
The Frailty Index and colon cancer: a 2-sample Mendelian-randomization study
Highlight box
Key findings
• Frailty might have no effect on the risk of cancer.
What is known and what is new?
• Frailty is characterized by a decline in multiple physiological functions, is often closely related to aging, and often coexists with disabilities and chronic diseases.
• Genetic changes in the Frailty Index were associated with the risk of colon cancer, but no significant heterogeneity between the genes was observed. In this 2-sample Mendelian-randomization study, no statistical relationship was found between frailty and the risk of cancer.
What is the implication, and what should change now?
• Frailty might have no direct relationship with the occurrence of cancer.
Introduction
Frailty is a state of extreme vulnerability to stressors that leads to adverse health outcomes (1,2). Frailty is characterized by a decline in multiple physiological functions, is often closely related to aging, and often coexists with disabilities and chronic diseases (3). Generally, it is recommended that individuals aged over 70 years and those who have lost >5% of their weight due to chronic diseases undergo a frailty assessment (4). In clinical practice or research, many methods are used to evaluate frailty, including the FRAIL Scale, the Vulnerability Elders Survey-13, the phenotypic framework, the Frailty Index, the Modified Frailty Index, and the Comprehensive Genetic Assessment.
In the current study, we examined the relationship between fragility, age, chronic disease, and tumors. A previous systematic evaluation integrated the data of 61,500 elderly people living in high-income countries, and reported that the total frailty rate was 11%, but the rates differed greatly among different studies (4–59%) (5). Among long-term care residents, this rate has been reported to be as high as 53% (6). About 37–46% of patients with end-stage renal disease (7,8). The results of a recent systematic evaluation and meta-analysis showed that among elderly hospitalized patients, the framework pooled validity and pre-feasibility rates were 47.4% [95% confidence interval (CI): 43.7–51.1%], and 25.8% (95% CI: 22.0–29.6%), respectively (9). Among patients with malignant tumors, the median rate was 42% (10). In a systematic evaluation of patients who were undergoing colorectal cancer (CRC) surgery, the overall frailty rate was 20%, and the included studies’ frailty rate ranged from 12–56% (11). At present, it is believed that frailty increases the risk of adverse events, including falls, hospitalization and even death, in the elderly, chronic disease patients, and cancer patients, and also increases the medical costs of inpatients (12).
The occurrence and development mechanism of cancer is complex. Cancer is caused by abnormal cell function and proliferation, which are mainly due to the continuous accumulation of chromosome and molecular abnormalities, which ultimately lead to gene changes. At present, it is believed that many risk factors, including environmental factors, endogenous factors, and exogenous factors, cause cancer (13).
Frailty is an abnormal state of the body caused by a variety of factors, including social factors, clinical factors, lifestyle factors, and biological factors (14,15), and is common in the elderly. In previous studies, especially those related to cancer, frailty is regarded as the co-existence or result of cancer. However, it is not yet known whether frailty is a predictive factor of cancer, especially in the elderly. In clinical practice, frail patients often have a short survival time (16,17). Prospective cohort studies need to be conducted to observe the relationship between different risk factors and cancer, but such studies often require a long follow-up time, which is difficult with frail patients.
Mendelian randomization (MR) uses genetic variations as instrumental variables (IVs) to investigate whether the observed correlations between risk factors and outcomes are consistent with the causal effects (18). The present study used a 2-sample MR approach to conduct a preliminary analysis of the relationship between frailty and the risk of colon cancer. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-134/rc).
Methods
Data sources
In this study, we conducted a MR analysis to identify the single-nucleotide polymorphisms (SNPs) associated with frailty, which were defined as the IVs. The database was derived from the Medical Research Council Integrative Epidemiology Unit (MRC-IEU) in 2021. The genome-wide association study (GWAS) data related to CRC was obtained from the GWAS website (http://gwas.mrcieu.ac.uk/datasets). The data set comprised the data of 462,933 individuals. As this study was based on open-access data, there was no need to acquire approval from the Ethics Committee affiliated with the authors. All the participants had previously provided their informed consent to the database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study design
In this study, the strictly selected SNPs were defined as the IVs. To avoid bias due to a linkage disequilibrium (LD) relationship in the analysis, the LD of the SNPs that was closely associated with the Frailty Index had to have an r2 value <0.001 and the minimum allele frequency had to be >0.01 (19). The confounding factors were selected using the PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk/phenoscanner). The parameters, including the effect allele (EA), non-effect allele (NEA), effect allele frequency (EAF), effect size (ES or β), standard error (SE), and P value, were extracted. F values >10 indicated no weak tool bias (20), and the following formula was used to calculate the F statistic: F statistic = R2(N–2)/(1–R2). R2 = 2 × EAF × (1-EAF) × β2.
