME) has lowered the rate of local recurrence even further (even when radiotherapy is not routinely utilised) to approximately 6% (1-3), which in turn may impact on distant metastases. However, only one European trial of chemoradiation published in the last decade impacted on disease free survival (DFS) (4) and none on overall survival (OS). Driving down the risk of local recurrence has in turn highlighted the risk of metastatic disease in 30-40% of cases, which appears now the predominant problem (5).
Chemoradiation has an important role for more locally advanced cases where surgery for complete tumour clearance is regarded as borderline, or in unresectable cases, where the mesorectal fascia (MRF) is breached or the pathological circumferential resection margin (CRM) potentially threatened according to the magnetic resonance imaging (MRI). In this advanced group selected by MRI, current chemoradiation schedules are only partially effective, since some patients still fail to achieve sufficient downstaging for surgery to be considered. Of those operated upon many do not achieve an R0 resection (6). Even with chemoradiation at least half the patients fail to achieve T-stage downstaging (4,6).
Response is therefore important not only for unresectable cancers. When downstaging is observed after radiochemotherapy, there are fewer recurrences and a better prognosis. Both combination chemotherapy and the use of targeted therapies in addition to chemotherapy have made a significant impact on the ability to resect initially unresectable liver metastases (7-9).
Yet attempts to increase response rates by integrating 2 cytotoxic drugs into CRT regimens have often been accompanied by excess toxicity and only minimal increases in efficacy. The integration of biological agents into chemoradiation is an attractive strategy both to improve local control and to reduce the high risk of metastases (in combination with or without chemotherapy) because of the targetted agents specificity and perceived lower levels of associated toxicity. However, it should be noted that Bevacizumab as a single agent was associated with a 36% overall incidence of grade 3 or 4 toxicity in the E3200 trial (10), and Cetuximab as a single agent was associated with a 43% overall incidence of grade 3 or 4 toxicity in the BOND trial (11).
It should be borne in mind that cytotoxic agents such as irinotecan and biologically targeted monoclonal antibodies such as bevacizumab and cetuximab despite their acknowledged efficacy in the metastatic setting, have consistently failed to show a benefit in DFS or OS when used as adjuvant chemotherapy in the postoperative setting in colon cancer (12-15). This observation underlines the principle that the use of combinations cannot simply be based on presumptions, but must be tested in prospective trials.
Novel biologically targetted agents may interact with cell signalling pathways involved in DNA repair, cellular proliferation apoptosis and angiogenesis which are differentially expresssed in tumour and normal tissues. This specificity could be exploited by integrating the targetted agent in combination with radiotherapy or chemoradiotherapy to achieve a therapeutic advantage i.e., to promote a greater cell kill for equivalent or less normal tissue toxicity. Dose response sigmoid curves plotting tumour control probability and normal tissue complication rates against radiation dose are often cited. This simple and attractive theory is the hallmark of cell line work which does not take into account the tumour environment, the fraction size and radiation field size. A huge number of small clinical phase 1/phase 2 studies have not been extended into the routine clinical setting. However, insufficient pre-clinical data to support the precise timing, sequence and optimal doses of these agents has bedevilled our efforts. Given that it takes several years to obtain mature results on LR, DFS and OS, there has been a tendency in phase I/II studies to use the primary endpoint of complete pathological response (PCR) as a surrogate for long-term clinical outcome.
Further speculation suggests that, because of principles of Darwinian evolution, the hypothesis goes that single targets are unlikely to apply to the majority of patients with common tumours because of inherent heterogeneity. Multiple targets are more likely to be effective in view of cross-talk between different cell signaling pathways. Although in rare cancers such as GIST tumours or subsets of a common cancer with a specific mutation, this strategy may be feasible. Radiobiologists would like to believe that because many tumours demonstrate a complete clinical response (but recur later) that we only need to kill a few radioresistant clones/stem cells to achieve clinically significant greater gains in locoregional control. Clinical experience seems less simplistic.
Moreover, early phase I clinical trials of novel agents in combination with RT raise difficult logistical, ethical and financial constraints. Despite the carrot that the novel agent may contribute to cure, the Pharmaceutical industry is often wary that treatment-related toxic events and the adverse publicity can tarnish or completely blight the future prospects of the novel agent—even if effective. Adding novel targeted drugs to either 5-FU-based, irinotecan based or oxaliplatin-based chemoradiation also adds considerable complexity to the interaction. Many concurrent CRT regimens are already close to the limits of normal tissue tolerance in terms of both acute and late effects. Further treatment intensification by integrating higher doses of the cytotoxic, delivering more frequent administration of the cytotoxic or even by the adding further different non-cross resistant cytotoxics with different toxicity profiles still carries considerable risks (16,17).
In this review, we examine the strategies of neoadjuvant chemoradiotherapy with cytotoxic agents, and the integration of additional biological agents which target EGFR and angiogenesis. There are no randomised phase III trials, so we will evaluate the few published phase I/II trials of targeted agents and assess if irinotecan or oxaliplatin was a more effective partner with cetuximab and radiotherapy. In order to integrate novel agents alongside RT, many possible study designs are possible. We will review their respective strengths and weaknesses and some of the key challenges to further development of the integration of targetted agents. Trial acronyms have often been used for the sake of brevity. Readers are encouraged to refer to the cited references for full details. We believe there is a need both new radiosensitizing agents and accurate predictive biomarkers to help optimize the use of existing strategies.
The evidence
Since the early 1980s, the fluororopyrimidine-5Fluorouracil (5-FU), and more recently combinations of cytotoxic chemotherapy using oxaliplatin or irinotecan, have represented the mainstay of chemotherapy treatment for patients with advanced and metastatic colorectal cancer (MCRC). Randomised trials have also confirmed the success of systemic regimens of 5-FU and oxaliplatin in dealing with distant micro-metastases in the adjuvant setting in colon cancer (18-20).
Four molecular targeted agents (cetuximab, panitumumab, bevacizumab and aflibercept) have now been integrated into standard chemotherapy regimens to improve response rates and extend progssion free (PFS) and overall survival (OS)—with varying success (8,21-24).
The combination of chemotherapy and radiotherapy aims to utilise both the independent effect of each modality and produce additive effects. Chemotherapy may enhance the initial DNA damage from radiation, inhibit DNA repair, or slow cellular repopulation during the latter part of fractionated radiotherapy. With some cytotoxic agents radiotherapy and chemotherapy may target different phases of the cell cycle, and radiosensitization may be partly dependent on cell cycle synchronization of the tumor cell population.
The fluoropyrimidines have attained a strong track record in chemoradiation schedules increasing the path CR rate by about 300% from 4-5% to 12-15% (25,26) with low toxicity over radiation alone (Table 1). Yet, these combinations have had only moderate success in improving outcomes in rectal cancer (27,28,31-34). Randomised phase III trials of neoadjuvant preoperative chemoradiation (CRT) in resectable rectal cancer (28,32) show that the addition of 5-FU to preoperative radiation improves loco-regional control (26,32), but has not extended disease-free survival (DFS) or overall survival.

Full Table
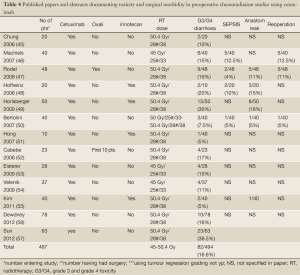
Early phase I/II trials integrating 5-FU and Irinotecan showed promise and PCR rates have ranged up to 38%. In the largest of these studies, the PCR rate was 14%. The RTOG 0012 study randomised 106 patients with T3/T4 rectal cancers between hyperfractionated radiotherapy with prolonged venous infusion of 5-FU or standard fractionation with PCR 5-FU and 50 mg/m2 of Irinotecan as a radiosensitizer per week but showed no benefit (33,35). Further advances have been limited by the observation that any additional increase in tumour control appears often to be balanced by an increase in acute and late normal tissue toxicity. The current national trial in the UK (ARISTOTLE) is examining the utility of the incorporation of irinotecan into pre-operative CRT in MRI defined unresectable/borderline resectable rectal cancer (www.controlled-trials.com/ISRCTN09351447).
Similar phase II trials with oxaliplatin appeared encouraging (36,37). However, preliminary results from randomized phase III trials, evaluating the addition of oxaliplatin to preoperative fluoropyrimidines-based CRT, have not shown a significant impact on early pathological response (STAR-01, ACCORD 12/0405-Prodige 2, NSABP R-04) with the exception of the German CAO/ARO/AIO-04 study. In addition, the PETACC-6 trial randomized patients between preoperative RT (50.4 Gray in 25 fractions) with capecitabine alone the same radiation schedule with capecitabine + oxaliplatin (50 mg/m2). Results have not yet been reported (Table 2).

Full Table
Efforts to improve the outcome from chemoradiotherapy further have focussed on adding biological agents to avoid overlapping toxicities. A landmark randomised phase III study in patients with locally advanced head and neck cancer showed that cetuximab in combination with radical radiotherapy significantly improved overall survival (41) compared to radiation alone. Many mechanisms have been postulated (42), including inhibition of repopulation during the latter phase of radiotherapy. Accelerated treatments improve outcome only in head and neck cancers, which have high EGFR expression (43). Yet, this benefit from cetuximab has not been extended to chemoradiation. In the Radiation Therapy Oncology Group (RTOG) 0522 trial patients with locally advanced head and neck showed a 2-year progression-free survival (PFS) of 63.4% with cetuximab versus 64.3% with cisplatin-based chemoradiation alone, and overall survival improved slightly but not significantly with cetuximab (44).
