Differential lymph node retrieval in rectal cancer: associated factors and effect on survival
Introduction
Colorectal cancer is the fourth leading cause of cancer and the second leading cause of cancer death in the U.S. each year. In the absence of distant metastatic disease, the status of the regional lymph nodes is the most powerful prognostic factor (1). Decisions regarding adjuvant chemotherapy and chemoradiotherapy are based, in large part, on the presence or absence of regional lymph node involvement. Given the importance of regional lymph node status, efforts to improve the accuracy of nodal staging are justified. The accuracy of lymph node staging improves as the number of lymph nodes pathologically examined increases (1). This observation, which has been made in both colon and rectal cancer, has led to consensus recommendations that at least 12 lymph nodes be identified and subjected to histological examination in both colon and rectal cancer (2). This recommendation has gained strength, and an additional degree of importance, since the more recent publication of studies that demonstrate that survival after resection for colorectal cancer improves as the number of lymph nodes examined increases. Indeed, those evaluating the quality of care delivered in colon and rectal cancers are becoming interested in using this recommendation as a quality benchmark for both diseases (3,4).
This identical recommendation for minimum lymph node examination in both colon and rectal cancer seems to ignore two important points. It is generally understood that lymph node counts are consistently lower in rectal cancer specimens compared to colon cancer specimens. Second, the body of evidence supporting an association between higher lymph node counts and improved survival is heavily weighted to analyses of colon cancer rather than rectal cancer. Since the impact of lymph node counts in rectal cancer seems less clear, we performed a retrospective review to determine whether lymph node counts correlated with 5-yr OS and to explore the relationship between lymph node counts and various clinical and pathologic factors.
Patients and methods
Through a search of our institutional tumor registry, we identified 190 patients with AJCC Stage 1, 2, or 3 rectal adenocarcinoma that underwent surgical resection in our hospital system over an eleven-year period (01/01/1995 through 12/31/2005). Institutional Review Board approval was obtained to extract information from the database. We defined rectal cancer patients as those with an invasive tumor with its distal edge <15 cm from the anal verge. We excluded 28 patients treated by transanal excision and three that represented a recurrence from another primary diagnosed before the beginning of our study period. We then conducted a retrospective review of the medical records of the remaining 159 patients to develop a database containing standard clinico-pathologic variables.
The clinico-pathologic data recorded included the following patient characteristics: age at diagnosis, gender, AJCC stage, histological grade, LNCs, period of diagnosis [1995-2000 and 2001-2005], administration of neoadjuvant therapy, and performance of a detailed mesorectal excision. LNCs were determined from the pathology report. For the purpose of analyses, LNCs were dichotomized in 4 different ways: ≥/<7, ≥/<10, ≥/<12, and ≥/<14, based on the median number of lymph nodes examined in the current study (i.e., 7) and values that appear in the literature (10, 12, and 14) (3,5,6).
Univariate analysis (Kruskal-Wallis test) was used to explore the relationship between lymph node counts and the following variables: age (<70 yrs/>70 yrs), AJCC Stage, time of diagnosis (early — 1995-2000/late — 2001-2005), gender, administration of neoadjuvant therapy and the performance of mesorectal excision. Five-year OS was estimated by the Kaplan-Meier method and log rank testing was used to assess potential differences between groups. Cox proportional hazards modeling was used to examine the relationship between lymph node counts and survival, adjusting for patient age and stage at diagnosis. P-values ≤0.05 were considered statistically significant.
