
Expression and clinical significance of ENC1 in gastrointestinal tumors: Bioinformatics analysis based on a public gene database
Highlight box
Key findings
• ENC1 expression is elevated in both gastric and colon cancers.
• ENC1 is not a key gene that affects the survival and prognosis of patients.
What is known and what is new?
• ENC1 expression was found to be significantly higher in several cancer tissues.
• ENC1 is not a key gene for gastrointestinal tumors.
What is the implication, and what should change now?
• Although ENC1 may be a gene that is involved with the development of gastrointestinal tumors, it does not affect the prognosis of patients.
• Other key genes should be further studied for targeted therapy.
Introduction
Gastrointestinal tumors include tumors arising in the stomach, small intestine, colon, and rectum, among which gastric cancer and colon cancer are the most common (1). Gastric carcinoma (GC) is a malignant tumor originating from the gastric mucosal epithelium, which can occur anywhere in the stomach and then spread to other parts of the body, and more than 60% of gastric cancers are caused by Helicobacter pylori infection (2). At present, the mortality rate of gastric cancer is extremely high, ranking third in cancer in the world and difficult to cure, but the 5-year survival probability of patients with early gastric cancer is as high as more than 90%, so early detection and treatment are the key. Colon adenocarcinoma (COAD) is a primary malignant tumor of the colonic epithelium with no obvious early symptoms. With the progression of the tumor, patients gradually exhibit abdominal pain, hematochezia, changes in stool characteristics, weight loss, and other symptoms. At present, comprehensive treatment regimens based on surgery for colon cancer have the best efficacy, but their therapeutic effect is still poor for patients with advanced or advanced disease (3,4). The symptoms of patients with early gastrointestinal tumors are insidious, and most patients have already reached the later stages by the time of diagnosis. Therefore, it is important to reveal the key molecular mechanism of gastrointestinal tumorigenesis for early diagnosis, prognosis, and precise treatment. Ectodermal-neural cortex 1 (ENC1) is a nervous system-specific expressed gene belonging to the KELCH family of genes, which was first discovered in mammals in 1997 by Hernandez et al. (5). This gene is involved in and encodes a binding protein for actin and plays an important role throughout nervous system development (6). In recent years, with the deepening of genetics in cancer research, it has been found that ENC1 may play a promoting role in the development of tumors. It has been shown that the expression of ENC1 is significantly increased in breast cancer compared with normal breast tissue, and is significantly associated with the proliferation, migration, and invasion of breast cancer cells (7). A study on cervical cancer patients showed that the higher the ENC1 expression level in cancer tissues, the worse the prognosis (8). Based on the few studies on the effect of ENC1 on gastrointestinal tumors, this study aimed to investigate the expression, clinical significance, and prognosis of ENC1 in gastrointestinal tumors by analyzing the data in The Cancer Genome Atlas (TCGA), providing a new target for the precise treatment of gastrointestinal tumors. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-217/rc).
Methods
Data acquisition
Data acquisition was logged on Xena website (https://xenabrowser.net/datapages/) and RNA sequence (RNA-seq) clinical expression profile data were obtained from stomach adenocarcinoma (STAD) and COAD samples source from the TCGA database (https://portal.gdc.cancer.gov/). The count data, fragments per kilobase million (FPKM) data, and survival data were downloaded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Software platform and analysis tools
The software platform and analysis tools were programmed in R language version 4.2.2 (The R Foundation for Statistical Computing Platform, Vienna, Austria) for data processing and figure presentation, the compilation tool used was Rtools42, and the integrated development environment was RStudio-2022.12.0-353.