Statistical analysis
We first harmonized the exposure and outcome data sets, and the retention EA was always associated with the same allele. We used different methods to obtain the MR estimates based on different validity assumptions. Additionally, to estimate the causal effect of the Frailty Index on cancer, the MR-Egger regression, weighted median (WM1), inverse-variance weighted (IVW) and weight mode (WM2) methods were used to calculate the SNP-Frailty Index and SNP-cancer estimates. IVW was applied on the premise that all the SNPs were valid IVs (21). WM1 and WM2 were used to obtain estimates of the causal effect when at least half of the SNPs were intravenously effective. We conducted a leave-one-out sensitivity analysis to evaluate the stability of the ES through IVW (each SNP was removed and its effect on the other SNPs was determined in turn). Heterogeneity was evaluated by Cochran’s Q statistic. R software (version 4.2.1) was used to conduct the statistical analysis. A two-sample Mendelian Randomization (TSMR) analysis was conducted using the “TwoSampleMR” and “plyr” packages in R software. A 2-tailed P value <0.05 was considered statistically significant.
Results
IV selection
A total of 12 SNPs were included in the analysis, but 4 SNPs (i.e., rs10891490, rs2071207, rs583514, and rs9275160) were excluded due to confounding factors. In the final analysis, 8 SNPs were selected as the IVs (Table 1). In relation to the strength of the selected single IVs, their F values ranged from 29.592 to 58.953. The F values were all >10, which indicated that our IVs were not biased by weak instrument (Table 1).
Table 1
| SNP | Chr | Position | EA | NEA | EAF | Association with the Frailty Index | Association with colon cancer | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta | SE | P value | F statistica | Beta | SE | P value | |||||||
| rs1363103 | 5 | 103917837 | C | T | 0.380 | –0.019 | 0.003 | 2.23E-08 | 29.811 | –1.28E-04 | 1.22E-04 | 0.290 | |
| rs17612102 | 15 | 52264094 | C | T | 0.593 | 0.019 | 0.003 | 2.85E-08 | 30.539 | –4.82E-05 | 1.20E-04 | 0.690 | |
| rs2396766 | 7 | 114318071 | A | G | 0.473 | 0.020 | 0.003 | 1.22E-09 | 34.950 | –1.14E-04 | 1.18E-04 | 0.330 | |
| rs374943348 | 6 | 32619856 | A | T | 0.361 | 0.027 | 0.004 | 3.23E-12 | 58.953 | –1.75E-04 | 1.39E-04 | 0.210 | |
| rs3959554 | 15 | 41443924 | G | A | 0.418 | 0.019 | 0.003 | 1.74E-08 | 30.783 | 3.94E-05 | 1.20E-04 | 0.740 | |
| rs4146140 | 10 | 61885362 | T | C | 0.381 | –0.020 | 0.003 | 6.83E-09 | 33.066 | 3.19E-04 | 1.22E-04 | 0.009 | |
| rs4952693 | 2 | 44151808 | T | C | 0.373 | –0.019 | 0.003 | 1.47E-08 | 29.592 | 2.61E-04 | 1.22E-04 | 0.033 | |
| rs82334 | 4 | 3225371 | C | A | 0.318 | –0.022 | 0.004 | 3.13E-10 | 36.794 | 5.79E-05 | 1.27E-04 | 0.650 | |
a, F statistic values were calculated using the following formula: R2(N–2)/(1–R2), where R2 were calculated using the following formula: 2×EAF×(1-EAF)×beta2, where EAF is the effect allele frequency, beta is the estimated effect on Frailty index and N is the sample size of the GWAS for the SNP-Frailty index association. SNP, single-nucleotide polymorphism; IVs, instrumental variables; Chr, chromosome; EA, effect allele; NEA, non-effect allele; EAF, effect allele frequency; SE, standard error.