Hence, with our increasing knowledge of molecular pathways in cancer, can we identify sufficient potential targets that may be manipulated to enhance the radiation response selectively in rectal cancers compared to normal tissues such as small bowel and the sphincter complex?
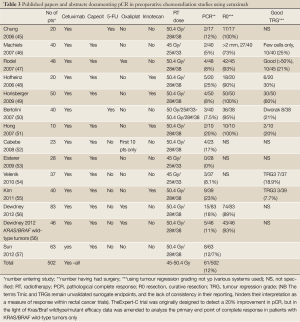
We found 13 papers documenting combinations of chemoradiotherapy with cetuximab, 2 with panitumumab and 15 with bevacizumab. Cetuximab-containing neoadjuvant chemoradiation has not been shown to improve tumor response/ pathologic complete responses in locally advanced rectal cancer patients in recent phase I/II trials (Tables 3,4). The data with panitumumab and small molecules is even sparser (Table 5). With bevacizumab (Table 6), although there are perhaps pointers to increased efficacy, concerns have been raised regarding an exacerbation of surgical morbidity (79).
Full Table
Full Table
Full Table
Full Table
The identification of biomarkers to tailor treatment to patients most likely to benefit has become the holy grail of investigation of novel treatments and regimens. While the selection of specific agents in a given combination has been based on biological considerations (including the role of the putative targets in cancer) and the interactions of the agents used in combination, there has been little exploration of the possible enhanced toxicity of combinations resulting from alterations in multiple signalling pathways in normal cell biology.
Any interruption in the delivery of CRT or even abandonment of this component of treatment in the case of severe unexpected toxicity could have a negative impact on local tumour control (80). If risks are to be minimised, clinical programmes need to be based on sound preclinical data and early phase studies in the palliative setting in patients with metastases. Investigators should recognise this is not the same scenario as locally advanced rectal cancer, and responses may be less. In order to reassure study sponsors and regulatory agencies, additional preclinical evaluation of the combinations is therefore essential, prior to initial evaluation of radiation-novel drug combination.
Owing to the complex networks and crosstalk that govern normal and tumour cell proliferation, inhibiting multiple pathways with targeted agent combinations can result in unpredictable disturbances in normal physiology. While numerous combination trials of targeted agents that target dysregulated pathways have been conducted, there has been little exploration of the molecular vulnerability of normal tissues to these combinations.
The epidermal growth factor receptor (EGFR) pathway
The epidermal growth factor receptor (EGFR) is a 170-kD trans-membrane glycoprotein. It is one of 4 members of the Erb-B family of proteins, and is also known as Erb-B1 or HER-1 receptor. In addition there are Erb-B2 (HER-2), HER-3 and HER-4. These receptors are part of a complex and inter related downstream signalling pathway deregulation of which is commonly seen in a number of malignant phenotypes. EGFR ligands include EGF, amphiregulin, epiregulin, neuregulin, transforming growth factor-a (TGF-a) and heparin binding EGF-like growth factor (HB-EGF) (81). There is also receptor cross-activation. The main downstream signalling pathways include the ras-raf mitogen activated protein kinase (MAPK), which controls cell-cycle progression and proliferation; and the phosopho-inosotide 3 kinase (PI3K-AKT) pathway, which is anti-apoptotic and promotes cell survival (82).
EGFR has a putative role in the repair of sublethal DNA-damage and can potentially influence DNA repair by translocation of DNA dependent protein kinase (DNA-PK) from cytoplasm to the nucleus (83), and by transcription and phosphorylation of repair genes (XRCC1 and ATM) (84).
EGFR appears to be over-expressed in 60-80% of tumours (85), either by ligand overproduction, receptor overproduction, extended receptor lifespan or constitutive overactivation of the receptor. This over-expression has been associated with a more aggressive tumour phenotype associated with adverse patient survival (86-88) and a poor tumour response to conventional therapy with acquired resistance to both chemotherapy and radiotherapy (69,89).
The rationale of integrating EGFR into chemoradiation schedules
Pre-clinical studies have shown that inhibiting EGFR signalling slows cell proliferation in vitro and in vivo and also additive effects are observed with radiotherapy (90), with enhanced radiocurability (91). There is speculation that hypoxic cells express more EGFR and are more sensitive to EGFR inhibition (92). Some investigators found a correlation between EGFR expression and complete pathologic response, disease-free and metastasis-free survival (85). However, most clinical studies showed the opposite—with low rates of pCR and shorter DFS (50,93-95). The risk of loco-regional recurrence may also be increased (96). In a study by Debucquoy, tumour proliferation decreased, as measured by Ki67 expression, following a loading dose of cetuximab (97). EGFR expression was upregulated in 55% of cases, downregulated in 30% (10/33), and remained unchanged in 15% (5/33). In patients with an upregulated EGFR expression an improved DFS was demonstrated (P=0.02).
Cetuximab and chemoradiation for rectal cancer
The EGFR pathway can be targeted either through monoclonal antibodies, the small molecule tyrosine kinase inhibitors (TKIs), anti-sense nucleotides, ligand toxins and inhibitors of downstream effects of the EGFR signalling pathway. Current monoclonal antibodies in clinical use include cetuximab and panitumumab. Cetuximab is a chimeric monoclonal antibody against the extracellular domain of the epidermal growth factor receptor (EGFR) leading to competitive inhibition of ligand-binding, which then prevents the dimerisation and activation of the receptor and inhibits the downstream signalling pathway. Binding of the antibody also stimulates the cell to internalise and degrade the receptor. The mechanism or action of these monoclonal antibodies appears to involve cell cycle arrest at G1, promotion of pro-apoptopic factors, decrease in levels of anti-apoptopic factors, and inhibition of angiogenesis. Cetuximab has also been suggested to also induce antibody-mediated cellular cytotoxicity (ADCC) due to its human IgG1 backbone, which may contribute to its anti-tumor effects. In contrast, two oral tyrosine kinases Gefitinib and Erlotinib act by inhibiting ATP binding and prevent phosphorylation in downstream signalling proteins.
Cetuximab and panitumumab have activity as single agents and increased response rates are achieved when these are added to standard chemotherapy schedules. Clinical studies in colorectal cancer have confirmed the efficacy of cetuximab in irinotecan refractory patients in terms of response rate and progression free survival (11), and have shown a significant benefit in response rates and progression free survival for the addition of cetuximab to FOLFIRI (98,99). Among patients with wild-type KRAS tumours, OS and PFS were significantly greater with the addition of cetuximab to FOLFIRI than with FOLFIRI alone (99). However, these results have not been replicated in the COIN study or the Nordic study, where in contrast cetuximab was added to oxaliplatin and 5-FU or capecitabine in the first-line setting (100,101).
The common side-effects of cetuximab include an acneiform rash and diarrhoea, which could prove a problem of overlapping toxicity with pelvic radiation. However, in rectal cancer the crude rate of G3/G4 gastrointestinal toxicity, in terms of diarrhoea, does not appear increased by the addition of cetuximab to chemoradiation.
It is now recognised that patients with mCRC and KRAS mutations are unlikely to benefit from the addition of cetuximab to standard chemotherapy (99,102-104). There is also evidence from a Spanish study that the combination of cetuximab and capecitabine is clearly active in wild type K-ras patients with metastatic disease and doubles the response rates from 24% to 48% over capecitabine alone (105).
Recent results of the preliminary use of cetuximab in the adjuvant setting, combined with 5-FU and oxaliplatin in colon cancer, have demonstrated excess toxicity in the over 70s. No advantage in DFS has been demonstrated and indeed some patients in the over 70s age group may well have been disadvanataged by this approach.
Cetuximab has been successfully combined with radical radiotherapy alone in head and neck cance, but combinations of cetuximab, chemotherapy and radical radiotherapy in head and neck cancer show no advantage to the addition of cetuximab (44).
However, in rectal cancer, the role of KRAS mutation status on tumour response when cetuximab is combined with chemoradiation is more opaque. None of the studies selected patients according to Kras status, so data is founded on retrospective analyses.
In addition, the proportion of patients with rectal cancer (as opposed to colon cancer) with mutant K-ras varies between only 12% (106) and 30% (107).
Several small studies are either equivocal (108-112) or suggest a negative association (113) for the presence of tumour KRAS mutations and tumour regression (either clinical or histopathological) and/or survival in patients with rectal cancer undergoing preoperative CRT. In a recent preoperative chemoradiation study using cetuximab, K-ras mutant type was found in 9/39 (23%) patients. Only one of these nine K-ras mutant patients (11%) demonstrated a good pathological regression (TRG3 and 4) compared to 11/30 (37%), (P=0.12) in patients with wild-type K-ras (114). In contrast, K-ras status did not significantly influence response in a Belgian study using cetuximab prior to and concurrent with capecitabine and (97). In a pooled analysis of 2 phase II studies, KRAS mutations were detected in 20 of the 82 patients (24.4%). 3-year DFS was higher i.e., 86.6% versus 75.0% but not significantly improved for patients receiving cetuximab with chemoradiation and chemoradiation alone, The lack of difference in outcomes remained whether assessed in KRAS wild-type or mutant patients (115).