Gene differential expression analysis
R language was used to filter out the messenger RNA (mRNA) genes in the dataset, and the matrix was divided into 2 groups: “Tumor” and “Normal” according to the last 3 digits of “01A” and “11A” listed in the organization of the matrix. The “DESeq2” module of BiocManager software package was used to perform differential analysis on the 2 groups of gene matrices. The screening threshold was |log2 fold change (FC)| >1, false discovery rate (FDR) <0.001. Eligible patients were those understood to possess a significantly differentially expressed gene (DEG), and the line named “ENC1” in the search results was used to obtain the up-regulation information of this gene. ENC1 expression in the “Tumor” and “Normal” groups was extracted, and group comparison plots were used to describe the information of the 2 groups.
Prognostic survival analysis
We obtained the survival data of STAD and COAD Phenotype from the same Xena website, collated the data using R language, and performed Cox regression analysis using the “survival” package, and the difference was considered significant at P<0.05. The hazard ratio (HR) information of ENC1 for overall survival (OS) could be found in the results. These samples were divided into a high expression group and a low expression group according to the median ENC1 expression value. The survival rate of patients in the high and low expression groups was compared. A Kaplan-Meier (KM) survival curve was drawn using the R survival package and log rank test was performed for the curve. P<0.05 suggested that the ENC1 expression level had a statistically significant difference in the prognosis and survival between the 2 groups.
Gene function enrichment analysis
We used the “DESeq2” model again to perform the difference analysis between the high expression group and low expression group divided by gene ENC1 and obtained the DEGs. Gene function enrichment analysis was performed by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the dataset through the “clusterprofiler” package based on these DEGs, and the results with P<0.05 were screened as significant enrichment results.
Correlation between gene expression and tumor immune infiltration
The FPKM datasets of STAD and COAD were divided into high and low expression groups according to the median expression of ENC1, and immune cell calculation and analysis were performed using the “xCell” package and plotted using “ggpubr”.
Construction of protein-protein interaction (PPI) network interacting with ENC1
A PPI network was constructed by predicting proteins interacting with ENC1 using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database. We entered “ENC1” in the database search page “Protein by name” option, selected “Homo sapiens” in the “organism” option, and selected the “Medium confidence (0.400)” option in the option of “minimum required interaction score” in the result set to obtain the analysis results and a PPI network was constructed.
Statistical analysis
The difference of ENC1 gene expression between gastric cancer, colon cancer, and normal tissues was analyzed by t-test. The relationship between ENC1 expression and the prognosis of gastric cancer and colon cancer was analyzed by Log rank test with test parameter P=0.05, and all tests were statistically analyzed by SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Results
Data acquisition results
A total of 405 samples were collected from the STAD data chip set in the TCGA database, including 373 tumor tissue samples and 32 normal tissue samples; a total of 494 samples were collected from the COAD data chip set, including 453 tumor tissue samples and 41 normal tissue samples.
Differential gene expression analysis and display between tumor tissues and normal tissues
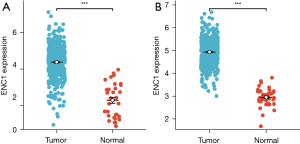
Through data mining and analysis of the dataset, it was found that the expression of ENC1 in tumor tissues of both cancer patients was significantly higher than that in normal tissues, and its Log2FC values were 1.97 and 2.06, respectively (as shown in Table 1), as represented by punctate plots in Figure 1.
Table 1
| Cancer type | Log2FC | P adj. | Change |
|---|---|---|---|
| STAD | 1.97 | 9.08e-31 | Up |
| COAD | 2.06 | 1.10e-109 | Up |
STAD, stomach adenocarcinoma; COAD, colon adenocarcinoma; FC, fold change.
Prognostic survival analysis
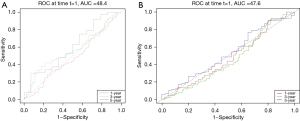
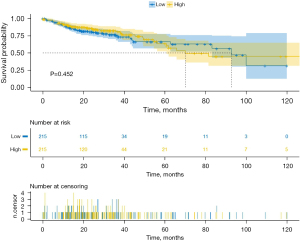
Cox regression analysis of the survival data of STAD and COAD using the R language’s survival package revealed that high expression of ENC1 was not significantly associated with the prognosis and survival time of gastric cancer patients and colon cancer patients: OS HR: 1.039, 95% confidence interval (CI): 0.890–1.213, P=0.627 for gastric cancer; OS HR: 0.886, 95% CI: 0.702–1.111, P=0.306 for colon cancer, and its 1-, 3-, and 5-year survival receiver operating characteristic (ROC) curves are shown in Figure 2 and timeROC curves are shown in Figure 3.
