
A case series of 10 patients undergone linear cutter/stapler guiding device-led overlapped esophagojejunostomy: a preliminary study
Highlight box
Key findings
• Application of the linear cutter/stapler guiding device (LCSGD) in overlap esophagojejunostomy (EJS) after laparoscopic total gastrectomy is safe and feasible, with satisfactory clinical effectiveness.
What is known and what is new?
• Overlap EJS after laparoscopic total gastrectomy is a highly safe procedure, with a small incidence of postoperative anastomotic stenosis. However, the procedure is difficult to perform.
• According to the design, LCSGD was used in EJS, so that the linear cutting/stapler can complete the technical action quickly and efficiently within the narrow lower posterior mediastinum via the esophageal hiatus; meanwhile, it avoids the formation of an esophageal pseudotract.
What is the implication, and what should change now?
• Overlap EJS is difficult to perform and involves a long anastomosis time, along with a high risk of creating an esophageal pseudotract. Our team designed and developed the LCSGD.
Introduction
Compared with laparoscopic-assisted total gastrectomy (LATG), totally laparoscopic total gastrectomy (TLTG) provides more room for surgical operation, reduces intraoperative blood loss, and dissects more lymph nodes, with smaller incisions and comparable short-term outcomes (1-3). However, TLTG, especially esophagojejunostomy (EJS), requires high surgical skills (4). EJS is mainly performed using a linear stapler, whereas overlap anastomosis is one of the most popular reconstruction methods for intraperitoneal EJS. Overlap EJS requires neither the use of purse-string sutures nor the insertion of anvils and has a good surgical field. The postoperative EJS is highly safe; in particular, the size of the anastomosis is not limited by the esophageal and jejunal transverse diameters, which effectively avoids anastomotic stenosis after surgery. Since its first description in 2010, overlap EJS has been associated with fewer anastomosis-related complications and satisfactory short-term effectiveness. However, overlap EJS is difficult to perform and involves a long anastomosis time, along with a high risk of creating an esophageal pseudotract. In addition, a large common opening after EJS is associated with more difficult closure, slower anastomosis, and increased surgical risk (5). Accordingly, our team designed and developed the linear cutter/stapler guiding device (LCSGD) (patent number: 202122912972.6) (Figure 1). The present study retrospectively analyzed the clinical data of 10 patients with gastric cancer who were admitted to the Third Department of Surgery of the Fourth Hospital of Hebei Medical University during the period from July 2021 to November 2021 and underwent overlap EJS after radical laparoscopic total gastrectomy under the guidance of LCSGD. We aimed to further clarify the value of LCSGD in this procedure. Through the LCSGD docking nail warehouse and stomach tube, nail warehouse into the esophageal cavity is more convenient and faster. The LCSGD skillfully connects the nail warehouse and the gastric tube, so that the nail warehouse in the abdominal cavity-the guiding device of the linear cutting stapler-the gastric tube pierced out of the esophageal cavity forms an integrated connecting device, and then moves into the esophageal cavity. The head end of the nail warehouse does not contact the esophageal wall, so the formation of the esophageal ‘false passage’ can be fundamentally avoided. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-193/rc).
Methods
General data
A retrospective, descriptive design was adopted. The clinical data of 10 patients (including 8 males and 2 females, aged 50–75 years) with gastric cancer were collected. Their mean body mass index (BMI) was 25.2±4.3 kg/m². The lesions were located in the gastric body (n=7) or in the cardia (n=3), and the average maximum diameter of the tumors was 3.1±1.4 cm. None of these 10 patients had a history of abdominal surgery; 2 patients also had hypertension, and 1 had diabetes (Table 1). All patients underwent LCSGD-guided overlap EJS after laparoscopic total gastrectomy.
