
Effects of magnetic resonance imaging (MRI)-detected extramural vascular invasion (mrEMVI) and tumor deposits (TDs) on distant metastasis and long-term survival after surgery for stage III rectal cancer: a retrospective study grouped based on the relationship between the bottom of the tumor and peritoneal reflection
Highlight box
Key findings
• In the group of under the peritoneal reflection, the combination of mrEMVI and TDs seems to play a certain guiding role in predicting distant metastasis and long-term survival after rectal cancer surgery.
What is known and what is new?
• Effects of mrEMVI and TDs on the prognosis of rectal cancer is known.
• We’ve added a new grouping method based on the relationship between the bottom of tumor and peritoneal reflection.
What is the implication, and what should change now?
• The author believes that after applying the results to clinical work, by combining existing guidelines, patients’ own conditions, physical conditions, family conditions and their own wishes, new options for clinical work will gradually increase. It also greatly improves the accuracy of postoperative risk stratification of these patients to some extent. For postoperative adjuvant therapy, both treatment methods and treatment plans will be more diversified and individualized.
Introduction
According to Global Cancer Statistics (1), colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer-specific deaths worldwide. In 2020, there were an estimated 1.9 million new cases of CRC and 935,000 deaths, accounting for about one in 10 cancer cases and deaths. Among them, rectal cancer accounts for about 30% of CRCs. With the development of rectal cancer treatment, accurately predicting the prognosis of rectal cancer is crucial. The tumor, lymph node, metastasis (TNM) staging system is the most widely used prognostic system for rectal cancer (2). Tumor deposits (TDs) were first proposed in 1997 in the fourth edition of the American Joint Committee on Cancer (AJCC) TNM system. TDs are currently defined as microscopic or macroscopic focal aggregates of cancer cells located in the subserosa, mesentery, and non-peritonealized pericolic or perirectal fat (3). In the latest version of the TNM system, the presence of TDs constitutes a new pathological N stage (pN) category (i.e., pN1c) for adenocarcinomas with TDs but without lymph node metastasis (LNM) (i.e., N0). N1c accounts for about 5–10% of rectal cancers and TDs can be seen in 20% of rectal adenocarcinomas, which is higher than the proportion of TDs in colon cancer (15–18%) (4). The presence of TDs in CRC is not only associated with poor prognostic factors but also may lead to worse disease-free survival (DFS) and overall survival (OS), which is particularly obvious in rectal cancer (4-6).
Peritoneal reflection represents a vital transition between peritoneal and non-peritoneal, which divides the rectum into two parts, the intraperitoneal region and the extraperitoneal region, taking into account the factors of rectal venous return and the lymphatic drainage system. The prognosis of rectal tumors may differ significantly depending on the location of the relationship between rectal tumors and peritoneal reflection (7-9). In addition, peritoneal reflection may be an appropriate marker to identify patients with rectal cancer who receive radiotherapy. The 5-year local recurrence rates of intraperitoneal and extraperitoneal rectal cancers are 4.2% and 13.3%, respectively (7-9).
The present study aimed to evaluate the effect of magnetic resonance imaging (MRI)-detected extramural vascular invasion (mrEMVI) and TDs on postoperative distant metastasis and long-term survival of stage III rectal cancer by grouping the relationship between the lower end of the tumor and peritoneal reflection. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-222/rc).
Methods
Patients
A hospital-based retrospective study was performed on 694 patients who underwent radical resection for rectal cancer at the Harbin Medical University Tumor Hospital from October 2016 to October 2021. The inclusion criteria were as follows: (I) radical resection and regional lymph node dissection for rectal cancer were performed at our hospital; (II) preoperative MRI imaging data in our hospital were available for reference; (III) postoperative pathology confirmed rectal adenocarcinoma and the clinical staging was stage III; and (IV) no distant metastases at the time of diagnosis. The exclusion criteria were as follows: (I) postoperative pathology confirmed malignant tumors other than rectal adenocarcinoma; (II) palliative surgery only; (III) poor image quality; (IV) lost to follow-up; and (V) local recurrence after operation. The case screening flow chart is shown in Figure 1.
Follow-up visit
Patients were followed up by outpatient visits, telephone interviews, and in-person examinations. The follow-up included a physical examination (especially digital rectal examination), postoperative survival status of patients, preoperative and postoperative treatment methods, protocols and cycles, imaging examination, biochemical blood indicators, and whether distant metastasis had occurred. Hematological tests were performed every month, including routine blood tests, blood biochemistry, carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9). Imaging examinations were performed every 3 months, including chest and abdomen enhanced computed tomography (CT) and pelvic enhanced MRI. OS was defined as from the date of radical surgery to death or the last follow-up, while DFS was defined as from the date of radical surgery to tumor recurrence, progression, or death. All patients were followed up until October 31, 2021.