Functional enrichment analysis
For gene ENC1, KEGG pathway enrichment analysis showed that ENC1 was mainly involved in neuroactive ligand-receptor interaction, as shown in Figure 4.

Correlation with tumor immunity
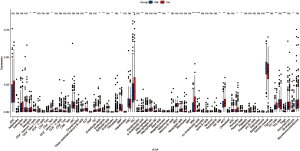
Through excavation analysis of tumor infiltration in the dataset, it was found that high expression of ENC1 was significantly associated with each immune cell and different T cells such as basophils, CD4+ memory T cells, CD4+ TEM, and MV endothelial cells in gastric and colon cancers, as shown in Figure 5.
PPI network interacting with ENC1
A search of the STRING database to investigate the ENC1 PPI network identified a total of 10 proteins interacting with ENC1, comprising KLHL20, KLHL25, SPOPL, LZTR1, SPOP, KLHL42, KEAP1, CUL3, RBX1, and TNFAIP1, as shown in Figure 6, suggesting that ENC1 may be involved in regulating neurite formation and neural crest cell differentiation.
Discussion
Gastric cancer and colon cancer are common tumors worldwide, but because their early symptoms are not obvious, most patients have reached an advanced stage by the time of diagnosis, resulting in poor prognosis and a serious threat to the health of patients (9,10). In recent years, the mechanism of various genes in the process of cancer development has been continuously revealed, which provides a solid theoretical basis for the treatment and diagnosis of gastric cancer and colon cancer, but no specific gene has been found to be used as an early diagnostic criterion or prognostic marker for these gastrointestinal malignant tumors.
Named for its expression pattern in the ectoderm and neurocortex, ENC1 is expressed in the expected neuroectodermal region of the ectoderm during early embryogenesis and continues to be expressed throughout the development of the nervous system (11). Analysis of ENC1 showed that it is a homolog of KELCH, an eosinophil gene essential for oogenesis, and ENC1, a BTB-Kelch protein belonging to the same family as Keap1, is mainly involved in the differentiation process of human central nervous cells and malignant transformation of cells and is abnormally expressed in a variety of brain tumor tissues including gliomas and astrocytomas (12). ENC1 is the only member of this family that is expressed in the nervous system and encodes a cytoplasmic protein that interacts with the actin cytoskeleton (13,14). ENC1 is overexpressed in a variety of malignancies and is thought to play an important regulatory role in the malignant transformation of a variety of tumors. It has also been found that abnormal expression of ENC1, including gene mutation, overexpression, and localization errors, can promote cell proliferation and inhibit apoptosis, thus promoting the occurrence of brain tumors. Fan et al. found that down-regulation of ENC1 expression significantly inhibited the proliferation, migration, and invasion of ovarian cancer cells, whereas low expression of ENC1 was associated with a good prognosis in ovarian cancer patients (15). Therefore, it is speculated that ENC1 is a crucial oncogene in tumor development.
