Hepatocellular carcinoma (HCC): beyond sorafenib—chemotherapy
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer. HCC is the sixth most common cancer and the second leading cause of cancer-related deaths worldwide (1). In contrast to most solid cancers, the incidence of HCC and HCC-related deaths have increased over the last several decades in many parts of the world including United States. In the United States, more than 39,230 new cases of HCC and more than 27,170 HCC-related deaths were predicted in 2016 (2). The prognosis of HCC is dismal with a 3-year survival rate of 12.7% and a median survival of 9 months (3).
HCC can be treated with surgical resection, liver transplantation, liver directed therapy and systemic therapy. Among these options, only surgical resection and liver transplantation are considered as potentially curative approaches. However, only 15% patients are eligible for the potentially curative treatments (4) since a majority of patients will present with advanced disease. In 2016, sorafenib is still the only FDA approved systemic therapy for advanced HCC. In contrast to other solid cancer, systemic chemotherapy has not been used routinely since HCC is considered as a chemotherapy resistant tumor with overexpression of dihydropyrimidine dehydrogenase, the P-glycoprotein gene product and the multidrug resistance gene, MDR-1 (5-7). Furthermore, patients with advanced HCC usually have significant underlying liver disease which is associated with poor tolerability to systemic chemotherapy. However, there have been many trials evaluating systemic chemotherapy in patients with advanced HCC prior to sorafenib era. In this paper, we will attempt to concisely summarize the historical perspective and the current status of systemic therapy development in HCC. The role of targeted therapy in HCC will not be covered in this review paper.
Chemotherapy
Single agent chemotherapy
Doxorubicin
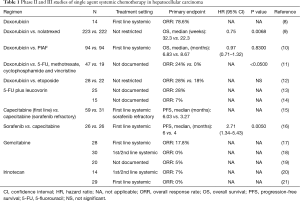
Prior to the approval of sorafenib, doxorubicin was commonly used in the treatment of advanced HCC. An initial phase II study of single agent doxorubicin demonstrated 79% (11/14) objective response rates including three complete responses in advanced HCC (Table 1) (8). However, subsequent studies demonstrated only limited efficacy (<20% clinical responses) without significant survival benefit (9-12,22). The reason for disparate outcome is unclear but patient selection in earlier trials may have contributed to better outcome. Furthermore acute and accumulative toxicity such as cardiotoxicity, limited adequate dosing of this compound as well. Absence of proven efficacy and toxicity explains why doxorubicin is not an approved HCC treatment and this is why it was not used for the control arm in the sorafenib trials.
Full table
To overcome the shortcomings of doxorubicin pegylated liposomal doxorubicin (PLD) was developed to improve anti-tumor activity and to reduce toxicity. PLD is a liposomal formulation of doxorubicin which reduces uptake by the reticulo-endothelial system. This formulation allows an extended circulation time and a reduces volume of distribution, thereby promoting tumor uptake with reduced cardiotoxicity (23). The efficacy of PLD was evaluated in several phase II trials in patients with advanced HCC. Although a favorable toxicity profile has been observed, the studies failed to show meaningful clinical outcome (24-27). The reduced uptake of PLD in organs including liver may be responsible for the lack of efficacy. Therefore PLD as a single agent has no significant activity against advanced HCC despite better toxicity profile.
5-fluorouracil (5-FU)
5-FU which is commonly been used in the treatment of colorectal cancer has been evaluated for advanced HCC as well. A phase II study of 5-FU combined with leucovorin showed modest activity with 28% response rates in advanced HCC (13). However, another phase II study of 5-FU with leucovorin showed poor clinical response with only 1 partial response (7%) (14).
