Cytopathology of the pancreatobiliary tract–the agony, and sometimes, the ease of it
Introduction
Pancreatic cytopathology is increasingly being recognized as a safe, rapid, reliable, accurate and cost effective modality in the evaluation of patients with a mass lesion. It has surpassed pancreatic wedge and core needle biopsies with their attendant increased risk of complications (fistulas, hemorrhage, and tumor seeding) as a first line pathologic investigative procedure (1). Optimal results require a dedicated approach, experience and expertise by all professionals involved. Ongoing, frequent, regular experience with a large number of patients over a period of time is necessary to acquire optimal diagnostic skills (radiologists, endoscopists, surgeons and cytologists). The American Society of Gastrointestinal Endoscopy (ASGE) has recommended mentoring of 25 or more cases of pancreatic FNA in order for endoscopists to achieve competence (2).
Pancreatic cancer is now the fourth leading cause of cancer - related deaths in the United States, and its incidence appears to be increasing. The disease is associated with a high mortality rate and a median survival of approximately four months in untreated patients. Unfortunately most patients with pancreatic cancer present at an advanced stage of the disease when surgical cure is no longer possible.
The regional anatomy of the pancreas is complex, making procurement of pathologic samples difficult. Traditionally, CT-guided fine needle aspiration (FNA), and endoscopic retrograde cholangiopancreatography (ERCP) has been used for biopsy of the pancreas. Both have been associated with a false negative rate of 20% and 30% respectively (3). Endoscopic ultrasound and fine needle aspiration (EUS) was developed in the 1980s, and allows identification of pancreatic lesions as small as 2-3 mm, as well as the detection of small, occult regional metastases in patients with pancreatic tumors, and may also be used for staging. There has been a recent nationwide trend towards EUS-FNA for the initial evaluation for pancreatic lesions. Also, recent investigations into EUS guided fine needle injection/ablation therapy are being conducted for treating unresectable tumors (4-9).
Accurate staging of patients with pancreatic cancer is critical to avoid the expense, morbidity, and mortality related to unnecessary surgery. The impact on cost and management of pancreatic cancers has been evaluated, and may be reduced by nearly $33,000 primarily by avoiding unnecessary surgical explorations (10).
Clinical considerations
Patient age, gender, social history, symptoms and clinical findings are essential. Also necessary are radiologic data: location of the lesion(s) in pancreas, is it solid, cystic, multicystic or mixed. Endoscopic findings should also be available. Is there any pertinent prior history (tumor, any treatment, has a stent been placed?). Also, what is the working diagnosis?
Techniques
Sampling techniques include:
Intraoperative FNA of the pancreas at the time of laparotomy;
Pre-operative CT/US guided FNA - percutaneous approach;
ERCP;
EUS FNA.
21 gauge or thinner needle (23 to 25 gauge are preferable, as there is less bleeding, without sacrificing diagnostic material). Increasing needle size correlates with increasing complications. Five to six passes are recommended for pancreatic aspirates (however diagnostic yield depends on many factors including type and cellularity of the lesion, quality of the pass, experience of the aspirator etc.).
Rapid on-site evaluation
Pathologist or cytologist should be available for rapid on-site assessment of adequacy and specimen triage, if necessary. A preliminary diagnosis is often provided, but it should be emphasized that this is only a preliminary interpretation. Final diagnosis involves examination of the whole sample, including cell block and possible ancillary testing.
The sample adequacy depends on the nature of the lesion sampled, and the experience of the aspirator - sometimes only two to three needle passes may be adequate to appropriately aspirate the target lesion.
The advantages of rapid on-site evaluation are a decrease in procedure time, less inadequate/non diagnostic specimens and diminished repeat interventional procedures (11). The assessment should be as confident and accurate as the sample permits.
Slide preparation and staining
Prepare smears rapidly. This is optimal if smears are prepared by trained professionals. Half of the slides may be stained for immediate assessment, the remainder quickly placed in 95% ethyl alcohol for later Papanicolaou staining. Needles should be rinsed in liquid medium for cell block preparation. This permits histologic assessment and special stains to be performed, if necessary. Extra smears may also be saved for possible immunochemistry.