Some authors have pointed out that there may be an optimal sequence of chemotherapy, biological agent and radiation if we are to avoid the potential for antagonism (58). The lack of additive effect can be explained if the addition of other agents leads to an over targeting of one target (? the endothelial cells within the tumour); if the novel agent leads to cell-cycle arrest protecting cells from the effects of 5-FU; if drug concentrations are suboptimal because of the low weekly doses being ineffectual; if there are antagonistic drug-drug interactions which could be more prominent in the presence of radiation [we know from the PACCE and CAIRO (23,116) trials that combinations of cetuximab and bevacizumab with 5-FU and oxaliplatin are antagonistic]; if biological agents and the apoptotic response and hence secondary immune phenomena are modified—after all neither bevacizumab nor cetuximab appear effective in the adjuvant setting; finally a heightened inflammatory response may simply attract more stem cells and actually assist repair. The multifactorial nature of these potential problems is obvious and poses a significant challenge if we wish to continue this form of biological, chemotherapeutic and radiation integration. However, some authors claim that target guided individualisation of treatment according to molecular markers can be successfully achieved (117).
A large multinational randomised phase II study EXPERT-C (NCT00383695) has compared neoadjuvant therapy comprising combination chemotherapy (oxaliplatin and capecitabine), with or without cetuximab followed by chemoradiotherapy with capecitabine with or without cetuximab in 164 patients (56). In the EXPERT-C trial, retrospective molecular analysis for KRAS/BRAF was successfully performed in 149 patients, of whom 90 (60%) were wild type. The pCR rate was not significantly higher with the addition of cetuximab to preoperative chemotherapy and CRT either for the group with locally advanced rectal cancer as a whole (18% versus 15% respectively), nor for KRAS wild-type - although this percentage is diluted by the fact that some samples achieving pCR were not available for Kras testing. Interestingly DFS in the selected KRAS wild-type group who received cetuximab was higher (56).
Preoperative treatment strategies for rectal cancer differ from radical chemoradiotherapy for head and neck cancer in a number of important areas. First there is radiation dose, where compared with doses of 60-70 Gy in head and neck cancer, 45-50 Gy is not considered as a radical curative dose, but potentially sufficient for microscopic disease. Tumour cell repopulation may be less crucial in a preoperative setting, when surgery is scheduled, than in squamous carcinomas of the head and neck. Certainly, repopulation does not appear to be such a major issue in adenocarcinoma of the rectum as in some squamous cancers. In treatment with radiation alone, neither overall treatment length nor a treatment interruption appear to impact on local control (118). Repopulation may also be less crucial in the presence of a continuous exposure to 5-FU, or capecitabine chemoradiation.
Cell cycle effects seem important to achieve these additive effects (90,119). 5 Fluorouracil (5-FU) is S-phase specific and acts by inhibiting thymidylate synthase and the synthesis of thymidine nucleotides required for DNA replication, thus preventing cell division. Additive effects can normally be observed by the addition of 5-FU to radiation at concentrations, which on their own are non-cytotoxic and when tumour cells have become resistant to 5-FU. Additive effects with 5-FU and RT may only occur in cells, with inappropriate progression through S-phase in the presence of 5-FU (120). When S-phase entry is blocked resulting in G1 arrest or the progression to S-phase is inhibited, additive effects are not observed from 5-FU and radiation, and cell cycle delay in the G1 and G1/S boundary may explain acquired resistance to 5-FU (121).
Slowing down the cell cycle time may increase the amount of time available for DNA repair extending G1-repair prior to S phase and mitosis, and thus could increase the potential for resistance to both 5-FU and radiation. The use of cetuximab prior to or concurrently with radiation might therefore abolish fluoropyrimidine-based radiosensitisation, if only a small proportion of cells arrest in G0/G1 or G2/M. High EGFR expression appears linked to high Ki-67 and PCNA, demonstrating increased rates of cell turnover (122). This study showed that significant decreases in proliferation with the addition of 5-FU, which were not seen with radiation alone. This finding also suggests that 5-FU does not recruit quiescent cells into proliferation.
Cetuximab can lead to G1 or G2/M cell cycle arrest, and if only a small proportion of cells within the tumour are affected, this decrease in proliferation could impact on the chance of achieving a complete pathological response. This hypothesis is supported by the evidence from one of the cited studies, which suggests that cetuximab up-regulated several genes involved in proliferation (PIK31, CGREF1 and PLAGL1) with a reduction in Ki67. This process might also affect oxaliplatin, which is mainly active in S phase, but would be less likely to be impacted by irinotecan.
Panitumumab
Panitumumab is a fully humanised IgG2 monoclonal antibody against human EGFR.
Recent studies have shown that with panitumumab median PFS times were similar for patients with negative, low, and high levels of EGFR expression (123). The efficacy of panitumumab monotherapy in patients with KRAS wild-type metastatic colorectal carcinoma refractory to standard chemotherapeutic agents has been shown in the pivotal open label phase III study (124,125) in which panitumumab significantly prolonged progression-free survival versus best supportive care (median 12.3 vs. 7.3 months, P<0.0001). Disease control was also improved with 51% versus 12% benefiting from treatment (PR, SD). OS was not significantly different between both groups- possibly because of crossover from the best supportive care alone to panitumumab after progression, which could confound the results. An exploratory analysis excluding crossover supports this hypothesis.
The combination of panitumumab and FOLFOX for first-line treatment has been investigated in a randomized study (PRIME) where 1,183 patients were randomized to FOLFOX4 with panitumumab every two weeks versus FOLFOX4 alone. Patients with wild-type KRAS in the panitumumab group had a median PFS of 9.6 months and a RR of 55% compared to a PFS of 8 months and a RR 48% respectively in patients with unmutated KRAS treated with FOLFOX4 alone (126).
The phase II multicentre, PACCE (Panitumumab Advanced Colorectal Cancer Evaluation) study evaluated the efficacy and safety of adding panitumumab to combination chemotherapy with bevacizumab for the first-line treatment of mCRC (116). A planned interim analysis revealed that PFS and OS were worse in the panitumumab plus bevacizumab and chemotherapy arm compared to the standard bevacizumab and chemotherapy arm.
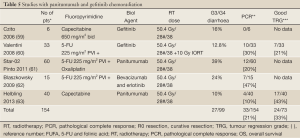
In the second-line setting, patients with wild-type KRAS were found to have significantly increased OS in the FOLFIRI/panitumumab group (127) with 14.5 versus 12.5 months in the wild-type KRAS group over FOLFIRI alone. No significant difference in PFS or OS was noted in patients with KRAS mutations. Two phase II trials have examined the integration of panitumumab into CRT schedules (61,63) see Table 5. In the StarPan (STAR-02) Study (61), pCR rate was 12/60 (20%), in the SAKK 41/07 trial this rate was 4/40 (10%) (63), which seems higher than the pCR achieved in the phase II studies based on cetuximab–fluoropyrimidine combination with or without oxaliplatin. Interestingly in the SAKK trial 43% achieved near complete regression (Dworak 3TRG) most of these residual cells were not apoptotic (63). The Italian group are intending to perfrom a further STAR Study (Rap Study/STAR-03) to evaluate panitumumab in combination with RT alone in low-risk LARC.
Gefitinib and erlotinib
Few results from clinical trials in mCRC patients are available which have examined treatment with downstream tyrosine kinase inhibitors of EGFR such as gefitinib and erlotinib—either a single agents (128,129) or in combination with standard chemotherapy regimens (130-132). Data suggests significant treatment related toxic effects without a strong clear message of additional benefit. There have been no successful studies to demonstrate the individual single agent activity of these agents except the multi-targetted agent rogarafenib (133) or any advantage with combination chemotherapy.
Pre-clinical studies with Gefitinib have shown that there are additive effects when combined with both radiotherapy and chemotherapy (134). In the clinical setting, a phase I trial combining gefitinib, capecitabine, and radiation in rectal cancer, resulted in significant toxicity, and no recommended phase II dose could be recommended (59). A small Italian study of 41 patients treated patients with ultrasound defined T3/T4 or N+ rectal cancer using a combination of prolonged venous infusion (PVI) of 5-FU and Gefitinib with pelvic radiotherapy (60). They reported a pCR of 30%. However, significant grade 3 toxicity was seen, 21% of these were GI symptoms and 26% hepatic, such that 61% of patients required a dose reduction. We did not find a single study integrating Erlotinib into radiotherapy in the neoadjuvant setting, either published or presented
Predictive markers
In other disease sites there is evidence of marked intratumour heterogeneity in samples obtained from a single tumour biopsy. Not all genetic aberrations (including mutations, allelic imbalance, and ploidy) present in the entire tumor specimen are demonstrated in a single biopsy. This observation sets major challenges to personalized—medicine and future biomarker development (135).
Although some less invasive clinical markers have been proposed for bevacizumab, such as circulating endothelial cells (CECS), circulating levels of VEGF and the development of overt hypertension, these biomarkers have not been validated and are observed to emerge only after a trial of the agent. For cetuximab, the appearance of an acneiform rash is associated with response, but low levels of magnesium appear more controversial.
