Signature of microsatellite instability, KRAS and BRAF gene mutations in German patients with locally advanced rectal adenocarcinoma before and after neoadjuvant 5-FU radiochemotherapy
Introduction
Despite improved survival rates for patients with rectal cancer due to molecular targeted agents (such as bevacizumab, panatinuman and cetuximab) and 5-fluorouracil (5-FU) based doublet chemotherapy, the overall prognosis is still bad. Colorectal cancer (CRC) accounts for 8% of all cancer deaths with about 608,000 deaths worldwide (1,2). Specific genetic changes in benign and malignant lesions presented the first multistep genetic model in colorectal carcinogenesis (20% develop in the rectum), which was proposed in 1990 by Fearon and Vogelstein (3). Sequential accumulation of genetic mutations in the RAS-/RAF signal- or repair pathway play an important role in the proliferation, angiogenesis, apoptosis and finally viability of the tumor (4-8). These features are common hallmarks of cancer, described by Hanahan and Weinberg (9). KRAS and BRAF mutations are not uncommon in colorectal cancer. In sporadic colorectal cancer (CRC) the frequency of mutations in KRAS proto-oncogenes is 30% to 50% (10,11). KRAS mutations found in rectal cancer are more frequent in exon 2 and to a lesser extent in exon 3, resulting in sustained signal transduction and consequent increased cell proliferation, maturation and decreased cell apoptosis. Several studies evaluated the KRAS mutation status in patients treated with cetuximab or panitumumab, which are monoclonal antibodies and inhibit the epidermal growth factor receptor (EGFR), thus blocking the signal transduction. A response in KRAS wild-type of 17-48%, but no response in patients with a mutation in KRAS codon 12 or 13 was observed (12,13). Currently, NCCN guidelines (version 4.2013) recommend KRAS gene testing in codon 12 and 13 in exon 2 for all patients with colorectal cancer to spare patients with mutations from unnecessary side effects through EGFR-tyrosine kinase inhibitors (EGFR-TKI) (14). KRAS and BRAF are described as being mutational exclusive (15,16). Recently, studies have shown that mutations in BRAF also confer resistance to anti-EGFR therapy. A clinical trial with patients positive for the mutation V600E in BRAF demonstrated that they also did not respond to treatment with cetuximab or panitumumab (17). The NCCN Clinical Practice Guidelines (version 4.2013) for rectal cancer have been updated and published regarding BRAF gene testing as an option if KRAS is non-mutated to determine the benefit from anti-EGFR monoclonal antibodies. There are limited data available (18), specifically for locally advanced rectal cancers and the presence of BRAF and KRAS mutations as potential prognostic and predictive biomarker are still a matter of debate and currently under focus. According to Gaedcke and colleagues, no V600E BRAF mutations were detected in rectal cancer (19). In addition to the complex accumulating sporadic acquired mutations (85%), chronic inflammatory intestinal diseases as well as hereditary genetic changes (around 10-15% are affected and are initiated by a mutation in one of the DNA mismatch repair (MMR) genes) may contribute to development of colorectal cancer (17,20-24). Hereditary genetic changes are characterized by a large number of mutations at microsatellite sequences (shot repeating nucleotide sequences) and arise from defects in the DNA mismatch repair systems responsible for the repair errors during DNA synthesis (24). The defects in DNA MMR system (MLH1, MSH2, MSH6, and PMS2) change the length of short repeating nucleotide sequences and can be detected in tumor when compared to normal tissue of the patient (24,25). MSI (microsatellite instability) testing is recommend for all patients younger than 50 with colorectal cancer and family history due to increased likelihood of Lynch syndrome, also known as hereditary non-polyposis CRC (HNPCC) in this population, which is further defined by Amsterdam (I and II) and Bethesda-criteria (26-28). According to NCCN, a reference panel of 5 loci markers (Bethesda panel) is sufficient for identifying MSI: BAT25, BAT26, D5S346, D2S123, and D17S250. We used an additional panel with further 5 loci: BAT40, D10S197, D18S69, D18S58, MYCL1, for improved sensitivity. MSI is defined when >2 of the 5 loci are unstable, or when ≥30% of >5 loci are unstable (29). MSI can be further classified into MSI-high (MSI in at least 2 of the 5 markers) and MSI-low (MSI in only 1 marker) (29). Many studies have consistently shown that MSI candidates show low or no response to 5-FU but there is a lot of education that needs to be done to determine whether MSI in stage II and III patients should be routinely tested (30). Samowitz and colleagues suggested that MSI-H rectal cancer are enriched for Lynch syndrome and are associated with an adverse prognosis (31). Currently, there is a lack of predictive information specifically for locally advanced rectal cancers concerning MSI tumors from patients treated with 5-FU/oxaliplatin. The already mentioned molecular features are still a matter of debate and will be further assessed in the current retrospective study before and after neoadjuvant 5-FU radiochemotherapy with respect to the tumor response and patients antecedent including nicotine abusus, familial history, and health care to further molecularly identify rectal cancer patients who may benefit from preoperative radiochemotherapy.