Pathologists should use the rapid stain with which they are most confident: (I) Romanowsky stain requires air dried smears - cell size and stromal components are better defined, however nuclear morphology is limited. (II) Rapid Papanicolaou stain - provides greater nuclear detail, ability to focus through thicker smears and overlapping cell groups. (III) Toluidine Blue stain - This is ultra fast, but requires subsequent destaining and restaining with a permanent stain. (IV) Hematoxylin and eosin - Requires rapid fixation and is more time consuming. However many non cytopathology subspecialty trained pathologists are familiar and more comfortable using this staining method.
Cells of the pancreas
The pancreas consists predominantly of the exocrine pancreatic acinar and ductal cells, and a smaller endocrine component consisting of the islets of Langerhans and scattered neuroendocrine Kulchitsky cells in the ducts.
Exocrine tissue
Serous acinar cells
These comprise 80% of the cellularity of the normal pancreas. They are pyramidal, polygonal shaped cells in small cohesive raspberry-like groups. Single serous acinar cells may be seen with aggressive smearing, similar to islet cell tumors. There are indistinct cell borders between cells, but margins of a group of cells are well defined. There is abundant cytoplasm containing coarse zymogen granules. The nucleus is eccentric, may be binucleated, small, round, and uniform (about the size of a red blood cell). Bare nuclei are common. Nuclear membranes are smooth, and there is granular, evenly distributed chromatin, with a prominent nucleolus.
Excretory ductal cells
This is a minor component in aspirates of normal pancreas. Ductal cells may be aspirated in monolayered sheets or as small intact cylindrical tubular structures. They are uniform columnar or cuboidal cells (from smaller ducts) with well demarcated pale, finely vacuolated cytoplasm. There may be occasional cytoplasmic mucin. Numerous, large, pleomorphic goblet cells suggest mucinous carcinoma. The sheets of ductal cells have a honeycomb pattern en face, with palisading at the periphery. Nuclei are basally situated, bland, round, uniform with smooth nuclear membranes and finely granular chromatin.
Nucleoli are small and inconspicuous (Figure 1).
A highly cellular aspirate composed entirely of ductal cells suggests ductal adenocarcinoma; whereas a highly cellular aspirate composed entirely of acinar cells suggests acinar cell carcinoma.
Endocrine tissue
Comprises 1% to 2% of the pancreatic mass, and is more abundant in the pancreatic tail region. Cells are sparse, and appear similar to acinar cells. Often seen as single cells, may be in streaks. Often present as bare nuclei. They have a salt and pepper chromatin pattern.
Special stains are required for identification.
Contaminint cells
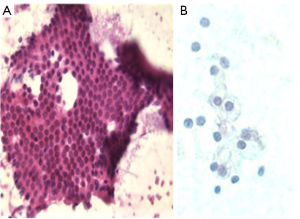
Bowel, particularly duodenal epithelium, presents as cohesive, uniform cell groups with starry sky pattern of clear goblet cells (Figure 2). There may be gastric epithelium or food debris.
Hepatocytes may also be sampled, look for lipofuscin granules, bile pigment, intranuclear inclusions, and macronucleoli. Pancreatic tail lesion sampling may include splenic tissue. Trans-abdominal sampling techniques often pick up mesothelium which will appear as flat sheets, with intercellular slits (windows). Other tissue including endothelial cells and adipose tissue may also be present.
Adequacy
At low magnification the entire slide should be quickly scanned to assess preservation and cellularity. The background should be checked for inflammation, blood, diathesis, or mucin. Note the cell population and architecture; see if there is cell crowding or a honeycomb pattern of benign cells. The cell size, presence of single cells, pleomorphism, nuclear membrane irregularities, nucleus to cytoplasmic ratios, chromatin pattern, and mitoses should be assessed.