TSMR analysis
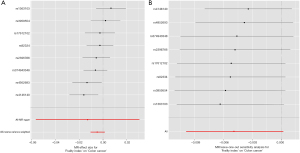
The IVW results [odds ratio (OR) =0.995, 95% CI: 0.990–1.001, P=0.052] showed that the genetic changes in the Frailty Index were not statistically associated with the risk of colon cancer, and no significant heterogeneity between the genes was observed (Q=7.382, P=0.184, Figure 1). The MR-Egger (OR =0.987, 95% CI: 0.945–1.031, P=0.581), WM1 (OR =0.995, 95% CI: 0.990–1.001, P=0.118), WM2 (OR =0.996, 95% CI: 0.988–1.004, P=0.356) and SM (OR =0.996, 95% CI: 0.987–1.005, P=0.449) results were also consistent with each other (Table 2). The effect values estimated using the TSMR method and the 95% CIs are shown in a forest map (Figure 2A). The MR-Egger regression results showed that genetic pleiotropy did not bias the results (P=0.231). The sensitivity analysis based on the leave-one-out method showed the individual SNPs had no effect on the robustness of the results (Figure 2B). The MR-PRESSO results showed that there were no outliers in the IVs, and once again proved that there was no horizontal pleiotropy (Table 1).
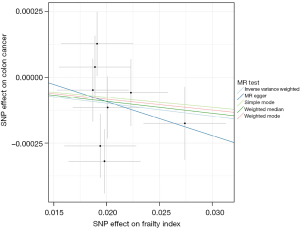
Table 2
| Exposure/outcome | Methods | NSNP | OR (95% CI) | P value | Heterogeneity test | Intercept term | Global test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | P value | Intercept | SE | P value | RSSobs | P value | |||||||
| Frailty Index/Colon cancer | MR Egger | 8 | 0.987 (0.945, 1.031) | 0.581 | 9.858 | 0.131 | 1.67E-04 | 4.59E-04 | 0.728 | 12.838 | 0.212 | ||
| Weighted median | 8 | 0.995 (0.990, 1.001) | 0.118 | ||||||||||
| IVW | 8 | 0.995 (0.990, 1.001) | 0.052 | 7.382 | 0.184 | ||||||||
| Simple mode | 8 | 0.996 (0.987, 1.005) | 0.449 | ||||||||||
| Weighted mode | 8 | 0.996 (0.988, 1.004) | 0.356 | ||||||||||
MR, Mendelian-randomization; IVW, inverse-variance weighted; NSNP, number of single-nucleotide polymorphisms; OR, odds ratio; CI, confidence interval; SE, standard error; RSSobs, Residual Sum of Squares observed.
Discussion
Research needs to be conducted to determine if the risk of cancer is increased in frail individuals, especially in the elderly and those with chronic diseases. At present, very little research has been conducted on this issue. This preliminary study analyzed the relationship between frailty and the risk of colon cancer through a TSMR, and found that the genetic changes in the Frailty Index were not statistically associated with the risk of colon cancer.
Previous studies have shown that age is closely related to cancer (22,23). As age increases, the adverse effects of risk factors continue to accumulate in the human body, resulting in the accumulation of gene mutations (24). Some previous studies have shown that chronic diseases increase the risk of cancer. A prospective cohort study of 405,878 participants found that some disease markers (e.g., blood pressure, proteinuria, and the glomerular filtration rate) corelated significantly with the risk of cancer death and that chronic disease risk scores (for 8 chronic diseases and markers) had a positive relationship with the risk of cancer (25). Diabetes is a common chronic disease. Many previous studies have investigated the risk of cancer in patients with diabetes and shown that diabetes increases the risk of many kinds of cancer, including liver cancer (26), pancreatic cancer (27), endometrial cancer (28), breast cancer (29), colon cancer (30), kidney cancer (31), and bladder cancer (32). Diabetes is closely related to frailty. Research has shown that the rate of diabetes is higher among patients with frailty, especially elderly patients (33). Frailty is also a common phenomenon in patients with diabetes (34).