Capecitabine which is an orally active form of fluoropyrimidine demonstrated no clinically significant influence on the pharmacokinetics in patients with mildly to moderately impaired hepatic function (28). Therefore, capecitabine has been evaluated in patients with advanced HCC which is generally associated with impaired hepatic function. In a phase II study, 59 treatment-naive patients and 31 sorafenib refractory patients with advanced HCC were treated with metronomic capecitabine (15). A median progression-free survival (PFS) and overall survival (OS) of previously untreated patients were 6.0 and 14.5 months with response rates of 5%, and those of sorafenib refractory patients were 3.3 and 9.8 months, respectively with no objective responses. Interestingly, metronomic capecitabine treatment prolonged OS of the treatment-naive patients cohort compared with matched historical controls (median OS: 15.6 vs. 8.0 months, P=0.043) in the trial. However, the survival benefit from capecitabine should be interpreted cautiously since the trial was not a randomized study.
Recently, a small randomized phase II study comparing sorafenib vs. capecitabine was conducted in patients with advanced HCC (16). The primary objective of this study was PFS. Unfortunately, capecitabine showed significant inferiority to sorafenib in terms of PFS (median PFS: 4 vs. 6 months, P<0.005) and OS (median OS: 5 vs. 7 months, P<0.016). Due to the lack of large randomized controlled studies, the antitumor activity of single agent 5-FU or capecitabine is unknown.
Gemcitabine
In contrast to other chemotherapeutic agents, gemcitabine is not subjected to the chemotherapy resistance mechanisms of HCC such as overexpression of dihydropyrimidine dehydrogenase, the P-glycoprotein or the MDR-1 protein (5-7). In several pre-clinical studies gemcitabine demonstrated strong antitumor effects on HCC (29-31). Therefore, based on pre-clinical rationale gemcitabine was evaluated in patients with advanced HCC. In a phase II study of gemcitabine in treatment-naive patients with advanced HCC, 5 patients (18%) had objective responses with mild toxicities (17). However, two subsequent phase II studies of gemcitabine failed to show meaningful clinical efficacy [0–5% overall response rate (ORR)] (18,19). The disparate outcomes may come from differences between Asian and Western patient population and the etiology of HCC.
Irinotecan
Irinotecan, a topoisomerase-1 inhibitor, has broad spectrum of antitumor activity in multiple malignancies. The active metabolite, SN-38 undergoes enterohepatic recirculation which leads to high local concentrations in the hepatobiliary tree (32). Based on these characteristics of irinotecan, irinotecan was evaluated in phase II studies in patients with advanced HCC. Unfortunately, antitumor activity of single agent irinotecan was not significant with objective response rates of only 0–7% (20,21).
Combination chemotherapy
Doxorubicin based combination chemotherapy
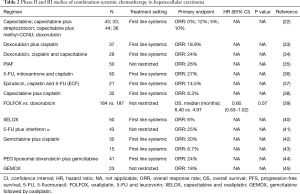
Several doxorubicin based combination regimens were evaluated in advanced HCC to enhance antitumor activity (Table 2). A phase II study of doxorubicin plus cisplatin resulted in marginal clinical benefit including the objective response rates of 18.9% (7/37), the median OS of 7.3 months and the median PFS of 6.6 months with tolerable adverse effects (33).
Full table
Adding capecitabine to doxorubicin and cisplatin also showed modest antitumor activity including 7 partial responses (24%) with the median PFS and median OS of 3.7 and 7.7 months, respectively (34).
While these doxorubicin based combination regimens showed only modest antitumor activity, the PIAF regimen, another doxorubicin based combination, demonstrated promising results. PIAF is an active and toxic combination chemotherapy regimen consisting of cisplatin, interferon α, doxorubicin and 5-FU. An initial phase II study of the PIAF regimen reported a relatively high objective response rate up to 26% (13/50) (35). Furthermore, 4 of 9 patients who underwent surgical resection after achieving partial response had complete pathological response with no viable tumor cells. The promising results from the phase II study led to a larger phase III study. Although the high response rate of the PIAF regimen was once again confirmed in a randomized phase III study comparing PIAF with doxorubicin (20.9% vs. 10.5%), there was no survival benefit (median OS: 8.7 vs. 6.8 months, P=0.83) with significant treatment-related grade 3/4 toxicities including neutropenia, thrombocytopenia and anemia (10). The failure to show a survival benefit despite the higher response rate might be explained by lack of patient selection in the study. The importance of patient selection for the PIAF regimen was confirmed by several retrospective studies. One retrospective study demonstrated that when the HCC patients with normal total bilirubin and non-cirrhotic livers were selected out, the ORRs were much higher (50.0% vs. 6.3%, P=0.004) compared with patients with abnormal bilirubin and liver cirrhosis (46). In another retrospective study from MD Anderson, the authors demonstrated that modified PIAF revealed higher objective response rate (36% vs. 15%, P=0.013), higher rate of resectability (33% vs. 10%, P=0.004) and longer median OS (21.3 vs. 10.6 months, P=0.002) than conventional high dose PIAF in patients with good performance status and without hepatitis or cirrhosis (47). Therefore, PIAF may be a reasonable option for patients with good performance status with normal liver function. However, toxicity of the regimen is a concern.