Reactive cells/pancreatitis
Associations
Alcoholism, biliary tract disease, trauma, medications, ulcers, acute and chronic pancreatitis as well as adjacent malignancy can cause reactive changes.
Acute pancreatitis is a clinical and biochemical diagnosis, the pancreas is usually not aspirated. There is a background of neutrophils, debris, macrophages, necrotic fat and calcium salts, degenerating acinar and reactive ductal cells. Chronic pancreatitis may present as a mass lesion. Aspirates show variable cellularity and are often scant due to fibrosis. There are mostly ductal cells, due to acinar atrophy. Islet cells may be identified, as they are unaffected by the pancreatitis. There may be squamous metaplastic cells, fat necrosis, and small calcified fragments. Inflammatory cells, usually chronic (lymphocytes, plasma cells), granulation tissue, multinucleate giant cells and fibroblasts as well as reactive mesothelial cells may be seen. There is ductal cell atypia, seen only in a few groups. There are flat sheets of cells, with preserved polarity, dissociation is not a feature. There may be cytomegaly, mild focal pleomorphism (do not show differences of 4:1 or greater). Nucleomegaly, with intact nuclear/cytoplasmic ratios, smooth nuclear membranes and prominent nucleoli. Macronucleoli suggest carcinoma. Only few mitoses should be present, a high mitotic rate suggests carcinoma.
Cysts
Non-neoplastic cysts
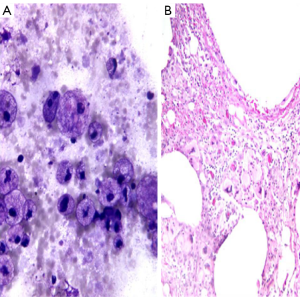
Pancreatic pseudocyts are rentention cysts that arise after pancreatitis, secondary to inflammation, necrosis, and hemorrhage. These may become infected and form an abscess. Aspiration may be therapeutic. They are usually small, solitary and unilocular and located adjacent to the pancreatic tail. They contain clear fluid, pus, semi-solid debris, mixed inflammatory cells, histiocytes with phagocytosed debris and hemosiderin. There is an absence or paucity of epithelial cells (Figure 3). Background shows cholesterol crystals, calcification, iron and bile pigments. Aspirated fluid has a high amylase and lipase content. CEA levels are low.
Lymphoepithelial cysts, congenital cysts, parasitic ecchinococcal cysts may also be seen.
Neoplastic cysts
Comprise 10% to 15% of all pancreatic cysts, 2% of all pancreatic neoplasms.
Serous cystadenoma/microcystic adenoma (SMAP)
Serous cystadenoma is usually benign, and has an excellent prognosis. Often presents as a palpable mass, with abdominal pain, weight loss, and jaundice, commonly in elderly women. There may be a history of diabetes mellitus. Some patients have von Hippel-Lindau syndrome.
These are frequently large and occur in the pancreatic head. There is a characteristic multicystc, honeycomb pattern with a central stellate scar and sunburst type calcifications.
Aspirates contain clear, watery fluid and are of low cellularity (may be missed, may be called non diagnostic!). Cuboidal to columnar single cells, or cohesive monolayered groups are seen with clear cytoplasm (due to glycogen). Nuclei are small, round with inconspicuous nucleoli.
Special studies: glycogen, PAS+, PAS-D, Alcian Blue–, EMA+, CK 7, 8, 18, 19+, CEA–, Neuroendocrine markers–.
Differential diagnosis includes lymphangioma (flattened cells, endothelial markers+), mucinous cystic neoplasms, serous cystadenocarcinoma.
Mucinous cystic neoplasms
These include mucinous cystic and intraductal papillary mucinous tumors.
Mucinous cystic tumor (MCT)
These are rare, usually indolent tumors. All are potentially malignant (benign, borderline, malignant – extensive sampling is required, as there are concomitant areas of benign appearing columnar epithelium, severe dysplasia, in situ and invasive carcinoma.