In this study, we analyzed the expression of ENC1 in gastric cancer tissues and colon cancer tissues by data mining of a large sample composed of multiple datasets in TCGA, and the results showed that the expression of ENC1 was higher than that in normal tissues in both STAD and COAD (Log2FC =1.97 and 2.06, respectively). However, survival analysis showed that high and low expression of ENC1 did not show a significant effect on the survival and prognosis of patients, and KM curves also showed that there was no significant difference between the 2 cancers, indicating that for gastrointestinal tumors, although ENC1 expression in tumor tissues was higher than that in normal tissues, ENC1 did not have a significant effect on prognosis. It is speculated that ENC1 is mainly involved in the differentiation and malignant transformation of nerve cells in human heat stroke, and plays an obvious role in the proliferation and regulation of craniocerebral tumors, but ENC1 does not play a major role in gastrointestinal tumors. As can also be seen from the KEGG functional enrichment of ENC1, ENC1 has a huge impact on the neuroactive ligand-receptor interaction, but remains too small for other pathways. The PPI network of ENC1 also showed that this gene was mainly involved in regulating neurite formation and neural crest cell differentiation, which echoed the aforementioned conclusions. However, although there was no significant correlation between ENC1 and the prognosis of gastrointestinal cancer, in immune infiltration analysis, we still found that high and low ENC1 expression showed significant differences at the level of some immune cells and T cells, suggesting that ENC1 can predict some immune infiltration levels. In summary, ENC1 does not become a key prognostic oncogene in the development of gastrointestinal tumors, nor a gene that significantly affects cancer cell proliferation, migration, and invasion.
In this study, only STAD and COAD datasets were mined, and no other types of gastrointestinal tumors were involved. In the study by Cui et al., ENC1 promoted the occurrence and metastasis of colorectal cancer through epithelial-mesenchymal transition mediated by the JAK2/STAT5/AKT axis, indicating that ENC1 may become a reasonable diagnostic marker and targeted therapy point for colorectal cancer (16). In another study on a mouse lung cancer model, reduced ENC1 levels inhibited lung tumor growth, suggesting that ENC1 is involved in proliferation, migration, and invasion of lung cancer cells and may therefore be an effective diagnostic target for lung cancer (17). A study by Zhang et al. also showed that ENC1 played an important role in the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway to promote cervical cancer (18). Evidence has shown that ENC1 also was one of the key genes involved in the pathogenesis of Alzheimer's disease (19). He et al. found that upregulated ENC1 predicts unfavorable prognosis and correlates with immune infiltration in endometrial cancer (8). Further investigation of ENC1 is warranted.
In this study, we mined and analyzed the STAD and COAD datasets in TCGA and found that ENC1 expression was increased in gastrointestinal tumors, but ENC1 did not affect the survival and prognosis of patients, and speculated that ENC1 was not a key gene affecting the occurrence and progression of gastrointestinal tumors. There are still some shortcomings in this study: (I) we only selected 2 datasets, STAD and COAD, and did not analyze the data of other types of gastrointestinal tumors (such as rectal cancer and small intestinal cancer), which may have biased the conclusions; (II) TCGA data have some shortcomings because they are provided by various countries or laboratories, the data of some tumors have not been updated recently, and due to sequencing technology or sequencing quality, RNA-seq data of TCGA do not fully represent the expression of tumor genes; (III) there is no significant demarcation line between the high and low expression of ENC1, and we used the median as the demarcation line, which may have caused data bias. Therefore, the conclusions reached in this study still need further experimental validation.