5-FU based combination chemotherapy
Due to the modest clinical efficacy of 5-FU or capecitabine as a single agent, 5-FU and capecitabine were combined with other chemotherapeutic agents including cisplatin and oxaliplatin. Two phase II studies of 5-FU, cisplatin and mitoxantrone reported objective response rates of 24–27% with median PFS of 2.5–4 months and median OS of 4.9–11.6 months (36,48). Other combination such as epirubicin, cisplatin and 5-FU (ECF) regimen or capecitabine and cisplatin have been evaluated as well. The combinations tend to have higher response rate but no definite conclusion can be drawn from these studies due to small number of patients (37).
Oxaliplatin, 5-FU and leucovorin (FOLFOX) and capecitabine and oxaliplatin (XELOX) which are commonly used in advanced colorectal cancer were evaluated in advanced HCC. In a randomized phase III study, FOLFOX showed improved ORR (8.2% vs. 2.7%, P=0.02) and improved PFS (median PFS: 2.9 vs. 1.8 months, P≤0.001) with a non-significant trend for increased OS (median OS: 6.4 vs. 5.0 months, P=0.07) compared with single agent doxorubicin (38). The most common grade 3 to 4 adverse events from FOLFOX were neutropenia (30.6%), leukocytopenia (8.7%), thrombocytopenia (7.7%) and anemia (4.9%) in the study. However, the interpretation of the trial is difficult as the trial was conducted in Asia where hepatitis B is the underlying causes of liver cirrhosis. Therefore, it is unclear if the results can be duplicated in the western hemisphere where hepatitis C is the number one cause of liver cirrhosis. A single arm phase II study of XELOX demonstrated better outcome with median PFS of 4.1 months and median OS of 9.3 months (39). However, this was not a randomized study thus limiting any definite conclusion.
5-FU was also evaluated in combination with interferon α which has direct antitumor activity by upregulation of MCH class I molecules on tumor cells, promotion of tumor cell apoptosis and antiangiogenic effects on tumor neovasculature (40) for treatment of advanced HCC. In a phase II study of 5-FU plus interferon α, 9 objective responses (25%) were observed with median OS of 19.5 months (49). However, another study failed to show any clinical efficacy of the combination of 5-FU and interferon α in heavily pretreated patients with advanced HCC (41).
Gemcitabine based combination chemotherapy
To enhance the antitumor activity, gemcitabine was combined with cisplatin. Gemcitabine plus cisplatin demonstrated modest clinical activity (25% objective response rates) with an acceptable toxicity profile in a retrospective study of 24 Indian patients with HCC (50). Similar with the retrospective study, a phase II study of gemcitabine plus cisplatin reported a partial response of 20% and a disease control rate of 63% (objective response plus stable disease) with median PFS of 18 weeks and median OS of 21 weeks in 30 patients with unresectable HCC (51). Observed grade 3 to 4 toxicities were anemia (44%), neutropenia (26%) and thrombocytopenia (14%) in the study. However, another phase II study with reduced dose of gemcitabine plus cisplatin showed poor clinical activity with only 1 partial response in 15 patients and extremely short PFS (median: 6 weeks) and OS (median: 18 weeks) (42). The reason for the poor outcome may be attributed to the fact that more than half of the patients had significant hepatic impairment (Child-Pugh B and C liver cirrhosis), and dose of gemcitabine and cisplatin was much lower than in the other study (42). Since patient selection plays a big role in the clinical outcome of the combination, gemcitabine plus cisplatin can be considered for selected patients who are not eligible for clinical trials and refractory to sorafenib with mild hepatic impairment.