Hypertension is also a common chronic disease. A systematic evaluation showed that hypertension increased the risk of breast cancer in postmenopausal women, but was not associated with the risk of breast cancer in premenopausal women (35). The prognosis of cancer patients with hypertension is also worse when compared with patients without hypertension (36). A prospective study carried out in Europe of 307,318 patients with hypertension with an average follow-up time of 13.7 years reported that hypertension increases the risk of esophageal squamous cell carcinoma, head and neck cancers, skin squamous cell carcinoma, colon cancer, postmenopausal breast cancer and uterine adenocarcinoma, but has no effect on esophageal adenocarcinoma, lung squamous cell carcinoma, lung adenocarcinoma, or uterine endometroid cancer (36). However, research has shown that hypertension might decrease the risk of cervical squamous cell carcinoma and lymphomas (37). Similarly, there is a close relationship between hypertension and frailty. A systematic evaluation reported that about 72% (95% CI: 66–79%) of frail individuals suffered from hypertension and about 14% (95% CI: 12–17%) of individuals with hypertension were frail (38).
Chronic obstructive pulmonary disease (COPD) is a common local chronic disease, which has been shown to increase the risk of lung cancer. A systematic evaluation of 11 studies showed that both COPD patients and emphysema patients had high risk of developing lung cancer, and this risk was higher for heavy smokers (39). COPD may also increase the risk of cancer in other parts of the body. A Danish cohort study of 236,494 individuals with COPD from 1980 through 2008 and an average follow-up time of 3.5 years showed that COPD patients had an increased risk of developing tobacco-related cancers, including cancers of the lung, larynx, tongue, oral cavity, pharynx, esophagus, stomach, liver, pancreas, cervix uteri, and urinary tract (40). The rate of frailty in patients with COPD is high. A Korean study showed that in 417 patients with COPD, 148 patients (35.5%) were frail, 156 (37.4%) were pre-frail, and 113 (27.1%) were not frail (41). Another MR study showed that impaired kidney function increase the risk of leukemia, cervical cancer, female renal cell carcinoma, and CRC, but decreased the risk of non-Hodgkin lymphoma (42).
Frailty is also a common problem in patients with chronic kidney disease (CKD). The results of a systematic evaluation showed that the prevalence of frailty ranged from 7% in community-dwellers with stage 1–4 CKD to 73% in those regularly receiving hemodialysis treatment (43). The incidence of frailty increased as the glomerular filtration rate decreased (43). Frailty has also been shown to be associated with an increased risk of mortality and hospitalization (43). However, frailty has rarely been included as a factor in many studies analyzing the risk of a disease and cancer. Thus, it is not clear whether it is a risk factor or predictive factor for the occurrence of cancer. Based on the above research, it is reasonable to speculate that frailty is a risk factor or predictive factor for cancer.
Our TSMR analysis found no statistically significant relationship between fragility and the risk of colon cancer. However, there may be a number of reasons for these results. First, fragility is a body state that involves multiple organs and tissues of the body. The effect of fragility on cancer may involve extremely complex mechanisms. Fragility is not a single disease, and its effect on cancer may represent a comprehensive effect of functional changes in various organs and tissues. Second, few studies have been conducted on the SNPs related to frailty. Of the 8 SNPs included in this study, only 2 SNPs (i.e., rs4146140 and rs4952693) were statistically related to colon cancer, and their β values were small (3.19E-04 and 2.61E-04, respectively); the other 6 SNPs had no clear biological relationship with colon cancer. Third, as mentioned above, it often takes a long time to observe the effect of a factor on the risk of cancer. When a patient has a fracture, their life expectancy is often short. Even if frailty has an impact on the occurrence of cancer, the impact may take a long time to manifest.
This study had some limitations. First, the GWAS colon cancer data were all derived from European populations; thus, comprehensive research needs to be conducted among different ethnic groups in different countries and districts. Second, this research included relatively few SNPs as the IVs, and the ability to detect causal correlations was limited. Third, some unknown confounding factors, including those not reported in the literature, cannot be completely excluded. Fourth, the data of each patient could not be obtained in the study; thus, further subgroup analyses could not be carried out in this study.