Table 1
| No. | Gender | Age (years) | Body mass index (kg/m2) | Tumor location | Maximum tumor diameter (cm) | Co-existing diseases |
|---|---|---|---|---|---|---|
| 1 | Male | 73 | 30.1 | Gastric body | 2.2 | No |
| 2 | Male | 57 | 22.9 | Gastric body | 1.0 | Hypertension |
| 3 | Male | 65 | 34.2 | Cardia | 4.0 | Diabetes |
| 4 | Male | 75 | 24.5 | Gastric body | 5.0 | No |
| 5 | Female | 64 | 22.5 | Gastric body | 3.0 | No |
| 6 | Male | 62 | 22.2 | Cardia | 2.5 | No |
| 7 | Male | 58 | 22.9 | Gastric body | 3.0 | No |
| 8 | Female | 63 | 18.4 | Gastric body | 6.0 | No |
| 9 | Male | 50 | 27.4 | Gastric body | 2.0 | Hypertension |
| 10 | Male | 61 | 26.9 | Cardia | 2.5 | No |
inclusion and exclusion criteria
The inclusion criteria were as follows: (I) pathologically confirmed gastric cancer before surgery; (II) tumors located in the cardia, fundus, or stomach body; (III) naïve to chemotherapy, immunotherapy, targeted therapy, and/or before surgery; (IV) without distant metastasis in clinical staging; (V) without obvious contraindications to surgery; (VI) aged ≥18 years; and (VII) with an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 points. Patients with surgical contraindications such as cardiopulmonary insufficiency were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2021KS011). Individual consent for this retrospective analysis was waived.
Observation indicators
The intraoperative observation indicators were total operative duration, time from the entry of the linear cutter/stapler into the abdominal cavity to the completion of anastomosis, time for manual suturing of esophageal-jejunal common opening, and number of suture needles. The postoperative observation indicators were time to first ambulation, time to first postoperative exhaust/defecation, time to semi-liquid diet, and length of postoperative hospital stay. The postoperative complications included reoperation, bleeding, anastomotic fistula and duodenal stump fistula. We followed up 10 patients every 3 or 6 months by telephone to ask about their eating and postoperative reflux.
Statistical analysis
SPSS (version 26.0) software was used for the statistical analysis. The measurement data are expressed as mean ± standard deviation.
Surgical techniques
Tracheal intubation was performed under general anesthesia. The patient was placed in the supine position, with their legs apart. The head was positioned higher than the feet by 15°–30° degrees. The operator stood at the left side of the patient, the assistant at the right side of the patient, and the camera holder between the legs of the patient. A trocar was placed below the umbilicus as a camera port. After the pneumoperitoneum was established, the air pressure was maintained at 12–14 mmHg (1 mmHg =0.133 kPa). A total of 4 operating ports were created at the intersections between the left and right midclavicular lines and the upper 2 cm of the umbilical level and at the sites 2 cm below the costal arch of the left and right anterior axillary lines. After the liver was lifted, the domes of the diaphragm were divided longitudinally to below the pericardium to create more surgical space. Stomach mobilization and D2 lymph node dissection were routinely performed. The lower esophagus was fully mobilized for 6–8 cm, then the esophagus was transected 2–3 cm above the upper edge of the tumor using a linear cutter/stapler. A 4-cm midline incision was made to harvest the specimen, which was immediately observed morphologically or via frozen sections to ensure the upper remnant was safe. Subsequently, routine histopathological examination was performed. The upper jejunum was lifted via the superior incision, and a linear cutter/stapler was used to dissociate the jejunum about 20 cm away from the Treitz ligament. The jejunal mesentery was fully mobilized, then the jejunal side-to-side anastomosis was performed 40–50 cm away from the jejunal blind loop. After the reconstruction of the pneumoperitoneum, the esophagus was fixed directly above the diaphragm angle by using a multi-spot suture fixation (overlap method, using W-type suture), and the distal jejunum was fixed behind the lower posterior mediastinal esophagus (6). The lower remnant of the esophagus was fixed to the blind end of the jejunum (about 6–8 cm in length) using absorbable sutures (2–3 stitches).