EGFR
Tumours are heterogeneous with regards to EGFR expression, but it is now accepted that testing for level of expression is irrelevant, and does not predict response (136,137), nor clinical outcome in trials of EGFR-positive mCRC utilising cetuximab. However, patients lacking any EGFR expression were ineligible. It is difficult to explain how a tumor with perhaps less than 1% of cells expressing low levels of EGFR has the same likelihood of response to an agent that supposedly only targets that population, than a tumor where 90% of cells express high levels of the target. In contrast interest has centred on K-ras status, because K-ras mutations appear constitutively to activate the signalling pathways, and stimulate cell proliferation (138).
KRAS, BRAF and PIK3CA mutations are commonly found in colorectal cancers.
PIK3CA mutations appear associated with a worse prognosis in stage I to III colon cancer (139), but the impact of KRAS and BRAF mutations on clinical outcome of patients is less clear.
BRAF, Ligand production of epiregulin and amphiregulin, the other RAS genes (NRAS and HRAS) have also been proposed as potential predictive markers, but have not been validated. It should be noted that it is much easier to define whether a mutation is present (or not) in a gene than to standardise the methodology for measurement of over expression or amplification.
Wild-type KRAS is an imperfect biomarker, because only 30-50% of such patients respond to cetuximab, or achieve any improvement in PFS or OS, but some (97,140) have found no correlation between wild-type Kras status and tumour pathological complete response in CRT trials.
BRAF
BRAF mutations are mutually exclusive to KRAS mutations and are found rarely in colonic carcinomas (approximately 10%), and may be even less frequent in rectal cancer (140), but few studies distinguish between rectal and colon cancer. The majority of the BRAF mutations are located at codon 600 with a conversion of valine to glutamic acid (V600E).
There are no effective drugs available for the specific and direct inhibition of KRAS. Several agents designed to inhibit the kinase activity of BRAF have been explored in melanoma, but have not been effective in CRC studies. There are suggestions that Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer (140). The treatment of patients with BRAF-mutated tumors using cetuximab/panitumumab in combination with a BRAF-inhibitor, are both possible and logical, but this strategy has not been used.
PI3K
In patients in the Dutch TME trial (141), DNA mutations in PIK3CA, KRAS, and BRAF were investigated in 240 stage I to III rectal tumors obtained from non irradiated patients. PIK3CA mutations at exons 9 and 20 were found in 19 (7.9%) rectal tumors, with 12 cases in exon 9 (5%) and 7 cases in exon 20 (2.9%); in 81 (33.8%) in exon 1 rectal tumors. BRAF V600E mutation was identified in 5 (2.1%) cases. PIK3CA mutations independently prediced local recurrences (hazard ratio, 3.4; 95% confidence interval, 1.2-9.2; P=0.017), next to tumor-node-metastasis stage.
PIK3CA mutations could be predictive with regard to SCPRT benefit (142). PIK3CA mutation in exons 9 and 20 was analyzed on 30 tumor samples out of all 32 patients who developed a LR in the irradiated arm of the Dutch TME study. In contrast to previous incidence of 20.8% (5/24) PIK3CA mutations in the nonirradiated patients (141), investigators identified only 6.7% (2/30) mutations in the irradiated patients experiencing local recurrence. The interaction odds ratio (OR) of 0.3 although not significant because of small numbers, does suggest a relative benefit from SCPRT among carriers of the PIK3CA mutation compared with non-carriers. Although others suggest the mutation may only represent 4% of patients with rectal cancer (143).
In addition further upstream in EGFR signalling pathways, over expression or very high expression of the EGFR ligands amphiregulin and epiregulin appear to be associated with a response to cetuximab (144,145). Possibly the increased expression of these epidermal growth factor (EGF) ligands is responsible for driving the growth of these tumours.
Recent reviews (146) and a pooled analysis (147) showed that both on univariate and multivariate analysis, there is a significantly lower tumour regression grade and a non significant trend towards a lower pCR rate (9% vs. 16%) when Cetuximab was added to a combination of 5-FU/ Capecitabine and Oxaliplatin. Recent work on the same data set has evaluated functional germline polymorphisms of EGF and TS (148), and biomarkers such as Kras status in combination with TS, VEGFR1 and VEGFR2d expression (149) which appear to predict for histopathological response. Other potential markers of response include the TP53 mutation (150).
In an Italian study the EGFR gene copy number was found to correlate significantly with tumour regression and response to cetuximab (108) but is not prognostic in standard chemoradiotherapy (114).
Even though in rectal cancer there will be no concern that expression in the primary tumour and metastatic sites will be different, rectal and colon cancers do have different gene expression profiles, different cytokeratin profiles, different levels of MSI-H, and different levels of mutations in Kras and BRAF (151-154). Thus extrapolating results from colon cancer trials to the treatment in rectal cancer might not demonstrate the same outcome.
Pre-clinical data suggests that the sequencing of chemotherapy, EGFR inhibition and radiation may be clinically significant and that the sequence of oxaliplatin followed by cetuximab may be more effective than cetuximab prior to oxaliplatin [Morelli 2005 (155)]. Better efficacy might be achieved by integrating cetuximab in the latter portion of the radiotherapy, or following chemoradiation. This strategy has already been proposed when integrating anti-metabolites such as gemcitabine with EGFR inhibitors and radiation [Shewach 2007 (156)]. Finally, better selection for the potential efficacy of EGFR inhibition by molecular markers could be appropriate in the future (140). A recent study in rectal cancer examining a combined analysis of VEGF and EGFR identified a subgroup of EGFR-negative and VEGF-positive patients who appeared resistant to radiotherapy, of whom only 2/34 (6%) achieved a pCR (95).
Early endpoints in terms of efficacy at the level of the primary tumor (e.g., pCR), may not in themselves be coupled to longer-term endpoints such as DFS and OS. Phase III randomised studies are the best way to define the advantages of a novel treatment. It should be borne in mind that 50% of patients are in their 70s and more than 70% of patients with colorectal cancer are over 65. Many of these patients have extensive other co-morbidity including cardiac problems.
Her-2
Her-2 (ErbB2) represents a further member of the EGFR family which is overexpressed in approximately 30% of patients with breast cancer, where overexpression heralds worse prognosis and poorer survival. Recent studies suggest Her-2 is overexpressed in 8-27% of rectal cancers. Positive Her-2 status was found in 12.4% of initial rectal cancer biopsies and in 26.7% of resected specimens (157). So HER-2 amplification is detectable in a relevant proportion (26.7%) of rectal cancer patients. HER-2 represent a possible target and should be further assessed within prospective clinical trials.
Bevacizumab
The integration of bevacizumab into CRT schedules also has considerable preclinical rationale. Both hypoxia and vascular endothelial growth factor (VEGF) can confer radioresistance, and promote angiogenesis ie the formation of new blood vessels.
Tumour growth, tumour invasion and the development of distant metastases appear dependent on this process of angiogenesis. Experimental studies in human tumor xenograft models have shown that VEGF blockade serves as a potent and nontoxic enhancer of radiation.
There are two main types of agents targetting angiogenesis—vascular disrupting agents (which cause rapid dysfunction of tumour vasculature) and antiangiogenic agents. Anti-angiogenic agents modify and normalise the existing vasculature and inhibit new blod vessel formation. Pre-clinical and clinical studies suggest that VEGF is the predominant angiogenic factor in this development. VEGF has direct effects on endothelial cell function including activation survival proliferation and migration. It also may have some effects by inhibiting dendritic cell maturation and enhancing the adhesion of natural killer cells to tumour microvessels. Bevacizumab is a recombinant humanized monoclonal antibody, which binds to the VEGFR ligand VEGF-A, and prevents VEGF-A from interacting with its target receptor. Aflibercept, a VEGF trap is a fully humanized recombinant fusion protein that binds VEGF-A, VEGF-B, and placental growth factor (PGF)-1 and 2 with high affinity, preventing their binding to VEGF receptors, has demonstrated efficacy in the recent Phase III trial (VELOUR) in second-line treatment of patients with mCRC, in combination with FOLFIRI chemotherapy [OS hazard ratio (HR): 0.82, P=0.0032)] (24).
Solid tumours commonly manifest an elevated interstitial fluid pressure (IFP) and regions of hypoxia as compared to normal tissues, which contribute to a decreased transcapillary transport, and lead to the poor delivery of cytotoxic drugs. A clinical study in locally advanced rectal cancer, demonstrated that tumour IFP was lowered by the use of the anti-VEGF monoclonal antibody bevacizumab (158). Experimental studies in human tumor xenograft models have shown that VEGF blockade enhances the effects of radiation reversing radiation resistance conferred by hypoxia. VEGF inhibition has showed a benefit in terms of overall survival for patients with metastatic colorectal cancer (10,21), but randomised studies have not proven the advantage of anti-VEGF therapy in the adjuvant setting (14,15).
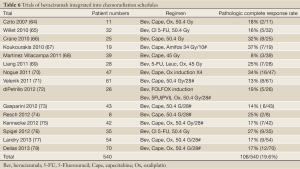
The recombintant humanised anti VEGF monoclonal antibody Bevacizumab has been extensively investigated in CRT schedules in rectal cancer. In a Phase I/II trial in rectal cancer patients receiving bevacizumab and CRT (158), provided direct evidence of the antivascular effect of anti-VEGF treatment by functional, cellular, and molecular investigations. Briefly, bevacizumab decreases the tumor vascular density, tumor perfusion, tumor interstitial fluid pressure, and the number of viable circulating endothelial and progenitor cells, which results into a significant increase in apoptosis of cancer cells (158). Several phase I/II trials reported on the feasibility of adding bevacizumab to 5-FU based CRT in the neo-adjuvant setting of locally advanced rectal cancer, and provided encouraging pCR rates with moderate toxicity (66,159). The reported incidence of postoperative wound complications in up to 36% of the patients is however concerning and consistent with other reports utilizing bevacizumab with CRT before a major surgical procedure (72).