Results
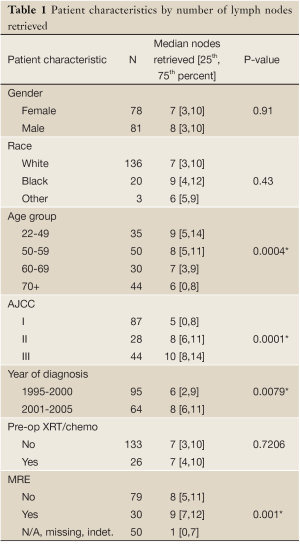
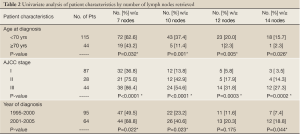
Age, stage at diagnosis, year of diagnosis, and performance of MRE were patient characteristics that were significantly associated with LNCs. Patients less than 70 years old had more lymph nodes retrieved compared to those ≥70 years old (P<0.05). In univariate analysis, there was no statistically significant difference in LNCs by gender or by the use of neoadjuvant therapy (P>0.05). Patients treated during the later years of the study were more likely to have more nodes retrieved (P>0.05). Patients with MRE performed had higher LNC, but not uniformly statistically significant for each cut point of LNCs used (Tables 1,2).
Full table
Full table
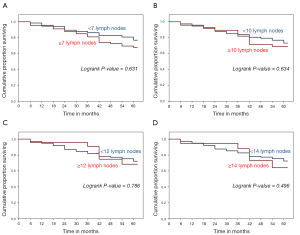
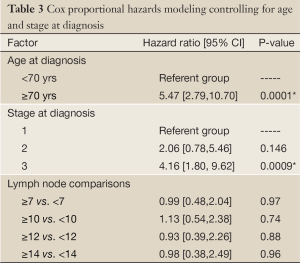
In our survival analysis, we observed that higher LNCs were associated with lower survival rates. Although these differences in survival were not statistically significant, they were consistent for each cut point of LNCs used (Figure 1). In multivariate survival analysis using the Cox proportional hazards model, the apparent negative effect of increasing LNCs on survival did not persist. In this analysis, only Stage III disease and age over 70 yrs proved to be independent predictors of the risk of death (Table 3). The performance of MRE was not significant using the Cox proportional hazards model and subsequently not used in survival analysis.
Full table
Discussion
Colorectal cancer represents the second leading cause of cancer related death in the U.S., resulting in 55,000 deaths each year. In the absence of distant metastatic disease, the status of the regional lymph nodes is the single most powerful prognostic factor (1). The presence of lymph node involvement, when matched for similar T-stage, results in a decrease in 5-yr OS. Since the NIH consensus statement regarding adjuvant therapy for colon and rectal cancer was published in 1990, patients with node positive colon or rectal cancer generally have been offered a 5-FU based adjuvant chemotherapy regimen (7). The presence of nodal involvement also increases the risk of regional recurrence after rectal cancer resection, a risk that can be mitigated by pelvic 5-FU based chemoradiotherapy. Accordingly, Stage III rectal cancer patients are routinely offered such chemoradiotherapy as part of a curative treatment regimen.
Given the importance of lymph node status in determining prognosis and guiding treatment in colon and rectal cancer, accurate staging of these diseases is an important issue, both in the public health arena and for individual patients and their physicians. Multiple studies have demonstrated that the accuracy of staging in colorectal cancer improves when more lymph nodes are histologically examined (3,4). This fact, observed in both colon and rectal cancer, has led to consensus recommendations to identify and examine at least 12 lymph nodes from the resected colon or rectal cancer specimen (2). The interest in LNCs has escalated recently after the publication of a similar observation that the probability of survival after treatment for colon or rectal cancer improved in patients in whom more lymph nodes were histologically examined (8). Because of the relationship between LNCs and staging accuracy and LNCs and survival, minimum LNCs are an obvious target for those interested in evaluating the quality of care in colorectal cancer (3).