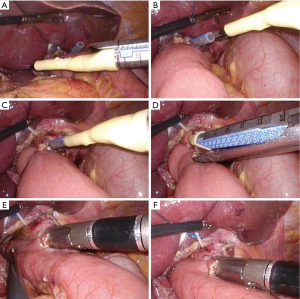
The LCSGD was assembled in vitro and then used according to the following steps: an itinerant nurse inserted the gastric tube into the esophagus, and the front end of the gastric tube was slightly pressed into the esophageal stump. The operator was guided to create a small opening next to the esophageal stump (about 1/4 to 1/3 of the stump). The gastric tube extended about 2–3 cm from this incision to dock with the LCSGD (Figure 2A). Using an electronic needle, the operator created a small opening in the jejunal loop (opposite to the mesentery) 0.5 cm below the esophageal-jejunal mutual fixation site, and the linear cutter/stapler was placed into the abdominal cavity through the upper right trocar. The LCSGD on the reload was docked with the gastric tube through an anterior dock line (Figure 2B,2C). The operator then moved the linear cutter/stapler towards the esophageal opening. As the anvil approached the opening at the jejunal loop, it was inserted into the jejunal opening. The linear cutter/stapler continued to move upwards, and the anvil and reload smoothly entered the jejunum and the esophagus, respectively. After their locations had been satisfactorily adjusted, the linear cutter/stapler was closed. The itinerant nurse withdrew the gastric tube upwards for 10–15 cm (Figure 2D). The operator ensured that the stapler did not clamp the gastric tube, after which they performed the esophageal-jejunal side-to-side anastomosis using the stapler (Figure 2E,2F). After anastomosis, both the reload and the LCSGD were withdrawn. How the anastomotic limbs overlap is shown in Figure 3. Finally, the common opening was sutured manually with absorbable barbed sutures under laparoscope to complete the esophageal jejunal anastomosis. Specific LCSGD usage method is shown in Video 1.
Results
Intra-operative conditions
A total of 10 patients received overlap EJS after radical laparoscopic total gastrectomy. Both D2 lymphadenectomy and R0 resection were achieved in these patients. No combined multiple organ resection was performed. No conversion to laparotomy or thoracotomy was required. Overlap EJS was guided by LCSGD in all 10 patients. The reload was successfully inserted into the esophageal lumen after a single attempt, with satisfactory position and angle, and the EJS was able to be immediately performed using the stapler. After the operation, the patient was successfully discharged from the hospital and followed up for 9–12 months. The average time from the entry of the linear cutter/stapler into the abdominal cavity to the completion of the firing of the stapler was 1.8±0.4 minutes, the average time for manual suturing of esophageal-jejunal common opening was 14.4±2.1 minutes (mean suturing: 18±2 stitches), and the average operative time was 255±52 minutes.
Postoperative conditions
The time to the first ambulation in these 10 patients was 1.9±1.4 days, the average time to the first postoperative opening of the bowels was 3.5±1.3 days, the average time to a semi-liquid diet was 3.6±0.7 days, and the average postoperative hospital stay was 10.4±4.1 days. All 10 patients were smoothly discharged, without any secondary surgery, bleeding, anastomotic fistula, or duodenal stump fistula.
Follow-up results
The telephone follow-up lasted 9–12 months. No eating disorders or anastomotic stenosis were reported. One patient experienced Visick grade II heartburn (acid reflux), and the condition of the remaining 9 patients was classified as Visick grade I (Table 2).
Table 2
| No. | Total operative duration (min) | Time from the entry of the linear cutter/stapler into the abdominal cavity to the completion of anastomosis (min) | Time for manual suturing of esophageal-jejunal common opening (min) | Stitches | Time to first ambulation (days) | Time to first postoperative exhaust/defecation (days) | Time to semi-liquid diet (days) | Postoperative hospital stay (days) | Postoperative Visick grade |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 336 | 2.5 | 18.4 | 20 | 2 | 3 | 3 | 11 | I |
| 2 | 285 | 1.8 | 15.1 | 18 | 1 | 2 | 4 | 7 | I |
| 3 | 212 | 2.2 | 15.2 | 19 | 2 | 3 | 3 | 8 | I |
| 4 | 251 | 2.0 | 16.8 | 16 | 1 | 3 | 4 | 8 | I |
| 5 | 245 | 1.6 | 14.8 | 21 | 1 | 2 | 5 | 8 | I |
| 6 | 194 | 2.1 | 13.5 | 17 | 6 | 3 | 3 | 22 | I |
| 7 | 220 | 1.4 | 14.1 | 15 | 1 | 5 | 4 | 9 | II |
| 8 | 179 | 1.5 | 12.8 | 18 | 2 | 6 | 4 | 9 | I |
| 9 | 318 | 1.1 | 12.4 | 17 | 2 | 3 | 4 | 11 | I |
| 10 | 310 | 1.8 | 10.7 | 19 | 1 | 5 | 3 | 11 | I |
Discussion