Another gemcitabine based combination chemotherapy, gemcitabine plus PLD treatment induced 10 objective clinical responses (24%) including 3 complete responses with median PFS of 5.8 months and median OS of 22.5 months in a phase II study (43). The results are very promising especially with the significantly improved median OS. However, further randomized studies are needed to confirm the findings.
Gemcitabine followed by oxaliplatin (GEMOX) is an attractive option for patients with advanced HCC since GEMOX has the lack of renal and hepatotoxicity. Although GEMOX was well-tolerable with the most common grade 3/4 toxicities of thrombocytopenia (27%) and neutropenia (24%) in patients with advanced HCC, the clinical outcome was not impressive with ORR of 18% (n=6) in a phase II study (44). Interestingly, all the objective responses were observed in patients with nonalcoholic underlying liver disease (6/21) but not with alcoholic liver disease (0/13).
Hormonal therapy
Previously, expression of estrogen receptors and somatostatin receptors was reported in HCCs (45,52), suggesting potential role of estrogen and somatostatin in HCC. Therefore, hormonal therapy such as tamoxifen, megestrol, octreotide and lanreotide was extensively studied for the treatment of advanced HCC. Initial early phase studied demonstrated modest clinical activity of these agents. However, subsequent randomized trials failed to show antitumor activity of hormonal therapy (53-59). Based on in vitro data demonstrating tamoxifen can reverse multidrug resistance in human cancer cells (60), the combination of tamoxifen and chemotherapy such as doxorubicin was investigated in patients with advanced HCC which highly expresses multidrug resistance gene, MDR-1. However, tamoxifen plus doxorubicin was not superior to doxorubicin alone in overall clinical response or OS (61). Another phase II study of the combination of tamoxifen and doxorubicin showed 12 partial responses (33.3%), and the median OS of the responders was only 10 months (62).
Interestingly, recent data have revealed that estrogen exerts protective effects against HCC through IL-6 restriction, STAT3 inactivation and tumor associated macrophage inhibition (63), which may explain the lack of efficacy of hormonal therapy in advanced HCC. Currently, hormonal therapy is not recommended for advanced HCC.
Systemic therapy plus targeted therapy
Combination with sorafenib
Since sorafenib was approved for advanced HCC, multiple therapeutic agents targeting vascular endothelial growth factor (VEGF) and/or epidermal growth factor receptor (EGFR) pathway have been studied. Since studies with the targeted agents including sorafenib are covered in accompanying reviews, we will discuss the combination of chemotherapy with targeted therapy (Table 3). To enhance anticancer activity of sorafenib, the combination of sorafenib and doxorubicin was evaluated in several studies. Sorafenib plus doxorubicin is an attractive regimen since inhibition of Ras/Raf/MEK/ERK signaling pathway by sorafenib may suppress expression of MDR-1 (70). Therefore, the combination can increase area under the curve (AUC) of doxorubicin without worsening toxicities (71). In a double-blind phase II study, sorafenib plus doxorubicin resulted in prolonged PFS (median PFS: 6.0 vs. 2.7 months, P=0.006) and OS (median OS: 13.7 vs. 6.5 months, P=0.006) compared with doxorubicin monotherapy (72). Based on the promising results of the phase II study, Cancer and Leukemia Group B (CALGB) conducted a phase III trial randomizing sorafenib plus doxorubicin compared to sorafenib alone. However, the preliminary report presented in 2016 demonstrated that the combination of sorafenib and doxorubicin was associated with shorter OS (median OS: 9.3 vs. 10.5 months) and higher toxicities than single agent sorafenib (64).