Materials and methods
Patients and tissue samples
Archival formalin fixed paraffin embedded (FFPE) human rectal tissue samples of 25 patients were investigated pre- and posttherapeutically. Clinical data were collected retrospectively. According to Dworak and colleagues, a 5-point tumor regression grading system was applied to assess the histopathologic response: grade 0, no regression; grade 1, minimal regression; grade 2, moderate regression; grade 3, good regression; and grade 4, total regression (31). Assessing genomic and tissue quality was essential for analytical processes, therefore the isolated tissue area was characterized regarding the presence of necrosis, viable tumor cells and inflammatory cells such as lymphocytes and granulocytes. Additionally, the quality of isolated DNA and amplified products was determined by optical density (OD260/280) measurements and agarose gel electrophoresis, respectively. All compiled mutations were carefully reviewed in regard to pattern potentially resulting from formalin fixation or paraffin embedded artifacts.
DNA isolation
DNA was prepared from 8-14 serial 3 µm-thick and HE (Haematoxylin & Eosin) stained paraffin sections. The microdissected material was manually isolated from deparaffinizated samples using QIAamp DNA Micro Tissue Kit (Qiagen) with the addition of a 16-hour proteinase K lysis step for protein degradation.
Mutation analysis for KRAS and BRAF
Polymerase-chain-reaction (PCR)
The KRAS and BRAF gene amplification was conducted by Primus 96 Advanced PCR-instrument (PeqLab). Primers and fragment details are described in Table 1. For all 50 samples, (25 samples before and 25 samples after neoadjuvant radiochemotherapy), the existence of amplified KRAS and BRAF fragment was revealed by 2% agarose gel electrophoresis prior to SNaPshot- and sequence analysis.
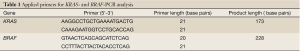
Full table
Sequencing and SNaPshot
Sequencing analysis was based on Sanger method and the SNaPshot analysis on single base extension (Table 2. Applied primers) carried out according to the recommendation of Applied Biosystems, Germany. Different sets of primers were used to amplify KRAS and BRAF genes, (Table 2). The GeneMapper® software v4.0 and the Sequencing Analysis Software v5.2 was applied to size and genotype the data. The GeneScan™-120 LIZ® size standard was used to indicate the size of labeled fragments. The SNaPshot reaction was purified by 1 µL SAP (1 U/mL) and the sequence-product by the application of the Dye Ex Kit 2.0 (QIAGEN, Germany).
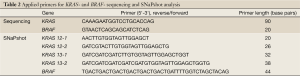
Full table
Microsatellite instability analysis
The microsatellite analysis was conducted using a fluorescent multiplex PCR-based method. Typical allelic profiles of microsatellite markers (as listed in Table 3), generated by amplification of matching tumor and normal tissue, were compared. Panel 1 and panel 2 (Table 3) include two distinct analyses of five microsatellite systems, respectively. Therefore in total 10 microsatellite markers were used for MSI testing.
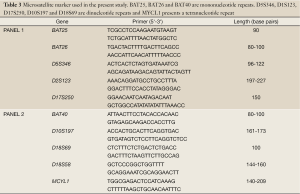
Full table
If more than 30% of a tumor’s markers are unstable, it is scored as MSI-H. The tumor is designated as MSI-L if at least one, but fewer than 30% of markers are unstable (Table 3).
Statistics and mathematics
The JMP statistical software version 6.0 (JMP, Germany) and SPSS 17.0 (IBM, Germany) were used for all statistical analyses. A P-value of 0.05 or less was usually regarded as relevant.