The past decades have witnessed the tremendous development of laparoscopic total gastrectomy with the advances in laparoscopic technology. However, there is no uniform standard for digestive tract reconstruction after laparoscopic total gastrectomy, among which the technical difficulties involved in EJS for surgeons have prevailed. The EJS methods used after TLTG include esophageal-jejunal functional end-to-end anastomosis, π-shaped anastomosis, and overlap anastomosis, which have their own strengths and weakness. There are many ways of digestive tract reconstruction after total gastrectomy, but which surgical method is more suitable for patients with total gastrectomy is still controversial. At present, the ideal digestive tract reconstruction method should have the function of food storage bag and keep the function of food storage bag. It can maintain the patency of the patient’s food channel, slow down the speed of food entering the small intestine, avoid the patient’s duodenal secretions returning to the esophagus, and accelerate the emptying of the bag food to the small intestine in a gradient. In addition, the implementation of reconstruction requires simple operation, less trauma to patients and less postoperative complications, which is conducive to postoperative rehabilitation of patients. Overlap anastomosis has several advantages: (I) it is an iso-peristaltic anastomosis; (II) it is more beneficial to determine whether the upper remnant is negative after the resection of a tumor invading the tooth line; and (III) folding of the jejunum and the mesangium is not required. However, it has some limitations: (I) due to the narrow field of view at the lower posterior mediastinum, the esophageal wall and jejunal wall need to be frequently stretched or clamped when looking for the optimal site for EJS (7); meanwhile, the positions of the anvil, reload, and esophagus/jejunum need to be repeatedly adjusted under the interference of the liver and diaphragmatic foot, so as to control the volumes of the anastomosis and the common opening. (II) The position of the common opening is high (near or above the level of the diaphragm), and the safety and efficiency of suturing is often reduced when the opening is too large. The relevant surgical risks include the following: (I) there is a risk of forming an “esophageal pseudo-tract” when the slender anvil enters the esophagus; (II) the length of the distal jejunal remnant is uncertain, and an excessively long remnant can easily render the jejunal blind loop vertical to or folded in the jejunum, showing an inverse “L” shape, which may increase the risk of the anastomosis at the highest point due to gravity and/or diaphragm obstruction. In view of the operational difficulties and risks in traditional overlap anastomosis, our team has made some optimizations and improvements based on our clinical practice. This article for the first time describes the application of LCSGD in overlap EJS after laparoscopic total gastrectomy.
During the traditional overlap anastomosis, the entry of reload into the jejunal side is usually required to prevent the relatively sharp anvil from piercing the jejunum, which, however, may also bring the relatively thick reload into the jejunal side, leading to a long common opening (8). It is well known that a longer jejunal margin greatly increases the chance and degree of jejunal mucosal exstrophy, thus impacting the quality and speed of closing the common opening. In contrast, the esophageal mucosa will not be significantly turned out because of the volume of the esophageal opening, and therefore a slightly larger esophageal opening will not significantly increase the difficulty in closing the common opening. Furthermore, when a reload enters the esophagus, it has minimal risk of causing an esophageal pseudotract due to its thick and blunt characteristics; in contrast, entry of the anvil into the esophagus has such a risk. Therefore, we used the method of hanging the jejunum (which is used in the multi-spot fixation method) to adjust the length and course of the jejunum, so as to systematically avoid the risk that the anvil pierces the jejunum. In fact, the unusual technique of the reload entering the esophagus and the anvil entering the jejunum has become an excellent option. However, 2 new problems still need to be addressed: (I) The reload and the anvil on the linear cutter/stapler need to enter the esophagus or jejunum almost simultaneously; (II) the thicker and blunt reload need to enter the opening smoothly and quickly at the lower end of the relatively small esophagus. Accordingly, we designed and applied the LCSGD that can be installed on the reload of the linear cutter/stapler.