Full table
Combination with bevacizumab
HCC is a hypervascular tumor, and neovascularization plays an important role in the growth and progression of HCC (65). Targeting angiogenesis in HCC has been studied, and bevacizumab, a monoclonal antibody against VEGF-A showed modest antitumor activity in advanced HCC (73). Bevacizumab in combination with chemotherapy such as GEMOX and XELOX were also evaluated in advanced HCC based on the fact that bevacizumab may enhance delivery and tumor uptake of drugs by alteration of tumor vasculature in tumors (74). A phase II study of gemcitabine, oxaliplatin and bevacizumab resulted in 6 objective responders (20%) with median PFS of 5.3 months and median OS of 9.6 months (75). Bevacizumab plus capecitabine as frontline treatment demonstrated modest antitumor activity (4 objective responses: 9%) with median PFS of 2.7 months and median OS of 5.9 months (66). When bevacizumab was combined with XELOX, median PFS and OS was 6.8 and 9.8 months, respectively with 8 partial responses (20%) (67).
Combination with cetuximab
EGFR is expressed on HCC cell lines, and the EGFR pathway has been reported as an essential player in the development of HCC (68). Several studies reported the modest clinical efficacy of erlotinib, an EGFR tyrosine kinase inhibitor in advanced HCC (76). An EGFR inhibitor (cetuximab) combined with chemotherapy was investigated in a phase II study (69). In the study, cetuximab plus GEMOX (gemcitabine and oxaliplatin) resulted in 20% confirmed response rates (9 patients) with median PFS of 4.7 and median OS of 9.5 months. The most common grade 3 to 4 toxicities were thrombocytopenia (24%), neutropenia (20%), cutaneous toxicity (16%) and neurotoxicity (11%). Large randomized trials are needed to confirm the efficacy of the combination regimen.
Conclusions
HCC is one of the increasing major health problems in both developing and developed countries. Unfortunately, only limited systemic treatment options are available for advanced HCC. Most of the trials using systemic chemotherapies were conducted in pre-sorafenib era and it has been difficult to interpret these studies due to small sample sizes, heterogeneous population and lack of randomization. Furthermore, most of the earlier studies did not stratify patients based on the severity of underlying cirrhosis or other factors, making comparison of study results difficult. Therefore, cytotoxic chemotherapy will play a minor role in the treatment of advanced HCC in the era of targeted therapy. Nevertheless, systemic chemotherapies may be considered in certain patients with good liver function test. Regimens such as PIAF can be used if aggressive therapy is desired with the high response rate and regimens including FOLFOX and gemcitabine plus cisplatin can be used in sorafenib refractory patients with good performance status if no clinical trials are available. Single agent such as capecitabine or 5-FU monotherapy can be considered for elderly and frail patients whose tumor is refractory to sorafenib. Finally, understanding the complex molecular biology of HCC with further studies and further evaluation of combination chemotherapy with other therapeutic agents such as molecular targeted therapy and immunotherapy may improve clinical outcome in this resilient tumor.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. R Kim receives honorarium from Lilly and Genentech. The other authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology 2015;61:184-90. [Crossref] [PubMed]
- Roxburgh P, Evans TR. Systemic therapy of hepatocellular carcinoma: are we making progress? Adv Ther 2008;25:1089-104. [Crossref] [PubMed]
- Kato A, Miyazaki M, Ambiru S, et al. Multidrug resistance gene (MDR-1) expression as a useful prognostic factor in patients with human hepatocellular carcinoma after surgical resection. J Surg Oncol 2001;78:110-5. [Crossref] [PubMed]