Experimental results
Baseline characteristics and pathologic evaluation
The project assessed the intratumoral mutation status of 25 pretherapeutic biopsies obtained prior to neoadjuvant therapy (Table 4), at the time of diagnosis, and 25 posttherapeutic samples, at the time of surgery. At diagnosis, the individuals ranged in age from 30-84 years with a median age of 67.5 years, 13 (52%) males and 12 (48%) females.
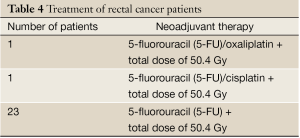
Full table
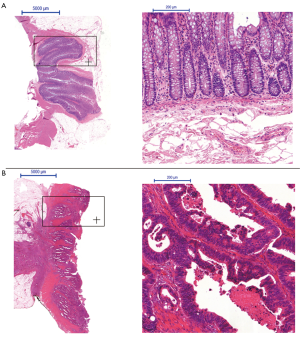
All carcinomas were histologically confirmed primary rectal adenocarcinomas (Figure 1). Most tumors were moderately differentiated [G1=1 (4%), G2=22 (88%), G3=2 (8%)]. Further pathohistological characteristics (TNM-classification) of the respective rectal cancer are listed in Table 5.

Full table
According to Dworak and colleagues, the histopathologic response (grade 0, no regression; grade 1, minimal regression; grade 2, moderate regression; grade 3, good regression; and grade 4, total regression) was as follows (31,32):
Regression grade 0→1 (4%)
Regression grade 1 → 5 (20%)
Regression grade 2 → 9 (36%)
Regression grade 3 → 10 (40%)
For further evaluation, the regression grades 0-1 were defined as non-response.
The following table (Table 6) includes further information concerning the patients antecedent.

Full table
The most frequent coexisting disease (found in 48% of the patients) was hypertension. 16% of the patients were smokers. These factors were not differently associated with the intratumoral mutation status. The diabetic patients were diagnosed with higher tumor (T3) and lymph node (N3) stages.
Mutation Analysis for KRAS and BRAF
KRAS and BRAF amplifications were electrophoresed on 2% agarose gel electrophoresis, (Figure 2), resulting in one visible band for each sample.
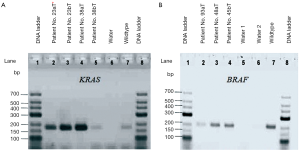
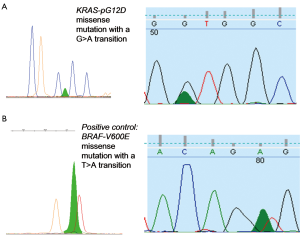
Figure 3 illustrates electropherograms of sequence and SNaPshot analysis of BRAF and KRAS genes, respectively. Mutations are found at the first, second and fourth base position of the wildtype sequence (Table 7).
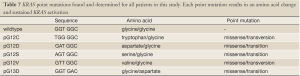
Full table
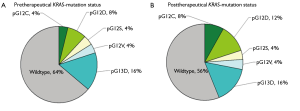
9 of 25 patients (36%) before and 11 of 25 individuals (44%) after neoadjuvant radiochemotherapy harboured KRAS mutations (Figure 4). Most mutations are transition ones. The above compiled mutation status changes for two patients from negative to KRAS mutation positive after therapeutic application, (presence of pG12C and pG12D after therapy). In 4 cases the mutation was missed through sequencing but detected by SNaPshot analysis.
The degree of tumor regression (Chi2-test, P=0.577), age (Chi2-test, P=0.249), sex (Chi2-test, P=0.566) and mutation status were not differently associated. The presence of KRAS mutations was correlated neither with tumor response, nodal or metastatic stage.
Microsatellite instability analysis
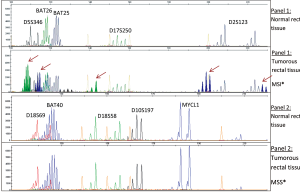
As shown in Figure 5, patients whose tumor DNA showed allelic pattern that was not present in the corresponding normal DNA were defined as MSI positive.
Full table
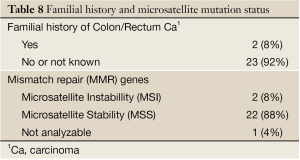
Among 25 patients analyzed, 2 (8%) exhibited a MSI+ phenotype, (Table 8), with early rectal cancer onset, familial recurrence of colorectal carcinomas and non-response to neoadjuvant 5-FU-therapy.