LCSGD is a soft conical rubber device that can be attached to the head end of the reload. The LCSGD-guided overlap EJS has the following advantages: (I) the reload can be placed into the esophageal cavity more easily and quickly after the LCSGD docks the reload and the gastric tube; (II) due to the insertion-typed connection between the device and the gastric tube, the insertion of the reload and the esophagus are predetermined, and thus subsequent attention can be paid to the placement of the anvil in the jejunum only, which is an extremely easy link. Thus, the anvil and the reload enter the jejunum or esophagus simultaneously. In addition, the reload and the anvil are placed into the esophagus or jejunum at satisfactory positions and angles, which help to reduce esophageal traction, narrow the common opening, reduce the iterations of reload placement into the esophageal lumen, and shorten the operative time; (III) the LCSGD delicately connects the reload and the gastric tube, so that the reload in the abdominal cavity, the LCSGD, and the gastric tube from the esophageal cavity are docked together to form an integrated connection device, which is then moved into the esophageal cavity again, during which the head end of the reload does not contact the esophageal wall, thus avoiding the formation of an esophageal pseudotract; (IV) since the reload and the LCSGD will push the gastric tube towards the oral side when they are successively entering the esophageal lumen and there is no direct contact between the stapler and the gastric tube, any accidental cutting or stapling of the gastric tube by the stapler can be avoided; (V) the anvil can enter the jejunum with the use of the LCSGD. As a result, the jejunal margin at the common opening is very small, and the jejunal mucosal exstrophy will easily appear or can be simply controlled once it occurs, which further lowers the difficulty in suturing the common opening, shortens the time of digestive tract reconstruction, increases the reliability of esophageal-jejunal anastomosis, and ensures the safety of surgery. Notably, the connection length must be ensured when the LCSGD is tightly installed onto the linear cutter/stapler, so as to avoid the guiding device being cut off when anastomosing. The biggest problem in this study is that the connection and removal of the linear cutting stapler and LCSGD need to be performed manually, which increases the operation time. We can study a complete linear cutting stapler containing LCSGD and put it into use.
Conclusions
In summary, the LCSGD can be safely and effectively applied in overlap EJS after laparoscopic total gastrectomy. However, the short-term performance of LCSGD needs to be further validated in prospective, randomized, and controlled studies with large sample sizes. Furthermore, the application of the LCSGD in some special populations including patients with large BMI, esophageal involvement, or neoadjuvant chemoradiotherapy warrants further investigations. At present, this study has just been put into use. In the future, it will increase the sample size, increase the use in different patient populations, and conduct bulk data research to further confirm the advantages of LCSGD, find deficiencies and make up for them.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-193/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-193/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-193/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-193/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (No. 2021KS011). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhao S, Zheng K, Zheng JC, et al. Comparison of totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy: A systematic review and meta-analysis. Int J Surg 2019;68:1-10. [Crossref] [PubMed]
- Liao G, Wang Z, Zhang W, et al. Comparison of the short-term outcomes between totally laparoscopic total gastrectomy and laparoscopic-assisted total gastrectomy for gastric cancer: a meta-analysis. Medicine (Baltimore) 2020;99:e19225. [Crossref] [PubMed]
- Gong CS, Kim BS, Kim HS. Comparison of totally laparoscopic total gastrectomy using an endoscopic linear stapler with laparoscopic-assisted total gastrectomy using a circular stapler in patients with gastric cancer: A single-center experience. World J Gastroenterol 2017;23:8553-61. [Crossref] [PubMed]
- Li Z, Liu Y, Bai B, et al. Surgical and Long-Term Survival Outcomes After Laparoscopic and Open Total Gastrectomy for Locally Advanced Gastric Cancer: A Propensity Score-Matched Analysis. World J Surg 2019;43:594-603. [Crossref] [PubMed]
- Fan H, Wang D, Ding P, et al. Application value of continuous seromuscular layer sutures in the reinforcement of esophagojejunostomy in total gastrectomy for gastric cancer: a retrospective comparative cohort study. J Gastrointest Oncol 2022;13:2749-57. [Crossref] [PubMed]
- Ding P, Wang D, Zhao Q, et al. Comparative study on the short-term efficacy and safety of multipoint fixed-Overlap method and traditional Overlap method after totally laparoscopic gastrectomy. Chinese Archives of General Surgery 2022;16:106-10. (Electronic Edition).
- Hu T, Wang D, Zhao Q, et al. Preliminary study of multi-point fixation-overlap method for esophagus-jejunal anastomosis after total laparoscopic total gastrectomy. Journal of Hebei Medical University 2022;43:99-102.
- Xinhua C, Tian L, Huilin H, et al. Application value of overlap guiding tube (OGT) in assisting overlap esophagojejunostomy during laparoscopic total gastrectomy for gastric/gastroesophageal junction (G/GEJ) tumors. Gastric Cancer 2022;25:827-36. [Crossref] [PubMed]
(English Language Editor: J. Jones)