Tumors usually are seen in middle-aged women in the body and tail of pancreas. Radiologic features are essential. These lesions are not in continuity with the pancreatic ductal system, and often present as multilocular cysts, usually 2 cm or more in diameter.
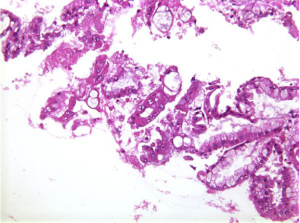
Aspirates show a mucinous background with moderate cellularity of regularly honeycombed epithelial sheets. Mucinous epithelial cells – goblet, signet ring cells are present. Well differentiated tumor cells resemble benign endocervical cells (Figure 4). Benign to obvious malignant cells may be seen. Mucinous macrophages and stroma may be present.
Special studies: EMA+, CK 7, 8, 18, 19+, CEA+, CA 19-9+, DUPAN-2+. Stromal component is vimentin, SMA, desmin, ER, PR, inhibin +. MUC 2 + in benign goblet cells, MUC 1 + in invasive tumors.
Intraductal papillary mucinous tumor (IPMT)
Rare, more frequent in males, 60-70 years old. These tumors are more commonly in head of pancreas. Radiological and clinical input is essential. Single or multiloculated cysts and dilated pancreatic ducts are seen. Characteristic feature is abundant mucin – seen flowing from a patulous ampulla at endoscopy.
Aspirates contain rounded, papillary cell islands and fragments of mucinous cells (Figure 5).
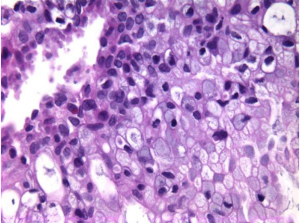
There may be obvious malignant cytologic features.
These tumors have a better prognosis than usual pancreatic cancer.
Special studies: EMA, CK+, Mucin+, MUC 2– adenomas, MUC 1– invasive tumors, PCNA, Ki 67 increased in malignant tumors, P 53+ in borderline tumors and carcinomas.
Cytologic diagnosis should be mucinous neoplasm NOS, unless obvious cytologic features of malignancy are present. They may be subclassified as MCT or IPMT depending on the radiological features.
Solid and papillary epithelial neoplasm
These are seen in adolescent girls and young women, and often involve the tail of the pancreas.
These are indolent, low grade lesions, curable by complete resection. They present as a multiloculated cystic and solid mass.
Smears are highly cellular, and show papillary formations (Figures 6,7), bare capillaries, and microacinar structures. Stromal cores are best visible on Papanicolaou stains. Tumor cells are monomorphic, with cytoplasmic processes. Nuclei are bland, and contain nuclear grooves. Intracellular or extracellular metachromatic hyaline globules may be seen.
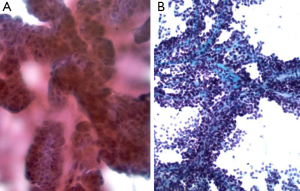
Special studies: Vimentin, Alpha 1 anti-trypsin, Alpha 1 antichymotrypsin, NSE+, Synaptophysin, chromogranin-, Beta catenin+, PR+, ER–, PAS+ hyaline globules (12,13).
Differential diagnosis includes other cystic lesions, and lesions with similar bland cytology such as endocrine tumors (SPT has better prognosis) and acinar carcinomas (may present with arthralgias, fat necrosis).
Ductal adenocarcinoma
These comprise 80% to 90% of pancreatic carcinomas. These tumors are usually seen in elderly patients. Patients may present with migratory thrombophlebitis, Trousseau’s syndrome, or as metastases. Ductal adenocarcinoma is a common source of metastasis from an unknown primary. Tumors arising in the pancreatic head present early with obstructive jaundice. Body and tail tumors have a late presentation, usually with metastases.