Discussion
KRAS and BRAF mutation status
These data show that the frequency of the KRAS oncogene mutation in a series of 25 CRC patients was 36% pretherapeutically and 44% posttherapeutically. All samples were diagnosed as V600E BRAF mutation negative. The KRAS mutation status was correlated neither with tumor response, sex, age or other histopathological features. According to the literature, oncogenic mutations affecting KRAS and BRAF occur in about 25-50% and approximately 4-12% of colorectal cancers, respectively (33). Gaedcke and colleagues detected no V600E BRAF mutations and 48% KRAS mutations in rectal cancer patients (n=94) consistent with our data (19). In two cases the mutation status in tumor DNA changed after therapy. This could be due to the fact that malignant tumors are genetically heterogeneous and different areas of the colonic tumor are taken from the patient or that the radiochemotherapy induces a mutation which is also common and relevant for further therapy decisions. In individual cases the KRAS mutation (most are transition ones) was missed by sequencing but detected using the SNaPshot analysis, thereby indicating the need to use highly sensitive molecular techniques. SNaPshot has a higher analytical sensitivity of approximately 5-10% as compared to the sequencing method which shows an allele detection sensitivity of 10-15% (34). Thus, the use of two independent analytical methods to ensure routinely efficient mutation detection was proven valuable.
The identification of mutationally activated KRAS and BRAF alleles in several tumor models supports the importance of this signaling pathway in cancer progression (35,36). It is known that KRAS and BRAF mutations may lead to a hyperactivation of the RAS/RAF/MAPK pathway. The detected somatic mutations predict resistance to monoclonal antibodies targeting epidermal growth factor receptor (EGFR). Therefore, promising treatments of combinations of anti-EGFR like cetuximab or panitumumab with 5-fluorouracil (5-FU)-based chemotherapy are not advisable. In contrast to colorectal cancer, rectal cancer missed V600E BRAF mutations, which seem to play no role in rectal cancer pathogenesis and consequently do not influence the tumor response to anti-EGFR or other therapies. In the current study, most patients have received a 5-FU therapy exclusively. No statistically significant correlation between the KRAS mutation status and the regression grade was detected. In a larger cohort the relation between KRAS mutation and EGFR status in metastases, secondary tumor and tumor cells in blood and stool related to primary tumor sample could be investigated.
Pre-/co-existing diseases and microsatellite instability
No significant differences were observed in the overall family history or nicotine abuse of rectal cancer patients regarding KRAS-/BRAF mutation. In another prospective study (n=37,399) cigarette smoking was associated with BRAF mutation-positive colorectal cancer subtypes indicating epigenetic modification, which may be functionally involved in smoking-related colorectal carcinogenesis (37). It is known that environmental, diet or lifestyle factors may contribute to or enhance the acquirement of gene mutations involved in carcinogenesis.
Two patients showed a positive familial history, were at the age of <50 and were diagnosed with microsatellite instable tumors. These two patients had the probability of a hereditary predisposition according to the clinical definition by means of Amsterdam and Bethesda criteria. Our data show a lower rate of MSI-H rectal cancer because rectal cancer is less likely to show MSI-H than colon cancer (38). Of significant clinical importance, patients with MSI-H/mismatch repair-deficient colorectal cancer do not appear to benefit from adjuvant 5-fluorouracil and leukovorin (or levamisole) chemotherapy, whereas approximately 85% of individuals with microsatellite stable (MSS) colon cancer do appear to benefit from this therapy, according to Gryfe et al. 2009 (39). Our data revealed a nonresponse of MSI-H rectal cancer to neoadjuvant 5-FU radiochemotherapy, which raises the question if rectal cancer patients should be routinely tested for microsatellites.
Other factors maybe such as age-related diseases, hypertension (48%) and diabetes (8%) may also contribute to or enhance the tumor development. A very important characteristic in the early stage of Type 2-diabetes or adult-onset diabetes is a high blood glucose level in context of insulin resistance or relative insulin deficiency. A high insulin dose is necessary to engage the insulin resistance. A principle function of insulin is to decrease the glucose level of the blood. Additionally, insulin assists the growth and proliferation of cells and may promote the cancer formation in a more aggressive form and the risk of relapse (40). As mentioned in the result part, the diabetic patients were diagnosed with higher tumor (T2-T3) and lymph node (N1-N3) stages, which agrees with the literature of CHEN Chuang-Qi et al., 2010 (40). According to the result part, the most frequent coexisting disease (found in 48% of the patients) was hypertension. The two distinct disease, rectal cancer and hypertension, may share some common pathophysiological mediators. Possible mediators linking hypertension and cancer could be nitric oxide, bradykinin or angiotensin II or elevated plasma levels of VEGF (41-44). Through these, hypertension might influence the promotion of tumourgenesis and malignant progression. The above mentioned issues could be further addressed by other studies including a larger collective, to maybe generate a clinically relevant risk-profile.