- Jiang W, Lu Z, He Y, et al. Dihydropyrimidine dehydrogenase activity in hepatocellular carcinoma: implication in 5-fluorouracil-based chemotherapy. Clin Cancer Res 1997;3:395-9. [PubMed]
- Soini Y, Virkajarvi N, Raunio H, et al. Expression of P-glycoprotein in hepatocellular carcinoma: a potential marker of prognosis. J Clin Pathol 1996;49:470-3. [Crossref] [PubMed]
- Olweny CL, Toya T, Katongole-Mbidde E, et al. Treatment of hepatocellular carcinoma with adriamycin. Preliminary communication. Cancer 1975;36:1250-7. [Crossref] [PubMed]
- Gish RG, Porta C, Lazar L, et al. Phase III randomized controlled trial comparing the survival of patients with unresectable hepatocellular carcinoma treated with nolatrexed or doxorubicin. J Clin Oncol 2007;25:3069-75. [Crossref] [PubMed]
- Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst 2005;97:1532-8. [Crossref] [PubMed]
- Choi TK, Lee NW, Wong J. Chemotherapy for advanced hepatocellular carcinoma. Adriamycin versus quadruple chemotherapy. Cancer 1984;53:401-5. [Crossref] [PubMed]
- Melia WM, Johnson PJ, Williams R. Induction of remission in hepatocellular carcinoma. A comparison of VP 16 with adriamycin. Cancer 1983;51:206-10. [Crossref] [PubMed]
- Porta C, Moroni M, Nastasi G, et al. 5-Fluorouracil and d,l-leucovorin calcium are active to treat unresectable hepatocellular carcinoma patients: preliminary results of a phase II study. Oncology 1995;52:487-91. [Crossref] [PubMed]
- Tetef M, Doroshow J, Akman S, et al. 5-Fluorouracil and high-dose calcium leucovorin for hepatocellular carcinoma: a phase II trial. Cancer Invest 1995;13:460-3. [Crossref] [PubMed]
- Brandi G, de Rosa F, Agostini V, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. Oncologist 2013;18:1256-7. [Crossref] [PubMed]
- Abdel-Rahman O, Abdel-Wahab M, Shaker M, et al. Sorafenib versus capecitabine in the management of advanced hepatocellular carcinoma. Med Oncol 2013;30:655. [Crossref] [PubMed]
- Yang TS, Lin YC, Chen JS, et al. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer 2000;89:750-6. [Crossref] [PubMed]
- Fuchs CS, Clark JW, Ryan DP, et al. A phase II trial of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer 2002;94:3186-91. [Crossref] [PubMed]
- Kubicka S, Rudolph KL, Tietze MK, et al. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology 2001;48:783-9. [PubMed]
- O'Reilly EM, Stuart KE, Sanz-Altamira PM, et al. A phase II study of irinotecan in patients with advanced hepatocellular carcinoma. Cancer 2001;91:101-5. [Crossref] [PubMed]
- Boige V, Taieb J, Hebbar M, et al. Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma: a multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur J Cancer 2006;42:456-9. [Crossref] [PubMed]
- Falkson G, Moertel CG, Lavin P, et al. Chemotherapy studies in primary liver cancer: a prospective randomized clinical trial. Cancer 1978;42:2149-56. [Crossref] [PubMed]
- Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet 2003;42:419-36. [Crossref] [PubMed]
- Valle JW, Dangoor A, Beech J, et al. Treatment of inoperable hepatocellular carcinoma with pegylated liposomal doxorubicin (PLD): results of a phase II study. Br J Cancer 2005;92:628-30. [Crossref] [PubMed]
- Halm U, Etzrodt G, Schiefke I, et al. A phase II study of pegylated liposomal doxorubicin for treatment of advanced hepatocellular carcinoma. Ann Oncol 2000;11:113-4. [Crossref] [PubMed]
- Hong RL, Tseng YL. A phase II and pharmacokinetic study of pegylated liposomal doxorubicin in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol 2003;51:433-8. [PubMed]
- Lind PA, Naucler G, Holm A, et al. Efficacy of pegylated liposomal doxorubicin in patients with advanced hepatocellular carcinoma. Acta Oncol 2007;46:230-3. [Crossref] [PubMed]
- Twelves C, Glynne-Jones R, Cassidy J, et al. Effect of hepatic dysfunction due to liver metastases on the pharmacokinetics of capecitabine and its metabolites. Clin Cancer Res 1999;5:1696-702. [PubMed]