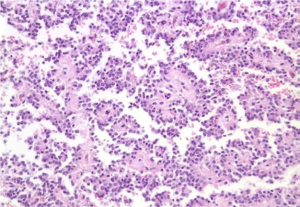
Aspirates show a necrotic background with superimposed features of pancreatitis. Specimens are cellular, consisting predominantly of ductal cells. There may be scattered few or abundant obviously malignant cells showing loss of polarity. Disordered sheets of cells, “drunken honeycombs” may be seen. There is pleomorphism, sometimes quite subtle. Cells may show cytoplasmic vacuolization, dense squamoid cytoplasm, or be large, tall columnar “tombstone cells” (Figures 8,9). Nucleomegaly (greater than red blood cells), anisonucleosis (4:1 or greater ratios seen within the same cell group), and irregular nuclear membranes: grooves, folds, clefts (“popcorn”, “tulip nuclei”) are present. Abnormal chromatin, thick nuclear membranes, nucleoli and mitoses (seen in many fields, or several mitoses seen in one HPF) are also features seen in ductal adenocarcinoma.
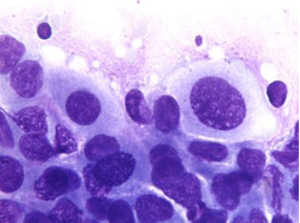
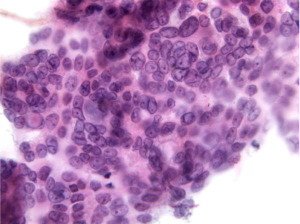
Special studies: Mucicarmine+, PASD+ mucin in tumor cells, EMA, Keratin (AE1/AE3), CK 7, polyclonal CEA, CAM 5.2+. Some CD10, CK 20+, focal chromogranin, pancreatic enzyme markers +, CA 19-9+.
K-ras mutation can be detected (14).
Acinar cell carcinoma
These tumors comprise 1% of pancreatic carcinomas. They occur anywhere in pancreas, and have an equally poor prognosis as pancreatic ductal carcinoma. Tumors are usually solid. They may be functional, secreting amylase, lipase or elastase. They are often associated with florid fat necrosis, polyarthralgia, eosinophilia or suppuration. Tumors are usually poorly differentiated.
Cytologically tumors show solid nests with overlapping, gland-like acinar clusters, and discohesive single cells. Tumor cells are large with abundant distinctive granular cytoplasm (zymogen granules), round nuclei, and irregular nuclear membranes. Nuclear/cyoplasmic ratios are increased. These tumors also often contain stripped nuclei in the background. Nucleoli are prominent. Mitoses are frequent. There is an absence of ductal cells (Figure 10).
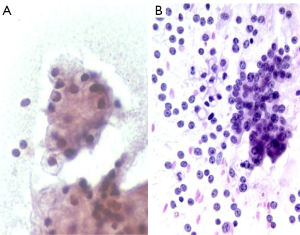
Differential diagnosis includes islet cell tumor. Zymogen granules of acinar cell tumors are larger than the fine, metachromatic neurosecretory granules in islet cell tumors. Neuroendocrine markers are negative in acinar cell carcinoma.
Endocrine neoplasms
Islet cell and carcinoid tumors
Aka Pancreatic endocrine tumors (PET).
These are rare, and occur in older adults. Tumors may be treatable; there is long survival even with metastases. However there may be life-threatening clinical manifestations due to excess hormone production. More commonly occur in body and tail of pancreas due to the greater number of Islets. Tumors are of variable size, and slow growing. They may be well or poorly differentiated.
These may be associated with clinical syndromes: MEN 1, insulin (hypoglycemia, benign), gastrin (Zollinger-Ellison syndrome, ulcers, benign if associated with MEN 1), glucagon (skin rash, large, often malignant), somatostatin (psammoma bodies common, NF 1), ACTH, parathormone (more likely to be malignant).