The more recent AVACROSS study selected 47 patients according to MRI criteria, and used 4 cycles of induction chemotherapy using capecitabine, oxaliplatin and bevacizumab, followed by chemoradiation with concurrent capecitabine and bevacizumab (70).Results are impressive with 98% having an R0 resection and 34% achieved a pCR, with an additional 17 patients (36%) achieving Dworak tumor regression grade 3. Besides pCR, 23% were downstaged to ypT1/T2N0. There was one sudden death during the induction, and surgical morbidity appears prominent, since 26/45 patients (58%) experienced at least one postoperative complication and 11/45 (24%) required surgical re-intervention (even though the median time from the last dose of bevacizumab to surgery was 2 months).
A phase 2 trial evaluate preoperative capecitabine, oxaliplatin, and bevacizumab with radiation therapy followed by surgery and postoperative 5-FU, leucovorin, oxaliplatin (FOLFOX) and bevacizumab for locally advanced rectal cancer in 57 patients (77). 17% achieved a pathologic complete response, but 47% of patients who underwent surgery experienced a surgical complication. A Canadian study achieved a pCR of 18%, but 4 patients (11%) required re-operation due to complications (75).
A further study evaluating bevacizumab/chemoradiation in the preoperative and adjuvant settings in 66 patients with stage II/III rectal cancer (76) achieved a pCR rate of 29%, but again showed frequent grade 3/4 toxicity and surgical morbidity.
A systematic review reported 15 trials over the past decade which incorporated bevacizumab into a neo-adjuvant CRT schedule (160). The pooled pCR rate of 21% is not better than in trials reported with 5-FU based CRT alone.
Taking into account the lack of phase III data in the neo-adjuvant setting of rectal cancer, the similar response rates as compared to 5-FU based CRT alone and the considerable treatment-related toxicities, bevacizumab as a radiation sensitizer in combination with 5-FU based CRT does not seem to provide additional benefits in the neo-adjuvant treatment of locally advanced rectal cancer.
Few phase II studies have reported in full, but the combination with CRT appears potentially deliverable usually with acceptable toxicity (158,159,161). Toxicity has been marked in some trials (74), such that grade 3/4 toxicity was observed in 19 of 25 patients (76%) in one study and led to termination of the study (72).
Pathological complete response rate remains below 20%, with actuarial 5-year local control and overall survival rates of 100% (159). Recent reports have highlighted a high incidence of postoperative wound infections (66,68,69,72)
None of these studies show a consistent definitive signal of improved efficacy.Yet, since the eligibilty criteria in the AVACROSS study (70), which achieved a pCR 36%, were similar to the GEMCAD GCR3 study (162) where a pcR of only 14% was observed with induction Xelox and capecitabine and oxaliplatin chemoradiation, it is possible that the addition of bevacizumab offers higher efficacy. However, several studies raise concerns that the combination of bevacizumab and radiation may impact on surgical morbidity. Future studies either need to leave a longer interval following the completion of bevacizumab before surgery or drop the bevacizumab from the chemoradiation component.
Novel biological targeted treatments
Topoisomerase I expression and increased EGFR gene copy number as possible predictors of response to irinotecan- and cetuximab-based chemoradiation, respectively, require further investigation.
As our understanding of tumour cell biology has advanced, so we have learnt of new targets and developed novel biological modifiers in terms of EGFR (EGFR, HER2, HER3, IGFR1, c-MET, VEGFR, BRAF and downstream PI3K, AKT and MTOR). In colorectal cancer, BRAF inhibitors have a very low activity. In view of observed HER2 expression in 8-10% of rectal cancers, Herceptin might be a target with lapatinib TDM1 and pertuzumab.
The insulin-like growth factor (IGF-1) and insulin like growth factor 1-receptor (IGF1R) signaling pathway has recently emerged as a potential determinant of radiation resistance in human cancer cell lines (163,164). IGF1-R overexpression is observed in colorectal cancers and is associated with a worse prognosis, but studies with these agents in colorectal cancer have not yet shown any benefit. Interestingly, normal rectal tissues express higher levels of insulin-like growth factor (IGF-1) and insulin like growth factor 1-receptor (IGF1R) than colon, and IGF-1 expression increases the further down the large bowel. The main downstream signalling pathways of IGF-1R are Ras-Raf-mitogen-activated protein kinase and PI3K/Akt signaling. IGF1/IGF1R mediates treatment resistance to cytotoxic agents, and may represent an escape /resistance mechanism from EGFR inhibition (165).
The MET proto-oncogene, encoding the tyrosine kinase receptor for Hepatocyte Growth Factor (HGF) regulates invasive growth, controls cell proliferation with invasion of the extracellular matrix and protection from apoptosis. The MET oncogene is overexpressed and/or genetically mutated in many tumors, thereby sustaining pathological invasive growth, a prerequisite for metastasis. The interplay between MET and the protease network provides potentially exploitable mechanisms which coulod inhibit growth. The signaling pathways linking MET activation and invasive growth appear partly shared with other growth factor receptors, i.e. MAP Kinase, PI-3 Kinase-AKT STAT3, p38, and NF-kB pathways. c-MET amplification is high in gastric cancer but virtually non existent in colorectal cancer.
We know from the Dutch study that the presence of PI3K mutations is prognostic for local recurrence but c-MET over expression may be better looking at distant metatases.
In addition, in colorectal cancer in particular there is the targeting of cycloxygenase 2 (Cox 2).
Conclusions
Currently, targeted agents which impact on angiogenesis and growth factors, (bevacizumab, aflibercept, cetuximab and panitumumab), when combined with conventional cytotoxic drugs, and their receptors, modestly increase response rates in metastatic disease, enhance resectability of liver metastases, and improve DFS. There is an associated G3/G4 toxicity even when used as single agents, and the long-term effects are unknown. Yet, insufficient understanding of the precise mechanisms from which their clinical efficacy derives, their innate and acquired resistance mechanisms, and the on-target and off-target effects on both tumour and normal tissues hamper further development/combining these agents with radiation or chemoradiation. To date, we lack a simple method of ongoing monitoring of ‘on target’ effects of these biological agents, which could determine and pre-empt the development of resistance, prior to radiological and clinical assessessments or even molecular imaging.
It is clearly feasible to combine cytotoxic drugs, targetted agents and radiation in rectal cancer. However, integration into chemoradiation schedules rationally, in the correct sequence, at the most appropriate time and in the most appropriate combinations remains difficult. Despite some evidence of preclinical activity, many trials have not confirmed additional activity in early clinical trials. There is little evidence that we have increased pCR in any of the larger clinical studies. This may not even be relevant—as increasing pCR rates did not improve DFS or OS in the trials comparing radiation with 5-FU based chemoradiation (25,26,166). Phase III trials have also been disappointing (44). The reason has been postulated in terms of radiation sensitization. Cetuximab and cytotoxic agents such as cisplatin probably have similar mechanisms of action, predominantly via inhibition of proliferation and DNA repair. Also the intensity of treatment in terms of any potential systemic effect has been low. We should perhaps therefore aim initially to develop novel clinical trial protocols combining biological agents and radiation in resectable cancers without chemotherapy, since chemoradiation has not impacted on survival. However, undertaking studies using agents with multiple targets before we understand the optimal dose and sequence of single targets may prove counter-productive.
The issues discussed above raise the question regarding what we are trying to achieve by adding targeted agents to chemoradiation. In randomised trials, when added to chemotherapy, these biological agents have increased response rates with only a modest effect on progression-free and overall survival in the metastatic setting, but have not been effective in the adjuvant setting. Hence, if the aim is to increase response rates, surely their integration into chemoradiation schedules should be directed only towards those patients where the MRI defines the rectal cancer extension as a threat to or having breached the MRF. If response is the main aim, then patients with resectable rectal cancer (cT3N0-N1) are unlikely to benefit, unless one is expecting abscopal/ immune effects.
Effective tolerable doses have also been difficult to deliver for inhibitors of VEGF, EGFR, mTOR, and HER2 pathways, owing to overlapping or unexpected toxicities. Although, recent improvements in delivery of radiation such as IMRT/VMAT may allow more precise dosing to the target volume (tumour and/or locoregional lymph nodes), while limiting radiation doses to critical normal structures.
We are unlikely to advance far until we are able to identify predictive biomarkers of activity, or understand the mechanisms of primary or secondary resistance so we can select the population of patients most likely to benefit from these targetted agents.
Relevant and robust biomarkers of efficacy and toxicity of molecular-targeted agent combinations are needed in future, and for preclinical pharmacokinetic and pharmacodynamic modelling to guide schedules and dose adjustments. We also need to incorporate imaging biomarker studies to assess in vivo activity/resistance as clinical and pathological response is a somewhat blunt measure.