Conclusions
ENC1 expression is elevated in both gastric and colon cancers, and ENC1 is associated with various immune cells and different T cells such as basophils, CD4+ memory T cells, CD4+ TEM, and MV endothelial cells in both gastric and colon cancers; however, ENC1 does not affect the survival and prognosis of patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-217/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-217/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-217/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gonzalez RS. Diagnosis and Management of Gastrointestinal Neuroendocrine Neoplasms. Surg Pathol Clin 2020;13:377-97. [Crossref] [PubMed]
- Wu D, Zhang P, Ma J, et al. Serum biomarker panels for the diagnosis of gastric cancer. Cancer Med 2019;8:1576-83. [Crossref] [PubMed]
- Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am 2008;37:1-24. v. [Crossref] [PubMed]
- Chen S, Shen X. Long noncoding RNAs: functions and mechanisms in colon cancer. Mol Cancer 2020;19:167. [Crossref] [PubMed]
- Hernandez MC, Andres-Barquin PJ, Martinez S, et al. ENC-1: a novel mammalian kelch-related gene specifically expressed in the nervous system encodes an actin-binding protein. J Neurosci 1997;17:3038-51. [Crossref] [PubMed]
- Worton LE, Shi YC, Smith EJ, et al. Ectodermal-Neural Cortex 1 Isoforms Have Contrasting Effects on MC3T3-E1 Osteoblast Mineralization and Gene Expression. J Cell Biochem 2017;118:2141-50. [Crossref] [PubMed]
- Zhou Y, Tang X, Niu L, et al. Ectodermal-neural cortex 1 as a novel biomarker predicts poor prognosis and induces metastasis in breast cancer by promoting Wnt/β-catenin pathway. J Cell Mol Med 2020;24:8826-35. [Crossref] [PubMed]
- He L, He W, Luo J, et al. Upregulated ENC1 predicts unfavorable prognosis and correlates with immune infiltration in endometrial cancer. Front Cell Dev Biol 2022;10:919637. [Crossref] [PubMed]
- Zhao Y, Li J, Li D, et al. Tumor biology and multidisciplinary strategies of oligometastasis in gastrointestinal cancers. Semin Cancer Biol 2020;60:334-43. [Crossref] [PubMed]
- Bacigalupo ML, Carabias P, Troncoso MF. Contribution of galectin-1, a glycan-binding protein, to gastrointestinal tumor progression. World J Gastroenterol 2017;23:5266-81. [Crossref] [PubMed]
- Fujita M, Furukawa Y, Tsunoda T, et al. Up-regulation of the ectodermal-neural cortex 1 (ENC1) gene, a downstream target of the beta-catenin/T-cell factor complex, in colorectal carcinomas. Cancer Res 2001;61:7722-6. [PubMed]
- Feng J, Hong L, Wu Y, et al. Identification of a subtype-specific ENC1 gene related to invasiveness in human pituitary null cell adenoma and oncocytomas. J Neurooncol 2014;119:307-15. [Crossref] [PubMed]
- Wang XJ, Zhang DD. Ectodermal-neural cortex 1 down-regulates Nrf2 at the translational level. PLoS One 2009;4:e5492. [Crossref] [PubMed]
- Lei H, Li J, Zhao Z, et al. Inhibition of Ectodermal-Neural Cortex 1 Protects Neural Cells from Apoptosis Induced by Hypoxia and Hypoglycemia. J Mol Neurosci 2016;59:126-34. [Crossref] [PubMed]
- Fan S, Wang Y, Sheng N, et al. Low expression of ENC1 predicts a favorable prognosis in patients with ovarian cancer. J Cell Biochem 2019;120:861-71. [Crossref] [PubMed]
- Cui Y, Yang J, Bai Y, et al. ENC1 Facilitates Colorectal Carcinoma Tumorigenesis and Metastasis via JAK2/STAT5/AKT Axis-Mediated Epithelial Mesenchymal Transition and Stemness. Front Cell Dev Biol 2021;9:616887. [Crossref] [PubMed]
- Wu C, Wang X, Wu X, et al. Ectodermal-neural cortex 1 affects the biological function of lung cancer through the MAPK pathway. Int J Mol Med 2021;47:79. [Crossref] [PubMed]
- Zhang P, Zhao F, Jia K, et al. The LOXL1 antisense RNA 1 (LOXL1-AS1)/microRNA-423-5p (miR-423-5p)/ectodermal-neural cortex 1 (ENC1) axis promotes cervical cancer through the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway. Bioengineered 2022;13:2567-84. [Crossref] [PubMed]
- Gns HS, Rajalekshmi SG, Burri RR. Revelation of Pivotal Genes Pertinent to Alzheimer's Pathogenesis: A Methodical Evaluation of 32 GEO Datasets. J Mol Neurosci 2022;72:303-22. [Crossref] [PubMed]
(English Language Editor: J. Jones)