- Graziadei I, Kelly T, Schirmer M, et al. Antitumor effect of the nucleoside analogs 2-chlorodeoxyadenosine and 2',2'-difluorodeoxycytidine on human hepatoma HepG2 cells. J Hepatol 1998;28:504-9. [Crossref] [PubMed]
- Haberkorn U, Bellemann ME, Brix G, et al. Apoptosis and changes in glucose transport early after treatment of Morris hepatoma with gemcitabine. Eur J Nucl Med 2001;28:418-25. [Crossref] [PubMed]
- Seong J, Kim SH, Suh CO. Enhancement of tumor radioresponse by combined chemotherapy in murine hepatocarcinoma. J Gastroenterol Hepatol 2001;16:883-9. [Crossref] [PubMed]
- Chabot GG, Robert J, Lokiec F, et al. Irinotecan pharmacokinetics. Bull Cancer 1998.11-20. [PubMed]
- Lee J, Park JO, Kim WS, et al. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother Pharmacol 2004;54:385-90. [Crossref] [PubMed]
- Park SH, Lee Y, Han SH, et al. Systemic chemotherapy with doxorubicin, cisplatin and capecitabine for metastatic hepatocellular carcinoma. BMC Cancer 2006;6:3. [Crossref] [PubMed]
- Leung TW, Patt YZ, Lau WY, et al. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin Cancer Res 1999;5:1676-81. [PubMed]
- Ikeda M, Okusaka T, Ueno H, et al. A phase II trial of continuous infusion of 5-fluorouracil, mitoxantrone, and cisplatin for metastatic hepatocellular carcinoma. Cancer 2005;103:756-62. [Crossref] [PubMed]
- Boucher E, Corbinais S, Brissot P, et al. Treatment of hepatocellular carcinoma (HCC) with systemic chemotherapy combining epirubicin, cisplatinum and infusional 5-fluorouracil (ECF regimen). Cancer Chemother Pharmacol 2002;50:305-8. [Crossref] [PubMed]
- Qin S, Bai Y, Lim HY, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol 2013;31:3501-8. [Crossref] [PubMed]
- Boige V, Raoul JL, Pignon JP, et al. Multicentre phase II trial of capecitabine plus oxaliplatin (XELOX) in patients with advanced hepatocellular carcinoma: FFCD 03-03 trial. Br J Cancer 2007;97:862-7. [PubMed]
- Floros T, Tarhini AA. Anticancer Cytokines: Biology and Clinical Effects of Interferon-alpha2, Interleukin (IL)-2, IL-15, IL-21, and IL-12. Semin Oncol 2015;42:539-48. [Crossref] [PubMed]
- Stuart K, Tessitore J, Huberman M. 5-Fluorouracil and alpha-interferon in hepatocellular carcinoma. Am J Clin Oncol 1996;19:136-9. [Crossref] [PubMed]
- Chia WK, Ong S, Toh HC, et al. Phase II trial of gemcitabine in combination with cisplatin in inoperable or advanced hepatocellular carcinoma. Ann Acad Med Singapore 2008;37:554-8. [PubMed]
- Lombardi G, Zustovich F, Farinati F, et al. Pegylated liposomal doxorubicin and gemcitabine in patients with advanced hepatocellular carcinoma: results of a phase 2 study. Cancer 2011;117:125-33. [Crossref] [PubMed]
- Louafi S, Boige V, Ducreux M, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer 2007;109:1384-90. [Crossref] [PubMed]
- De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol 2002;193:59-63. [Crossref] [PubMed]
- Leung TW, Tang AM, Zee B, et al. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer 2002;94:421-7. [Crossref] [PubMed]
- Kaseb AO, Shindoh J, Patt YZ, et al. Modified cisplatin/interferon alpha-2b/doxorubicin/5-fluorouracil (PIAF) chemotherapy in patients with no hepatitis or cirrhosis is associated with improved response rate, resectability, and survival of initially unresectable hepatocellular carcinoma. Cancer 2013;119:3334-42. [Crossref] [PubMed]
- Yang TS, Chang HK, Chen JS, et al. Chemotherapy using 5-fluorouracil, mitoxantrone, and cisplatin for patients with advanced hepatocellular carcinoma: an analysis of 63 cases. J Gastroenterol 2004;39:362-9. [Crossref] [PubMed]
- Patt YZ, Hassan MM, Lozano RD, et al. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol 2003;21:421-7. [Crossref] [PubMed]