Conclusions
In conclusion, the results of this study suggest that frailty may not be a risk factor for colon cancer and cannot be used as a predictor. The future study should focus on the predictive value of frailty on the prognosis of patients with colon cancer.
Acknowledgments
Funding: This work was supported by the Science and Technology Research Programme of the Health Commission of Hebei Province (No. 20170503).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-134/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-134/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-134/coif). The authors have no conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. As this study was based on open-access data, there was no need to acquire approval from the Ethics Committee affiliated with the authors. All the participants had previously provided their informed consent to the database. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Komici K, Bencivenga L, Navani N, et al. Frailty in Patients With Lung Cancer: A Systematic Review and Meta-Analysis. Chest 2022;162:485-97. [Crossref] [PubMed]
- Wang S, Yang T, Qiang W, et al. The prevalence of frailty among breast cancer patients: a systematic review and meta-analysis. Support Care Cancer 2022;30:2993-3006. [Crossref] [PubMed]
- Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255-63. [Crossref] [PubMed]
- Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013;14:392-7. [Crossref] [PubMed]
- Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487-92. [Crossref] [PubMed]
- Kojima G. Prevalence of Frailty in Nursing Homes: A Systematic Review and Meta-Analysis. J Am Med Dir Assoc 2015;16:940-5. [Crossref] [PubMed]
- Kojima G. Prevalence of frailty in end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol 2017;49:1989-97. [Crossref] [PubMed]
- Lee HJ, Son YJ. Prevalence and Associated Factors of Frailty and Mortality in Patients with End-Stage Renal Disease Undergoing Hemodialysis: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 2021;18:3471. [Crossref] [PubMed]
- Doody P, Asamane EA, Aunger JA, et al. The prevalence of frailty and pre-frailty among geriatric hospital inpatients and its association with economic prosperity and healthcare expenditure: A systematic review and meta-analysis of 467,779 geriatric hospital inpatients. Ageing Res Rev 2022;80:101666. [Crossref] [PubMed]
- Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol 2015;26:1091-101. [Crossref] [PubMed]
- McGovern J, Dolan RD, Horgan PG, et al. The prevalence and prognostic value of frailty screening measures in patients undergoing surgery for colorectal cancer: observations from a systematic review. BMC Geriatr 2022;22:260. [Crossref] [PubMed]
- Hoogendijk EO, Afilalo J, Ensrud KE, et al. Frailty: implications for clinical practice and public health. Lancet 2019;394:1365-75. [Crossref] [PubMed]
- Lewandowska AM, Rudzki M, Rudzki S, et al. Environmental risk factors for cancer - review paper. Ann Agric Environ Med 2019;26:1-7. [Crossref] [PubMed]
- Allum WH. Role of frailty assessment in selection for cancer surgery. BJS Open 2022;6:zrac117. [Crossref] [PubMed]
- Chen SY, Chou WC, Lin YC, et al. Performance of two frailty screening tools among patients with cancer in Taiwan. Biomed J 2022;45:361-9. [Crossref] [PubMed]
- Osatnik J, Matarrese A, Leone B, et al. Frailty and clinical outcomes in critically ill patients with cancer: A cohort study. J Geriatr Oncol 2022;13:1156-61. [Crossref] [PubMed]
- Shaw JF, Budiansky D, Sharif F, et al. The Association of Frailty with Outcomes after Cancer Surgery: A Systematic Review and Metaanalysis. Ann Surg Oncol 2022;29:4690-704. [Crossref] [PubMed]
- Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA 2017;318:1925-6. [Crossref] [PubMed]
- Pritchard JK, Przeworski M. Linkage disequilibrium in humans: models and data. Am J Hum Genet 2001;69:1-14. [Crossref] [PubMed]
- Burgess S, Butterworth A, Malarstig A, et al. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ 2012;345:e7325. [Crossref] [PubMed]
- Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol 2013;178:1177-84. [Crossref] [PubMed]
- Liao HY, Liao B, Zhang HH. CISD2 plays a role in age-related diseases and cancer. Biomed Pharmacother 2021;138:111472. [Crossref] [PubMed]
- Santucci C, Carioli G, Bertuccio P, et al. Progress in cancer mortality, incidence, and survival: a global overview. Eur J Cancer Prev 2020;29:367-81. [Crossref] [PubMed]
- Rozhok AI, DeGregori J. The evolution of lifespan and age-dependent cancer risk. Trends Cancer 2016;2:552-60. [Crossref] [PubMed]
- Tu H, Wen CP, Tsai SP, et al. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ 2018;360:k134. [Crossref] [PubMed]
- Teng PC, Huang DQ, Lin TY, et al. Diabetes and Risk of Hepatocellular Carcinoma in Cirrhosis Patients with Nonalcoholic Fatty Liver Disease. Gut Liver 2023;17:24-33. [Crossref] [PubMed]
- Yamaguchi A, Tazuma S, Tamaru Y, et al. Long-standing diabetes mellitus increases concomitant pancreatic cancer risk in patients with intraductal papillary mucinous neoplasms. BMC Gastroenterol 2022;22:529. [Crossref] [PubMed]
- Saed L, Varse F, Baradaran HR, et al. The effect of diabetes on the risk of endometrial Cancer: an updated a systematic review and meta-analysis. BMC Cancer 2019;19:527. [Crossref] [PubMed]
- Hossain FM, Danos DM, Fu Q, et al. Association of Obesity and Diabetes With the Incidence of Breast Cancer in Louisiana. Am J Prev Med 2022;63:S83-92. [Crossref] [PubMed]
- Xiao W, Huang J, Zhao C, et al. Diabetes and Risks of Right-Sided and Left-Sided Colon Cancer: A Meta-Analysis of Prospective Cohorts. Front Oncol 2022;12:737330. [Crossref] [PubMed]
- Undzyte G, Patasius A, Linkeviciute-Ulinskiene D, et al. Increased kidney cancer risk in diabetes mellitus patients: a population-based cohort study in Lithuania. Aging Male 2020;23:1241-5. [Crossref] [PubMed]
- Fang H, Yao B, Yan Y, et al. Diabetes mellitus increases the risk of bladder cancer: an updated meta-analysis of observational studies. Diabetes Technol Ther 2013;15:914-22. [Crossref] [PubMed]
- Assar ME, Laosa O, Rodríguez Mañas L. Diabetes and frailty. Curr Opin Clin Nutr Metab Care 2019;22:52-7. [Crossref] [PubMed]
- Kong LN, Lyu Q, Yao HY, et al. The prevalence of frailty among community-dwelling older adults with diabetes: A meta-analysis. Int J Nurs Stud 2021;119:103952. [Crossref] [PubMed]
- Han H, Guo W, Shi W, et al. Hypertension and breast cancer risk: a systematic review and meta-analysis. Sci Rep 2017;7:44877. [Crossref] [PubMed]
- Petrelli F, Ghidini A, Cabiddu M, et al. Effects of hypertension on cancer survival: A meta-analysis. Eur J Clin Invest 2021;51:e13493. [Crossref] [PubMed]
- Christakoudi S, Kakourou A, Markozannes G, et al. Blood pressure and risk of cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2020;146:2680-93. [Crossref] [PubMed]
- Vetrano DL, Palmer KM, Galluzzo L, et al. Hypertension and frailty: a systematic review and meta-analysis. BMJ Open 2018;8:e024406. [Crossref] [PubMed]
- Mouronte-Roibás C, Leiro-Fernández V, Fernández-Villar A, et al. COPD, emphysema and the onset of lung cancer. A systematic review. Cancer Lett 2016;382:240-4. [Crossref] [PubMed]
- Kornum JB, Sværke C, Thomsen RW, et al. Chronic obstructive pulmonary disease and cancer risk: a Danish nationwide cohort study. Respir Med 2012;106:845-52. [Crossref] [PubMed]
- Park SK. Frailty in Korean patients with chronic obstructive pulmonary disease, using data from the Korea National Health and Nutrition Examination Survey, 2015 and 2016. Appl Nurs Res 2021;59:151417. [Crossref] [PubMed]
- Tang L, Li C, Chen W, et al. Causal Association between Chronic Kidney Disease and Risk of 19 Site-Specific Cancers: A Mendelian Randomization Study. Cancer Epidemiol Biomarkers Prev 2022;31:1233-42. [Crossref] [PubMed]
- Chowdhury R, Peel NM, Krosch M, et al. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr 2017;68:135-42. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