This knowledge will allow more rationally designed preclinical and translational studies (with recognised negative predictive factors such as k-ras mutations, b-raf mutatations, EGFR and VEGF expression, and EGFR gene copy numbers) might therefore help select out inappropriate patients, and determine the optimal sequence of such chemotherapy and biological triple combinations. Only then can we move on to perform large randomised phase III trials.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Taylor FG, Quirke P, Heald RJ, et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg 2011;98:872-9. [PubMed]
- Frasson M, Garcia-Granero E, Roda D, et al. Preoperative chemoradiation may not always be needed for patients with T3 and T2N+ rectal cancer. Cancer 2011;117:3118-25. [PubMed]
- Bernstein TE, Endreseth BH, Romundstad P, et al. Improved local control of rectal cancer reduces distant metastases. Colorectal Dis 2012;14:e668-78. [PubMed]
- Braendengen M, Tveit KM, Berglund A, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26:3687-94. [PubMed]
- Engelen SM, Maas M, Lahaye MJ, et al. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer 2013;49:2311-20. [PubMed]
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic Resonance Imaging-Detected Tumor Response for Locally Advanced Rectal Cancer Predicts Survival Outcomes: MERCURY Experience. J Clin Oncol 2011;29:3753-60. [PubMed]
- Adam R, Wicherts DA, de Haas RJ, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 2009;27:1829-35. [PubMed]
- Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol 2010;11:38-47. [PubMed]
- Gruenberger T, Arnold D, Rubbia-Brandt L. Pathologic response to bevacizumab-containing chemotherapy in patients with colorectal liver metastases and its correlation with survival. Surg Oncol 2012;21:309-15. [PubMed]
- Giantonio BJ, Catalano PJ, Meropol NJ, et al. Eastern Cooperative Oncology Group Study E3200. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol 2007;25:1539-44. [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [PubMed]
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol 2007;25:3456-61. [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012;307:1383-93. [PubMed]
- Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol 2013;31:359-64. [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33. [PubMed]
- Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol 2007;25:4096-103. [PubMed]
- Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26:3582-9. [PubMed]
- Moertel CG, Childs DS Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 1969;2:865-7. [PubMed]
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [PubMed]
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [PubMed]
- Maughan TS, Adams RA, Smith CG, et al. MRC COIN Trial Investigators. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011;377:2103-14. [PubMed]
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563-72. [PubMed]
- Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499-506. [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Enhanced tumoricidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results of EORTC 22921. J Clin Oncol 2005;23:5620-27. [PubMed]
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620-5. [PubMed]
- Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP-R03. J Clin Oncol 2009;27:5124-30. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72:15-24. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomised trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [PubMed]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol 2010;28:1638-44. [PubMed]
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. [PubMed]
- Mohiuddin M, Winter K, Mitchell E, et al. Randomised phase II study of neoadjuvant combined modality chemoradiation in distal rectal cancer. Radiation Oncology Group Trials 0012. J Clin Oncol 2006;24:650-5. [PubMed]
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [PubMed]
- Mohiuddin M, Paulus R, Mitchell E, et al. Neoadjuvant Chemoradiation for Distal Rectal Cancer: 5-Year Updated Results of a Randomized Phase 2 Study of Neoadjuvant Combined Modality Chemoradiation for Distal Rectal Cancer. Int J Radiat Oncol Biol Phys 2013;86:523-8. [PubMed]
- Glynne-Jones R, Anyamene N. Just how useful an endpoint is complete pathological response after neoadjuvant chemoradiation in rectal cancer? Int J Radiat Oncol Biol Phys 2006;66:319-20. [PubMed]
- Sebag-Montefiore DJ, Rutten H, Rullier, et al. Three-year survival results of CORE (Capecitabine, Oxaliplatin, Radiotherapy, and Excision) study after postoperative chemotherapy in patients with locally advanced rectal adenocarcinoma. 2009 ASCO Gastrointestinal Cancers Symposium 2009:abstract 447.
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 2012;30:4558-65. [PubMed]
- Rödel C, Liersch T, Becker H, et al. German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012;13:679-87. [PubMed]
- Roh MS, Yothers GA, O’Connell MJ, et al. The impact of capecitabine and oxaliplatin in the preoperative multimodality treatment in patients with carcinoma of the rectum: NSABP R-04. J Clin Oncol 2011;29:abstr 3503.
- Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78. [PubMed]
- Baumann M, Krause M, Dikomey E, et al. EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms. Radiother Oncol 2007;83:238-48. [PubMed]
- Eriksen JG, Steiniche T, Overgaard J, et al. The role of epidermal growth factor receptor and E-cadherin for the outcome of reduction in the overall treatment time of radiotherapy of supraglottic larynx squamous cell carcinoma. Acta Oncol 2005;44:50-8. [PubMed]
- Ang KK, Zhang QE, Rosenthal DI, et al. A randomized phase III trial (RTOG 0522) of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III-IV head and neck squamous cell carcinomas. J Clin Oncol 2011;29:abstr 5500.
- Chung KY, Minsky B, Schrag D, et al. Phase I trial of preoperative cetuximab with concurrent continuous infusion 5-fluorouracil and pelvic radiation in patients with local-regionally advanced rectal cancer. J Clin Oncol 2006;24:abstr 3560.
- Machiels JP, Sempoux C, Scalliet P, et al. Phase I/II study of preoperative cetuximab, capecitabine and external beam radiotherapy in patients with rectal cancer. Ann Oncol 2007;18:738-44. [PubMed]
- Rödel C, Liersch T, Hermann RM, et al. Multicenter phase II trial of chemoradiation with oxaliplatin for rectal cancer. J Clin Oncol 2007;25:110-7. [PubMed]
- Hofheinz RD, Horisberger K, Woernle C, et al. Phase I trial of cetuximab in combination with capecitabine, weekly irinotecan, and radiotherapy as neoadjuvant therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2006;66:1384-90. [PubMed]
- Horisberger K, Treschl A, Mai S, et al. MARGIT (Mannheimer Arbeitsgruppe für Gastrointestinale Tumoren). Cetuximab in Combination with Capecitabine, Irinotecan, and Radiotherapy for Patients with Locally Advanced Rectal Cancer: Results of a Phase II MARGIT Trial. Int J Radiation Oncol Biol Phys 2009;74:1487-93.
- Bertolini F, Chiara S, Bengala C, et al. Neoadjuvant treatment with single-agent cetuximab followed by 5-FU, cetuximab, and pelvic radiotherapy: a phase II study in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2009;73:466-72. [PubMed]
- Hong YS, Kim DY, Lee KS, et al. Phase II study of preoperative chemoradiation (CRT) with cetuximab, irinotecan and capecitabine in patients with locally advanced resectable rectal cancer. J Clin Oncol 2007;25:abstr 4045.
- Cabebe EC, Kuo T, Koong M, et al. Phase I trial of preoperative cetuximab in combination with oxaliplatin, capecitabine, and radiation therapy for locally advanced rectal cancer. J Clin Oncol 2008;26:abstr 15019.
- Eisterer WM, De Vries A, Oefner D, et al. Neoadjuvant chemoradiation therapy with capecitabine plus cetuximab and external beam radiotherapy in locally advanced rectal cancer (LARC) ABCSG trial R03. J Clin Oncol 2009;27;abstr 4109.
- Velenik V, Ocvirk J, Oblak I, et al. A phase II study of cetuximab, capecitabine and radiotherapy in neoadjuvant treatment of patients with locally advanced resectable rectal cancer. Eur J Surg Oncol 2010;36:244-50. [PubMed]
- Kim SY, Hong YS, Kim DY, et al. Preoperative chemo-radiation with cetuximab, irinotecan, and capecitabine in patients with locally advanced resectable rectal cancer: a multicenter Phase II study. Int J Radiat Oncol Biol Phys 2011;81:677-83. [PubMed]
- Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol 2012;30:1620-7. [PubMed]
- Sun PL, Li B, Ye QF. Effect of neoadjuvant cetuximab, capecitabine, and radiotherapy for locally advanced rectal cancer: results of a phase II study. Int J Colorectal Dis 2012;27:1325-32. [PubMed]
- Nyati MK, Morgan MA, Feng FY, et al. Integration of EGFR inhibitors with radiochemotherapy. Nat Rev Cancer 2006;6:876-85. [PubMed]
- Czito BG, Willett CG, Bendell JC, et al. Increased toxicity with Gefitinib, Capecitabine and radiation therapy in pancreatic and rectal cancer. Phase I trial results. J Clin Oncol 2006;24:656-62. [PubMed]
- Valentini V, De Paoli A, Gambacorta MA, et al. Infusional 5-flourouracil and ZD1839 (Gefitinib-Iressa) in combination with preoperative radiotherapy in patients with locally advanced rectal cancer: a phase I and II trial (1839IL/0092). Int J Radiation Oncol Biol Phys 2008;72:644-9.
- Pinto C, Di Fabio F, Maiello E, et al. Phase II study of panitumumab, oxaliplatin, 5-fluorouracil, and concurrent radiotherapy as preoperative treatment in high-risk locally advanced rectal cancer patients (StarPan/STAR-02 Study). Ann Oncol 2011;22:2424-30. [PubMed]
- Blaszkowsky LS, Hong TS, Zhu AX, et al. A phase I/II study of bevacizumab, erlotinib and 5-fluorouracil with concurrent external beam radiation therapy in locally advanced rectal cancer. J Clin Oncol 2009;27:abstr 4106.