- Pande SB, Doval DC, Pavithran K, et al. Gemcitabine and cisplatin-based combination chemotherapy in advanced hepatocellular carcinoma: An Indian experience. Indian J Med Paediatr Oncol 2012;33:42-7. [Crossref] [PubMed]
- Parikh PM, Fuloria J, Babu G, et al. A phase II study of gemcitabine and cisplatin in patients with advanced hepatocellular carcinoma. Trop Gastroenterol 2005;26:115-8. [PubMed]
- Bläker M, Schmitz M, Gocht A, et al. Differential expression of somatostatin receptor subtypes in hepatocellular carcinomas. J Hepatol 2004;41:112-8. [Crossref] [PubMed]
- Castells A, Bruix J, Bru C, et al. Treatment of hepatocellular carcinoma with tamoxifen: a double-blind placebo-controlled trial in 120 patients. Gastroenterology 1995;109:917-22. [Crossref] [PubMed]
- Tamoxifen in treatment of hepatocellular carcinoma: a randomised controlled trial. CLIP Group (Cancer of the Liver Italian Programme). Lancet 1998;352:17-20. [Crossref] [PubMed]
- Chow PK, Tai BC, Tan CK, et al. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: A multicenter randomized controlled trial. Hepatology 2002;36:1221-6. [Crossref] [PubMed]
- Barbare JC, Bouche O, Bonnetain F, et al. Randomized controlled trial of tamoxifen in advanced hepatocellular carcinoma. J Clin Oncol 2005;23:4338-46. [Crossref] [PubMed]
- Chow PK, Machin D, Chen Y, et al. Randomised double-blind trial of megestrol acetate vs placebo in treatment-naive advanced hepatocellular carcinoma. Br J Cancer 2011;105:945-52. [Crossref] [PubMed]
- Barbare JC, Bouche O, Bonnetain F, et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: a phase III multicentre, randomised, double blind placebo-controlled study. Eur J Cancer 2009;45:1788-97. [Crossref] [PubMed]
- Becker G, Allgaier HP, Olschewski M, et al. Long-acting octreotide versus placebo for treatment of advanced HCC: a randomized controlled double-blind study. Hepatology 2007;45:9-15. [Crossref] [PubMed]
- Lavie Y, Cao H, Volner A, et al. Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J Biol Chem 1997;272:1682-7. [Crossref] [PubMed]
- Melia WM, Johnson PJ, Williams R. Controlled clinical trial of doxorubicin and tamoxifen versus doxorubicin alone in hepatocellular carcinoma. Cancer Treat Rep 1987;71:1213-6. [PubMed]
- Cheng AL, Yeh KH, Fine RL, et al. Biochemical modulation of doxorubicin by high-dose tamoxifen in the treatment of advanced hepatocellular carcinoma. Hepatogastroenterology 1998;45:1955-60. [PubMed]
- Shi L, Feng Y, Lin H, et al. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med 2014;12:93. [Crossref] [PubMed]
- Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol 2016;34:abstr 192.
- Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett 2006;242:151-67. [Crossref] [PubMed]
- Hsu CH, Yang TS, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer 2010;102:981-6. [Crossref] [PubMed]
- Sun W, Sohal D, Haller DG, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011;117:3187-92. [Crossref] [PubMed]
- Berasain C, Avila MA. The EGFR signalling system in the liver: from hepatoprotection to hepatocarcinogenesis. J Gastroenterol 2014;49:9-23. [Crossref] [PubMed]
- Asnacios A, Fartoux L, Romano O, et al. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer 2008;112:2733-9. [Crossref] [PubMed]
- McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul 2006;46:249-79. [Crossref] [PubMed]
- Richly H, Henning BF, Kupsch P, et al. Results of a Phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol 2006;17:866-73. [Crossref] [PubMed]
- Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA 2010;304:2154-60. [Crossref] [PubMed]
- Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol 2008;26:2992-8. [Crossref] [PubMed]
- Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 2001;7:987-9. [Crossref] [PubMed]
- Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:1898-903. [Crossref] [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol 2005;23:6657-63. [Crossref] [PubMed]