It is interesting that, in spite of the fact that there appear to be significant differences between colon cancer and rectal cancer, the minimum LNC recommendations do not discriminate between these two diseases (9). We believe that this is unfortunate, since considering these two disease as one disease process imprecisely characterizes each and ignores important differences between them (10). From an anatomical standpoint, the colon has a long abundant mesentery that contains vascular structures and rich lymphatics, while the rectal lymphatics are contained in a much more compact and shortened mesentery. In addition to these anatomic differences, the approaches to resecting the colon and rectal mesenteries differ. Standard teaching dictates that a 5 cm bowel wall margin is required on the proximal and distal ends of colon cancer resections. However, this bowel margin is never a practical issue as colon resections are based on the segmental, mesenteric blood supply and lymphatic drainage of the part of the colon to be resected. In rectal cancer resections, the technical considerations are more complicated. While most agree that the proximal bowel margin should be at least 5 cm, the acceptable distal margin has been a source of some disagreement/confusion (11). Historically, a 2 cm distal margin on the bowel wall was considered adequate. However, since Heald described the total mesorectal excision in 1982, there has been a growing recognition that the distal margin of importance in rectal resections is the one on the mesorectum, and that this should be at least 4 cm distal to the tumor (12). Our study suggests that attention to the distal mesorectal margin might be suboptimal, as TME was described in a minority of cases in our series. If this is true of community practice in general, this combination of mesenteric anatomic facts and differences in common surgical techniques for mesenteric resection might explain the gap in LNCs observed between colon cancer and rectal cancer resections. It also makes a compelling argument for additional studies that attempt to more clearly characterize both the operative treatment of rectal cancer and the impact this treatment has on outcome measures, such as LNCs, OS and regional recurrence.
This consistent gap in LNCs between colon cancer and rectal cancer makes it logical to pursue separate minimum LNCs for each disease. Since we understand that more appears to always be better when it comes to staging, we are not necessarily arguing to decrease the minimum for LNCs in rectal cancer. It might actually be more reasonable, however, to increase the minimum LNCs for colon cancer. This would then create some distinction between colon and rectal cancer that reflects the current data. It might also give those involved in quality oversight efforts a better perspective on what constitutes an acceptable and fair quality benchmark for LNCs in rectal cancer. It is also worthwhile to remember the LNC is not the only factor in determining outcomes after rectal cancer treatment (13). Ultimately, lymph node count will be but one of many factors considered in this disease. Because of the ease of determination of LNCs, however, and the described relationship between LNCs and survival, LNCs now occupy a central place in the discussion.
In an effort to better understand the factors that affect LNCs in rectal cancer, we explored the relationship between LNCs and several clinico-pathologic factors. While gender and race did not appear to affect LNCs, patient age did seem to affect LNCs, as patients under 70 had higher LNCs than those over 70. In addition, the period of diagnosis was also important, as patients in our cohort diagnosed after 2000 had higher LNCs. While this suggests some change over time, we cannot readily identify the source of that change. We suspect that increased awareness among treating physicians and pathologists might have contributed to the improvement in LNCs.
Another potential explanation for the increase in LNCs could be a shift in the operative techniques being employed. We did not observe any increase in the frequency of TME performance but noticed an increase in LNCs in those patients undergoing a TME. As suggested earlier, we believe the impact of surgical techniques of rectal resection on LNCs deserves more attention. Unfortunately, larger, population-based data sets do not provide this level of detail. Another potentially important factor in rectal cancer and LNCs is the delivery of preoperative, pelvic radiotherapy. Neoadjuvant radiotherapy is known to decrease LNCs in the resected rectal cancer specimen. Since neoadjuvant chemoradiotherapy has been accepted as a standard treatment for node positive and Stage II rectal cancers, efforts to use LNCs as a quality indicator will have to consider the impact of this approach on this metric. One would assume that minimum LNCs would necessarily be adjusted downward. Other clinical factors, such as the clinical and or pathologic response to the preoperative therapy might also have an impact on LNCs. Prior studies have not considered patients who had undergone neoadjuvant therapy (14,15). In the current study, there did not appear to be any difference in LNCs between patients who received preoperative chemoradiotherapy and those who did not. One possible explanation for this negative result might be that more patients received neoadjuvant chemoradiatherapy in the later period, during which LNCs increased. It is possible, therefore, that the negative impact on LNCs expected because of preoperative radiotherapy was masked by improved identification during the later period of the study. Another possible explanation is that our study simply lacked the power to detect a difference in LNCs caused by preoperative radiotherapy. In either case, future population-based studies should attempt to characterize LNCs in patients who have undergone preoperative radiotherapy and to determine whether LNCs in this clinical setting carry the same importance as they appear to carry in untreated patients.