Smears are hypercellular, with an equal mixture of single cells and cohesive groups. Rosettes, acinar like formations, trabeculae may be seen. Cells are monotonous, small to medium-sized. Benign tumors may show endocrine pleomorphism, malignant tumors may have entirely bland uniform cells. Plasmacytoid cells with finely granular cytoplasm, and red cytoplasmic granules may be seen as in other neuroendocrine neoplasms. Single cells, binucleation and multinucleated tumor giant cells, and often malignant spindle cells may be seen. Stripped, bare nuclei, salt and pepper chromatin pattern are characteristic features. Mitotic figures, necrotic debris, and tumor diathesis is rare. Primary small cell carcinoma (poorly differentiated neuroendocrine carcinoma) is rare (Figure 11).
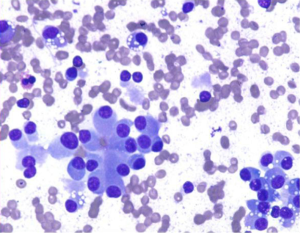
Special studies: Immunocytochemical stains for neuroendocrine markers (NSE, synaptophysin, chromogranin, insulin, gastrin).
Diagnosis is essential, as prognosis and surgical treatment is different from ductal adenocarcinomas.
Differential diagnosis includes acinar cell tumor, well differentiated ductal carcinoma, solid and papillary epithelial neoplasm, metastatic small cell carcinoma, lymphoma, plasmacytoma/myeloma.
Miscellaneous tumors
Anaplastic carcinoma of pancreas
Anaplastic carcinoma is an aggressive neoplasm, often arising in the body and tail of pancreas. It consists of large, multinucleated tumor giant cells, showing cellular cannibalism, and emperipolesis of inflammatory cells (neutrophils) (Figure 12). There are frequent mitoses, often atypical. Hyaline cytoplasmic inclusions may be seen.
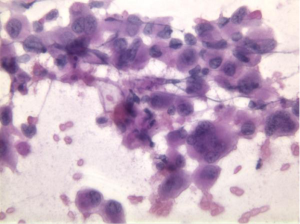
Differential diagnosis includes melanoma, hepatocellular carcinoma, pleomorphic sarcomas, poorly differentiated squamous cell carcinoma, and Hodgkin lymphoma.
Adenosquamous carcinoma
Comprise less than 5% of pancreatic neoplasms. There is a dual population of glandular and distinctly malignant squamous cells, rarely the squamous component may be predominant (Figure 13).
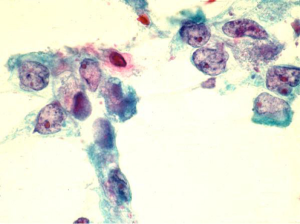
Smears show tumor cells with dense cytoplasm and distinct cell borders. Note focal squamous differentiation is not uncommon in ductal carcinomas.
Connective tissue tumors
Benign and malignant connective tissue tumors may rarely involve the pancreas.
Metastatic tumors
Metastatic tumors to the pancreas may include lung, kidney, breast, liver, GI, melanoma, prostate, sarcomas, myeloma, lymphoma (primary rare) (Figure 14).
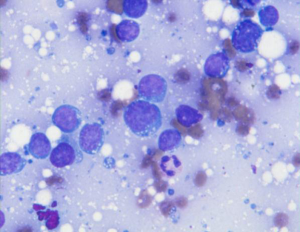
Consider metastases if tumor cells are not typical of primary pancreatic carcinoma, particularly if there are small cells or squamous cells, and in cases with known history of primary elsewhere. Ancillary studies should be performed.
Ampullary carcinoma
Ampullary carcinoma is similar to ductal type pancreatic carcinoma.
Common bile duct
Gallstones and stents may cause reactive cellular atypia. Tumors of the bile duct include granular cell tumor, cholangiocarcinoma, papillary bile duct neoplasms and embryonal rhabdomyosarcoma.
Triage for ancillary studies
Triage for ancillary studies requires on site evaluation and collection into appropriate media or fixatives. Tissue/cells may be collected for culture, special stains, immunochemistry, flow cytometry (RPMI solution), electron micoscopy (glutaraldehyde), and molecular studies (RPMI). Also fluid may be submitted for amylase and CEA levels.