- Helbling D, Bodoky G, Gautschi O, et al. Neoadjuvant chemoradiotherapy with or without panitumumab in patients with wild-type KRAS, locally advanced rectal cancer (LARC): a randomized, multicenter, phase II trial SAKK 41/07. Ann Oncol 2013;24:718-25. [PubMed]
- Czito BG, Bendell JC, Willett CG, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. Int J Radiat Oncol Biol Phys 2007;68:472-8. [PubMed]
- Willett CG, Duda DG, Ancukiewicz M, et al. A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer. Oncologist 2010;15:845-51. [PubMed]
- Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:824-30. [PubMed]
- Koukourakis MI, Giatromanolaki A, Tsoutsou P, et al. Bevacizumab, capecitabine, amifostine, and preoperative hypofractionated accelerated radiotherapy (HypoArc) for rectal cancer: a Phase II study. Int J Radiat Oncol Biol Phys 2011;80:492-8. [PubMed]
- Martinez Villacampa M, Santos C, Garcia M, et al. Phase II of preoperative bevacizumab, capecitabine and radiotherapy in locally advanced rectal cancer. J Clin Oncol 2011;177:abstr 516.
- Liang JT, Lai H, Chen K. Technical feasibility of laparoscopic total mesorectal excision for patients with low rectal cancer after concurrent radiation and chemotherapy with bevacizumab plus FOLFOX. Surg Endosc 2011;25:305-8. [PubMed]
- Nogué M, Salud A, Vicente P, et al. On behalf of the AVACROSS Study Group. Addition of Bevacizumab to XELOX Induction Therapy Plus Concomitant Capecitabine-Based Chemoradiotherapy in Magnetic Resonance Imaging-Defined Poor-Prognosis Locally Advanced Rectal Cancer: The AVACROSS Study. Oncologist 2011;16:614-20. [PubMed]
- Velenik V, Ocvirk J, Music M, et al. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open-label phase II study. Radiat Oncol 2011;6:105. [PubMed]
- Dipetrillo T, Pricolo V, Lagares-Garcia J, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:124-9. [PubMed]
- Gasparini G, Torino F, Ueno T, et al. A phase II study of neoadjuvant bevacizumab plus capecitabine and concomitant radiotherapy in patients with locally advanced rectal cancer. Angiogenesis 2012;15:141-50. [PubMed]
- Resch G, De Vries A, Öfner D, et al. Austrian Breast and Colorectal Cancer Study Group. Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer--a two stage phase II clinical trial. Radiother Oncol 2012;102:10-3. [PubMed]
- Kennecke H, Berry S, Wong R, et al. Preoperative bevacizumab, capecitabine, oxaliplatin and radiation among patients with locally advanced or low rectal cancer: a phase II trial. Eur J Cancer 2012;48:37-45. [PubMed]
- Spigel DR, Bendell JC, McCleod M, et al. Phase II study of bevacizumab and chemoradiation in the preoperative or adjuvant treatment of patients with stage II/III rectal cancer. Clin Colorectal Cancer 2012;11:45-52. [PubMed]
- Landry JC, Feng Y, Cohen SJ, et al. Phase 2 study of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: ECOG 3204. Cancer 2013;119:1521-7. [PubMed]
- Dellas K, Höhler T, Reese T, et al. Phase II trial of preoperative radiochemotherapy with concurrent bevacizumab, capecitabine and oxaliplatin in patients with locally advanced rectal cancer. Radiat Oncol 2013;8:90. [PubMed]
- Avallone A, Aloj L, Delrio P, et al. Multidisciplinary Approach to Rectal Cancer: Are We Ready for Selective Treatment Strategies? Anticancer Agents Med Chem 2013;13:852-60. [PubMed]
- Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys 2007;68:654-61. [PubMed]
- Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer 2001;37:S3-8 Suppl 4. [PubMed]
- Uberall I, Kolar Z, Trojec R, et al. The status and role of ErbB receptors in human cancer. Exp Mol Pathol 2008;84:79-89. [PubMed]
- Bandyopadhyay D, Mandal M, Adam L, et al. Physical interaction between epidermal growth factor receptor and DNA-dependent protein kinase in mammalian cells. J Biol Chem 1998;273:1568-73. [PubMed]
- Meyn RE, Munshi A, Haymach JV, et al. Receptor signalling as a regulatory mechanism of DNA repair. Radiother Oncol 2009;92:316-22. [PubMed]
- Giralt J, de las Heras M, Cerezo L, et al. The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy. Radiother Oncol 2005;74:101-8. [PubMed]
- Steel GG, Peckham MJ. Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys 1979;5:85-91. [PubMed]
- Mayer A, Takimoto M, Fritz E, et al. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer 1993;71:2454-60. [PubMed]
- Khorana AA, Ryan CK, Cox C, et al. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: a role for the host response in prognosis. Cancer 2003;97:960-8. [PubMed]
- Akimoto T, Hunter NR, Buchmiller L, et al. Inverse relationship between Epidermal Growth Factor Expression and radio-curability of murine carcinomas. Clin Cancer Res 1999;5:2884-90. [PubMed]
- Huang SM, Harari PM. Modulation of radiation response after epidermal growth factor receptor blockade in squamous cell carcinomas: inhibition of damage repair, cell cycle kinetics, and tumor angiogenesis. Clin Cancer Res 2000;6:2166-74. [PubMed]
- Nasu S, Ang KK, Fan Z, et al. C225 antiepidermal growth factor receptor antibody enhances tumor radiocurability. Int J Radiat Oncol Biol Phys 2001;51:474-7. [PubMed]
- Zips D, Krause M, Yaromina A, et al. Epidermal groth factor receptor inhibitors for radiotherapy: biological rationale and preclinical results. J Pharm Pharmacol 2008;60:1019-28. [PubMed]
- Li S, Kim JS, Kim JM, et al. Epidermal growth factor receptor as a prognostic factor in locally advanced rectal-cancer patients treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 2006;65:705-12. [PubMed]
- Kim JS, Kim JM, Li S, et al. Epidermal Growth Factor Receptor as a predictor of tumour downstaging in locally advanced rectal cancer patients treated with preoperative radiotherapy. Int J Radiation Oncol Biol Phys 2006;66:195-200.
- Zlobec I, Vuong T, Compton CC, et al. Combined analysis of VEGF and EGFR predicts complete tumour response in rectal cancer treated with preoperative radiotherapy. Br J Cancer 2008;98:450-6. [PubMed]
- Azria D, Bibeau F, Barbier N, et al. Prognostic impact of epidermal growth factor receptor (EGFR) expression on loco-regional recurrence after preoperative radiotherapy in rectal cancer. BMC Cancer 2005;5:62. [PubMed]
- Debucquoy A, Haustermanns K, Daemen A, et al. Molecular response to cetuximab and efficacy of preoperative cetuximab-based chemoradiation in rectal cancer. J Clin Oncol 2009;27:2751-7. [PubMed]
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311-9. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Madi A, Fisher D, Wilson RH, et al. COIN trial research group. Oxaliplatin/capecitabine vs oxaliplatin/infusional 5-FU in advanced colorectal cancer: the MRC COIN trial. Br J Cancer 2012;107:1037-43. [PubMed]
- Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755-62. [PubMed]
- Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663-71. [PubMed]
- Marchetti A, Gasparini G. K-ras mutations and cetuximab in colorectal cancer. N Engl J Med 2009;360:833-4. [PubMed]
- Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [PubMed]
- Sastre J, Grávalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist 2012;17:339-45. [PubMed]
- Hirvikoski P, Auvinen A, Servomaa K, et al. K-ras and p53 mutations and overexpressions as prognostic factors in female rectal carcinoma. Anticancer Res 1999;19:685-91. [PubMed]
- Luna-Pérez P, Segura J, Alvarado I, et al. Specific c-K-ras gene mutations as a tumor-response marker in locally advanced rectal cancer treated with preoperative chemoradiotherapy. Ann Surg Oncol 2000;7:727-31. [PubMed]
- Bengala C, Bettelli S, Bertolini F, et al. Prognostic role of EGFR gene copy number and KRAS mutation in patients with locally advanced rectal cancer treated with preoperative chemoradiotherapy. Br J Cancer 2010;103:1019-24. [PubMed]
- Gaedcke J, Grade M, Jung K, et al. KRAS and BRAF mutations in patients with rectal cancer treated with preoperative chemoradiotherapy. Radiother Oncol 2010;94:76-81. [PubMed]
- Davies JM, Trembath D, Deal AM, et al. Phospho-ERK and AKT status, but not KRAS mutation status, are associated with outcomes in rectal cancer treated with chemoradiotherapy. Radiat Oncol 2011;6:114. [PubMed]
- Erben P, Strobel P, Horisberger K, et al. KRAS and BRAF mutations and PTEN expression do not predict efficacy of cetuximab-based chemoradiotherapy in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2011;81:1032-8. [PubMed]
- Velenik V, Ocvirk J, Oblak I, et al. Cetuximab in preoperative treatment of rectal cancer - term outcome of the XERT trial. Radiol Oncol 2012;46:252-7. [PubMed]
- Garcia-Aguilar J, Chen Z, Smith DD, et al. Identification of a biomarker profile associated with resistance to neoadjuvant chemoradiation therapy in rectal cancer. Ann Surg 2011;254:486-92. [PubMed]
- Bengala C, Patelli S, Bertolini F, et al. Epidermal Growth Factor Receptor gene copy number KRAS mutation and pathological response to preoperative Cetuximab, 5-FU and radiation therapy in locally advanced rectal cancer Ann Oncol 2009;20:469-74. [PubMed]
- Kim SY, Shim EK, Yeo HY, et al. KRAS Mutation Status and Clinical Outcome of Preoperative Chemoradiation With Cetuximab in Locally Advanced Rectal Cancer: A Pooled Analysis of 2 Phase II Trials. Int J Radiat Oncol Biol Phys 2013;85:201-7. [PubMed]
- Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009;27:672-80. [PubMed]
- Cubillo A, Hernando-Requejo O, García-García E, et al. A Prospective Pilot Study of Target-guided Personalized Chemotherapy with Intensity-modulated Radiotherapy in Patients With Early Rectal Cancer. Am J Clin Oncol 2012; [PubMed]
- Brierley JD, Keane TJ, Cummings B, et al. The absence of an adverse effect of prolongation of radiation treatment of primary rectal adenocarcinoma. Clin Oncol (R Coll Radiol) 1996;8:97-101. [PubMed]
- Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitisation of conventional chemotherapeutic agents. Semin Radiat Oncol 2003;13:13-21. [PubMed]
- Lawrence TS, Davis MA, Tang HY, et al. Fluorodeoxyuridine-mediated cytotoxicity and radiosensitisation require S phase progression. Int J Radiation Biol 1996;70:273-80.