While the improvement in staging accuracy with increasing LNCs has been firmly established, the relationship between lymph node counts and survival is less settled. In the current study, no statistically significant improvement in 5-yr OS was detected with increasing LNCs. In fact, in Kaplan Meier analysis, higher lymph node counts correlated with worse survival, albeit not statistically significant either. This consistent observation, regardless of how LNCs were dichotomized, is likely because increasing LNCs were closely correlated with increasing stage. It is difficult to imagine, based on the current data, that an improvement in survival could be observed as LNCs increase, since increasing LNCs are so closely tied to increasing stage, and increasing stage is itself tied to worse OS. We recognize that our inability to demonstrate an improvement in survival with increasing LNCs does not preclude the existence of such a relationship. In fact, larger studies have provided more definitive information on this relationship (5,15). It is worth pointing out that large studies like these are crucial in detecting such phenomena since institutionally based studies would be much less likely to uncover them. Patient-level studies remain important; however, because they provide more granular clinical data that when analyzed teases out the why and the how behind observations from population-based studies. Combining individual institutional studies should improve the productivity of this type of study. Perhaps the most important role of these patient-level studies could be to inform and improve the population-based registries by suggesting which additional data should be collected by these organizations.
The current study examines the relationship between LNCs in resected rectal cancer and various clinico-pathologic factors. Higher LNCs were associated with younger age, higher stage, diagnosis in the later period of our study, and performance of MRE. We could not demonstrate a decrease in lymph node counts among patients treated with neoadjuvant chemoradiotherapy. Examination of the relationship between lymph node counts and 5-yr OS failed to demonstrate any improvements in survival with higher LNCs. In fact, the opposite effect of higher LNCs was observed. Based on the apparent differences between rectal cancer and colon cancer, we believe separate recommendations for minimum lymph node counts should be developed, based on population-based data. We also believe that LNCs in patients treated with preoperative chemoradiotherapy should be separately analyzed to determine appropriate quality benchmarks. Finally, recalling that LNC is not the only important factor, institutionally based studies should continue to identify other factors that influence outcomes after rectal cancer treatment. These factors could then be considered for inclusion in the data collection efforts of large population-based registries.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kim J, Huynh R, Abraham I, et al. Number of lymph nodes examined and its impact on colorectal cancer staging. Am Surg 2006;72:902-5.
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96.
- Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 2007;99:433-41.
- Carloss H, Huang B, Cohen A, et al. The impact of number of lymph nodes removed on five-year survival in stage II colon and rectal cancer. J Ky Med Assoc 2004;102:345-7.
- Pocard M, Panis Y, Malassagne B, et al. Assessing the effectiveness of mesorectal excision in rectal cancer: prognostic value of the number of lymph nodes found in resected specimens. Dis Colon Rectum 1998;41:839-45.
- Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001;19:157-63.
- Adjuvant therapy for patients with colon and rectum cancer. Consens Statement 1990;8:1-25.
- Cserni G, Vinh-Hung V, Burzykowski T. Is there a minimum number of lymph nodes that should be histologically assessed for a reliable nodal staging of T3N0M0 colorectal carcinomas? J Surg Oncol 2002;81:63-9.
- National Comprehensive Cancer Network Practice Guidelines in Oncology. V2. 2008.
- Li M, Li JY, Zhao AL, et al. Colorectal cancer or colon and rectal cancer? Clinicopathological comparison between colonic and rectal carcinomas. Oncology 2007;73:52-7.
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96.
- Visser O, Bakx R, Zoetmulder FA, et al. The influence of total mesorectal excision on local recurrence and survival in rectal cancer patients: a population-based study in Greater Amsterdam. J Surg Oncol 2007;95:447-54.
- Enker WE. The elusive goal of preoperative staging in rectal cancer. Ann Surg Oncol 2004;11:245-6.
- Baxter NN, Virnig DJ, Rothenberger DA, et al. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst 2005;97:219-25.
- Swanson RS, Compton CC, Stewart AK, et al. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003;10:65-71.