Conclusions and outlook
In the present study, age and sex of the patients were not associated with the mutation status. Contrarily to V600 E BRAF gene mutation, 44% of patients were KRAS mutation positive (most located at codon 12) and therefore a treatment with an anti-EGFR monoclonal antibody drug would not be advisable. SNaPshot analysis indicates the need to use highly sensitive molecular techniques to ensure detection of mutations in tumors conferring resistance to treatments. Mutational analysis after therapy in primary tumor or metastasis could be relevant for further treatment decisions.
To investigate these observations, a further detect study with larger series should be analyzed in order to definitely establish the clinical relevance. The fact that KRAS mutational alterations occur after therapy implicates the need to compare the mutational status and gene expression levels between primary tumors and metastases of the same patient. This might give information on the potential response to a chemotherapeutic reagent and will therefore be important in the future. Finally, metastases could be screened directly for the presence of alterations conferring either sensitivity or resistance to these targeted therapies and to reduce the risk of further tumor spread and invasion influencing the final prognosis of the patient.
It was interesting to note that the majority of cancer patients have coexisting diseases. Hypertension can maybe influence the promotion of tumourgenesis and malignant progression. Several studies documented a connection between hypertension and the risk of cancer (45). The process of neovascularization or angiogenesis is a phenomenon that plays a significant role in both hypertension and cancer. Different important pro-angiogenic factors such as VEGF, bFGF, TNF-alpha, TGF-alpha, IL-1, IL-6 and so on were often found to be secreted by tumor, inflammatory and stromal cells. The level of angiogenic factors is high in hypertension. Thus, more studies at the basic biological or pathophysiological would be interesting to improve the understanding about the relationship between hypertension, diabetes and cancer. Is there really a relation or are they just distinct coexisting diseases?
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- WHO. Cancer. 2009. Available online: http://www.who.int/mediacentre/factsheets/fs297/en/ (accessed on 20th October 2012)
- Globocan. Cancer Fact Sheet. Colorectal Cancer Incidence and mOrtality Worldwide in 2008 Summary. 2008. Available online: http://globocan.iarc.fr/factsheets/cancers/colorectal.asp (accessed on 14th September 2012)
- Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759-67.
- Wieduwilt MJ, Moasser MM. The epidermal growth factor receptor family: biology driving targeted therapeutics. Cell Mol Life Sci 2008;65:1566-84.
- Chapman MS, Miner JN. Novel mitogen-activated protein kinase kinase inhibitors. Expert Opin Investig Drugs 2011;20:209-20.
- Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 2008;27:5497-510.
- Weiss GA, Rossi MR, Khushalani NI, et al. Evaluation of phosphatidylinositol-3-kinase catalytic subunit (PIK3CA) and Epidermal Growth Factor Receptor (EGFR) gene mutations in pancreaticobiliary adenocarcinoma. J Gastrointest Oncol 2013;4:20-29.
- Kerkhoff E, Rapp UR. Cell cycle targets of Ras/Raf signalling. Oncogene 1998;17:1457-62.
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000;100:57-70.
- Smith G, Carey FA, Beattie J, et al. Mutations in APC, Kirsten-ras, and p53--alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci U S A 2002;99:9433-8.
- Calistri D, Rengucci C, Seymour I, et al. Mutation analysis of p53, K-ras, and BRAF genes in colorectal cancer progression. J Cell Physiol 2005;204:484-8.
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643-8.
- Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6.
- Cancer Connect. NCCN Updates Colorectal Cancer Guidelines to Include KRAS Testing. Available online: http://news.cancerconnect.com/nccn-updates-colorectal-cancer-guidelines-to-include-kras-testing/ (accessed on 23th September 2012)
- Mao C, Zhou J, Yang Z, et al. KRAS, BRAF and PIK3CA mutations and the loss of PTEN expression in Chinese patients with colorectal cancer. PLoS One 2012;7:e36653.
- Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 2007;67:2643-8.
- Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008;26:5705-12.