Complications
Pain, bleeding which is self limited, rarely requiring transfusion. Acute pancreatitis following aspiration cytology is rare and usually mild. There may be sepsis, following aspiration of a pseudocyst. Tumor seeding of needle track and peritoneal spread is extremely rare due to the smaller diameter of the aspirating needle (15,16).
False negative diagnoses
These are usually due to technical difficulties. There may be sampling errors or interpretive errors. Hypocellularity with lack of sufficient diagnostic cells may be due to small tumor size and desmoplasia. These may be minimized by on site evaluation of adequacy by pathology.
Adequacy
Pancreatic FNA is more difficult than other sites, likely due to the fibrotic and inflammatory nature of many pancreatic tumors. Cystic tumors may be hypocellular, and result in false negative results. Five to six separate FNAs may be necessary to achieve maximal accuracy compared to only two to three for other organ targets. It has been recommended that for optimal results, a pancreatic mass should be sampled with seven aspirations (1,17,18). If an onsite pathologist/cytologist is present to assess adequacy, the number of aspirations required may be reduced (19).
Needle gauge does not appear to make a difference.
Follow up
Every effort should be made to review follow up as well as previous pathology on patients undergoing diagnostic or therapeutic procedures. Subsequent repeat cytologic biopsy specimens that are diagnostic, as well as correlation with the surgical specimens (if available) should be routinely performed. Also, information should be available to compare patient outcomes with the diagnoses previously rendered.
Conclusions
There are two major indications for pancreatic FNA, evaluation of a pancreatic mass, and documentation of malignancy prior to chemotherapy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Brugge WR. Pancreatic fine needle aspiration: to do or not to do? JOP 2004;5:282-8.
- Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc 2001;54:811-4.
- Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med 2001;134:459-64.
- Noh KW, Wallace MB. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic adenocarcinoma. MedGenMed 2005;7:15.
- Goldberg SN, Mallery S, Gazelle GS, et al. EUS-guided radiofrequency ablation in the pancreas: results in a porcine model. Gastrointest Endosc 1999;50:392-401.
- Chang KJ, Nguyen PT, Thompson JA, et al. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer 2000;88:1325-35.
- Hecht JR, Bedford R, Abbruzzese JL, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unre sectable pancreatic carcinoma. Clin Cancer Res 2003;9:555-61.
- Nori D, Merimsky O, Osian AD, et al. Palladium-103: a new radioactive source in the treatment of unresectable carcinoma of the pancreas: a phase I-II study. J Surg Oncol 1996;61:300-5.
- Wallace MB, Hawes RH. Emerging indications for EUS. Gastrointest Endosc 2000;52:S55-60.
- Bhatia V, Garg PK, Gupta SD, et al. Demonstration of peristalsis in gastric duplication cyst by EUS: implications for diagnosis and symptomatology (with videos). Gastrointest Endosc 2008;68:183-5.
- Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol 2011;106:1705-10.
- Schmitt F, Barroca H. Role of ancillary studies in fine-needle aspiration from selected tumors. Cancer Cytopathol 2011. [Epub ahead of print].
- Fowler LJ, Lachar WA. Application of immunohistochemistry to cytology. Arch Pathol Lab Med 2008;132:373-83.
- Pellisé M, Castells A, Ginès A, et al. Clinical usefulness of KRAS mutational analysis in the diagnosis of pancreatic adenocarcinoma by means of endosonography-guided fine-needle aspiration biopsy. Aliment Pharmacol Ther 2003;17:1299-307.
- Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc 2003;58:690-5.
- DeMay RM. The Art and Science of Cytopathology. Chigaco: ASCP Press;2012.
- Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc 2000;51:184-90.
- Binmoeller KF, Rathod VD. Difficult pancreatic mass FNA: tips for success. Gastrointest Endosc 2002;56:S86-91.
- Klapman JB, Logrono R, Dye CE, et al. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol 2003;98:1289-94.