- Guo X, Goessl E, Jin G, et al. Cell cycle perturbation and acquired 5-fluorouracil chemoresistance. Anticancer Res 2008;28:9-14. [PubMed]
- Willett CG, Warland G, Cheek R, et al. Proliferating cell nuclear antigen and mitotic activity in rectal cancer: predictor of response to preoperative irradiation. J Clin Oncol 1994;12:679-82. [PubMed]
- Hecht JR, Mitchell E, Neubauer MA, et al. Lack of correlation between epidermal growth factor receptor status and response to Panitumumab monotherapy in metastatic colorectal cancer. Clin Cancer Res 2010;16:2205-13. [PubMed]
- Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64. [PubMed]
- Amado RG, Wolf M, Peeters M, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626-34. [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [PubMed]
- Rothenberg ML, LaFleur B, Levy DE, et al. Randomized phase II trial of the clinical and biological effects of two dose levels of gefitinib in patients with recurrent colorectal adenocarcinoma. J Clin Oncol 2005;23:9265-74. [PubMed]
- Townsley CA, Major P, Siu LL, et al. Phase II study of erlotinib (OSI-774) in patients with metastatic colorectal cancer. Br J Cancer 2006;94:1136-43. [PubMed]
- Meyerhardt JA, Zhu AX, Enzinger PC, et al. Phase II study of capecitabine, oxaliplatin, and erlotinib in previously treated patients with metastastic colorectal cancer. J Clin Oncol 2006;24:1892-7. [PubMed]
- Messersmith WA, Jimeno A, Jacene H, et al. Phase I trial of oxaliplatin, infusional 5-fluorouracil, and leucovorin (FOLFOX4) with erlotinib and bevacizumab in colorectal cancer. Clin Colorectal Cancer 2010;9:297-304. [PubMed]
- Carlomagno C, Daniele G, Bianco R, et al. Addition of erlotinib to fluoropyrimidine-oxaliplatin-based chemotherapy with or without bevacizumab: Two sequential phase I trials. Exp Ther Med 2011;2:449-55. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Williams KJ, Telfer BA, Stratford IJ, et al. ZD1839 (‘Iressa’) A specific oral epidermal growth factor receptor tyrosine kinase inhibitor potentiates radiotherapy in a human colorectal cancer xenograft model. Br J Cancer 2002;86:1157-61. [PubMed]
- Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012;366:883-92. [PubMed]
- Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors which do not express the epidermal growth factor receptor by immunocytochemistry. J Clin Oncol 2005;23:1803-10. [PubMed]
- Hebbar M, Wacrenier A, Desauw C, et al. Lack of usefulness of epidermal growth factor receptor expression determination for cetuximab therapy in patients with colorectal cancer. Anticancer drugs 2006;17:855-7. [PubMed]
- Bos JL. Ras oncogenes in human cancer: a review. Cancer Res 1989;49:4682-9. [PubMed]
- Ogino S, Nosho K, Kirkner GJ, et al. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol 2009;27:1477-84. [PubMed]
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12. [PubMed]
- He Y, Van’t Veer LJ, Mikolajewska-Hanclich I, et al. PIK3CA mutations predict local recurrences in rectal cancer patients. Clin Cancer Res 2009;15:6956-62. [PubMed]
- He Y, Van’t Veer LJ, Lopez-Yurda M, et al. Do rectal cancer patients with PIK3CA mutations benefit from preoperative radiotherapy with regard to local recurrences? Clin Cancer Res 2010;16:6179. [PubMed]
- Derbel O, Wang Q, Desseigne F, et al. Impact of KRAS, BRAF and PI3KCAmutataions in rectal carcinomas treated with neoadjuvant radiochemotherapy and surgery. BMC Cancer 2013;13:200. [PubMed]
- Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007;25:3230-7. [PubMed]
- Pentheroudakis G, Kotoula V, De Roock W, et al. Biomarkers of benefit from cetuximab-based therapy in metastatic colorectal cancer: interaction of EGFR ligand expression with RAS/RAF, PIK3CA genotypes. BMC Cancer 2013;13:49. [PubMed]
- Glynne-Jones R, Mawdsley S, Harrison M. Cetuximab and chemoradiation for rectal cancer-is the water getting muddy? Acta Oncol 2010;49:278-86. [PubMed]
- Weiss C, Arnold D, Dellas K, et al. Preoperative radiotherapy of advanced rectal cancer with capecitabine and oxaliplatin with or without cetuximab: a pooled analysis of three prospective phase I-II trials. Int J Radiat Oncol Biol Phys 2010;78:472-8. [PubMed]
- Hu-Lieskovan S, Vallbohmer D, Zhang W, et al. EGF61 polymorphism predicts complete pathologic response to cetuximab-based chemoradiation independent of KRAS status in locally advanced rectal cancer patients. Clin Cancer Res 2011;17:5161-9. [PubMed]
- Grimminger PP, Danenberg P, Dellas K, et al. Biomarkers for cetuximab-based neoadjuvant radiochemotherapy in locally advanced rectal cancer. Clin Cancer Res 2011;17:3469-77. [PubMed]
- Oden-Gangloff A, Di Fiore F, Bibeau F, et al. TP53 mutations predict disease control in metastatic colorectal cancer treated with cetuximab-based chemotherapy. Br J Cancer 2009;100:1330-5. [PubMed]
- Fransén K, Klintenäs M, Osterström A, et al. Mutation analysis of the BRAF, ARAF and RAF-1 genes in human colorectal adenocarcinomas. Carcinogenesis 2004;25:527-33. [PubMed]
- Birkenkamp-Demtroder K, Olesen SH, Sorensen FB, et al. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut 2005;54:374-84. [PubMed]
- Jönsson M, Ekstrand A, Edekling T, et al. Experiences from treatment-predictive KRAS testing; high mutation frequency in rectal cancers from females and concurrent mutations in the same tumor. BMC Clin Pathol 2009;9:8. [PubMed]
- Kalady MF, Sanchez JA, Manilich E, et al. Divergent oncogenic changes influence survival differences between colon and rectal adenocarcinomas. Dis Colon Rectum 2009;52:1039-45. [PubMed]
- Morelli MP, Cascone T, Troiani T, et al. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol 2005;16 Suppl 4:iv61-68. [PubMed]
- Shewach DS, Lawrence TS. Antimetabolite Radiosensitizers. J Clin Oncol 2007;25:4043-50. [PubMed]
- Conradi LC, Styczen H, Sprenger T, et al. Frequency of HER-2 positivity in rectal cancer and prognosis. Am J Surg Pathol 2013;37:522-31. [PubMed]
- Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004;10:145-7. [PubMed]
- Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 2009;27:3020-6. [PubMed]
- Shaalan Beg M, Meyer J, Balch GC, et al. Pathologic complete response rates after neoadjuvant chemoradiation (CRT) for rectal cancer: do novel agents have a role? J Clin Oncol 2012;30:abstr 597.
- Czito BG, Bendell JC, Willett CG, et al. Bevacizumab, oxaliplatin, and capecitabine with radiation therapy in rectal cancer: Phase I trial results. Int J Radiat Oncol Biol Phys 2007;68:472-8. [PubMed]
- Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cáncer de Recto 3 study. J Clin Oncol 2010;28:859-65. [PubMed]
- Perer ES, Madan AK, Shurin A, et al. Insulin-like growth factor I receptor antagonism augments response to chemoradiation therapy in colon cancer cells. J Surg Res 2000;94:1-5. [PubMed]
- Cosaceanu D, Budiu RA, Carapancea M, et al. Ionizing radiation activates IGF-1R triggering a cytoprotective signaling by interfering with Ku-DNA binding and by modulating Ku86 expression via a p38 kinase-dependent mechanism. Oncogene 2007;26:2423-34. [PubMed]
- Jones HE, Goddard L, Gee JM, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer 2004;11:793-814. [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Enhanced tumoricidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results of EORTC 22921. J Clin Oncol 2005;23:5620-7. [PubMed]