- National Comprehensive Cancer Network. Available online: http://www.nccn.org/network/business_insights/flash_updates/flash_update_information.asp?FlashID=31. 275 (accessed on 6th December 2012)
- Gaedcke J, Grade M, Jung K, et al. KRAS and BRAF mutations in patients with rectal cancer treated with preoperative chemoradiotherapy. Radiother Oncol 2010;94:76-81.
- Trichopoulos D, Lipworth L, Petridou E. Epidemiology of Cancer. Philadelphia: Lippincott Williams & Wilkins, 1997.
- Skibber J, Minsky B, Hoff P. Cancer of the colon and rectum. In: DeVita VT Jr, Hellmann S, Rosenberg SA. eds. Cancer: principles & practice of oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2001:1216-71.
- Lynch HT, Smyrk T, Lynch J. An update of HNPCC (Lynch syndrome). Cancer Genet Cytogenet 1997;93:84-99.
- Ilyas M, Straub J, Tomlinson IP, et al. Genetic pathways in colorectal and other cancers. Eur J Cancer 1999;35:335-51.
- Wu C, Bekaii-Saab T. CpG Island Methylation, Microsatellite Instability, and BRAF Mutations and Their Clinical Application in the Treatment of Colon Cancer. Chemother Res Pract 2012;2012:359041.
- Boland CR, Koi M, Chang DK, et al. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedside. Fam Cancer 2008;7:41-52.
- NCCN Clinical Practice Guidelines. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. (accessed on 10th December 2012)
- Schmiegel W, Pox C, Reinacher-Schick A, et al. S3-Leitlinie “Kolorektales Karzinom” 2004/2008. Z Gastroenterol 2008;46:1-73.
- Review of microsatellite instability (MSI) in colorectal cancer 2012. Available online: http://pathlabmed.typepad.com/surgical_pathology_and_la/2012/02/review-of-microsatellite-instability-msi-in-colorectal-cancer.html (accessed on 8th October 2012)
- SRL Global knowledge. Colorectal Cancer-Personalized Medicine, Now a Clinical Reality 2012. Available online: http://www.srlworld.com/innersense/Voice-135-Colorectal-Cancer-Sept-2012-IS.pdf (accessed on 2nd October 2012)
- Samowitz WS, Curtin K, Wolff RK, et al. Microsatellite instability and survival in rectal cancer. Cancer Causes Control 2009;20:1763-8.
- Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19-23.
- Demes M, Bartsch H, Scheil-Bertram S, et al. A. Real-Time PCR Data Processing Shown by the Analysis of Colorectal Specific Candidate Genes, ERCC1, RRM1 and TS in Relation to β2M as Endogenous Control. Appl Sci 2012;2:139-59.
- Zhang W, El-Khoueiry A, Yang D, et al. K-ras mutation status associated with clinical outcome in metastatic colorectal cancer patients treated with 5-fluorouracil/oxaliplatin. Gastrointestinal Cancers Symposium 2009, abstr 340.
- Hurst CD, Zuiverloon T, Hafner C, et al. Cancer Research UK Clinical Centre, 2009.
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54.
- Rajagopalan H, Bardelli A, Lengauer C, et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002;418:934.
- Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst 2010;102:1012-22.
- Ishikubo T, Nishimura Y, Yamaguchi K, et al. The clinical features of rectal cancers with high-frequency microsatellite instability (MSI-H) in Japanese males. Cancer Lett 2004;216:55-62.
- Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69-77.
- Chen CQ, Fang LK, Cai SR, et al. Effects of diabetes mellitus on prognosis of the patients with colorectal cancer undergoing resection: a cohort study with 945 patients. Chin Med J (Engl) 2010;123:3084-8.
- Carrizo PH, Dubin M, Stoppani AO. Physiopathologic effects of nitric oxide and their relationship with oxidative stress. Medicina (B Aires) 1998;58:367-73.
- Harris MB, Ju H, Venema VJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem 2001;276:16587-91.
- Egami K, Murohara T, Shimada T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest 2003;112:67-75.
- Yoshiji H, Yoshii J, Ikenaka Y, et al. Suppression of the renin-angiotensin system attenuates vascular endothelial growth factor-mediated tumor development and angiogenesis in murine hepatocellular carcinoma cells. Int J Oncol 2002;20:1227-31.
- Grossman E, Messerli FH, Boyko V, et al. Is there an association between hypertension and cancer mortality? Am J Med 2002;112:479-86.