Statistical analysis
SPSS 25.0 software and R 4.2.1 software were used for statistical analysis. Chi-squared tests were utilized to compare differences between two groups of counting data, whereas the rank-sum tests were employed for comparison between two groups of grading data. A Kaplan-Meier curve was used to estimate the survival time, while the log-rank test was applied to compare the median survival time. Univariate and multivariate logistic regression models were applied to correlate different factors with postoperative distant metastasis. Univariate and multivariate Cox proportional risk models were used to correlate different factors with long-term postoperative survival. Forest maps were employed for subgroup analysis. P<0.05 was considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Review Board of Harbin Medical University (No. KY2022-19). All patients signed the informed consent for the second use of medical history data/biospecimens in the process of diagnosis and treatment and agreed to provide diagnosis and treatment information for scientific research. The privacy and identity information of patients was protected.
Study process
Firstly, we divided all of the patients into three groups based on the surgical records according to the relationship between the bottom of the tumor and peritoneal reflection: on the peritoneal reflection group; across the peritoneal reflection group; and under the peritoneal reflection group. Next, the Kaplan-Meier curve was used to evaluate the effect of TDs on postoperative distant metastasis and long-term survival in each group. We defined the following categories by combining mrEMVI and TDs: mrEMVI+TDs− [1], mrEMVI−TDs− [2], mrEMVI+TDs+ [3], and mrEMVI−TDs+ [4]. The Kaplan-Meier curve was used to evaluate the effects of the four categories on postoperative distant metastasis and long-term survival in the above three groups. Subsequently, the following analyses were performed in the non-neoadjuvant subgroup and the mrEMVI and lymph vascular invasion (LVI) consistent subgroup: the effects of the presence or absence of TDs and the four categories on postoperative distant metastasis and long-term survival were evaluated in the three groups using the Kaplan-Meier curves.
Also, univariate and multivariate analyses were performed to investigate the association between the relevant clinicopathological variables and the presence or absence of TDs. Finally, to identify the risk factors for predicting postoperative distant metastasis and long-term survival after rectal cancer surgery, univariate analysis was performed for the following factors in the whole study population and the non-neoadjuvant subgroup, and the statistically significant factors were selected for multivariate analysis: age, sex, tumor length to diameter, degree of tumor differentiation, pathological T stage (pT), pN, clinical stage, the relationship between the lower part of the tumor and peritoneal reflection, mrEMVI, mesorectal fascia (MRF), LNM, nerve invasion, LVI, postoperative distant metastasis, neoadjuvant therapy, postoperative adjuvant therapy, TDs, preoperative serum CEA, preoperative serum CA19-9, etc.
Results
Patient characteristics and groups
A total of 694 cases were recruited in this study, including 460 males and 234 females, aged 26–85 years (median: 62 years). The median maximum tumor diameter was 4 cm. The clinical stage of the 694 patients was stage III (55 cases of stage IIIA, 538 cases of stage IIIB, and 101 cases of stage IIIC). All patients underwent radical resection of rectal cancer and regional lymph node dissection, and the pathological type was rectal adenocarcinoma. In total, 140 patients received neoadjuvant therapy, and 507 patients received postoperative adjuvant therapy. Among the 694 patients, 174 patients had TDs+ and 296 patients had mrEMVI+. Postoperative distant metastases occurred in 78 patients. The patients’ characteristics are summarized in Table 1.
Table 1
| Characteristics | Total number | TD+ (n=174) | TD− (n=520) | 95% CI | OR | P |
|---|---|---|---|---|---|---|
| Sex | 0.623–1.280 | 0.893 | 0.537 | |||
| Male | 460 | 112 | 348 | |||
| Female | 234 | 62 | 172 | |||
| Age | 0.562–1.130 | 0.797 | 0.203 | |||
| ≤60 years | 304 | 69 | 235 | |||
| >60 years | 390 | 105 | 285 | |||
| Tumor length to diameter | 0.825–1.041 | 0.927 | 0.198 | |||
| ≤4 cm | 353 | 94 | 259 | |||
| >4 cm | 341 | 80 | 261 | |||
| Degree of tumor differentiation | 0.943–1.295 | 1.105 | 0.217 | |||
| High differentiation | 9 | 1 | 8 | |||
| High-medium differentiation | 24 | 4 | 20 | |||
| Medium differentiation | 492 | 133 | 359 | |||
| Medium-low differentiation | 112 | 23 | 89 | |||
| Medium-high differentiation | 2 | 0 | 2 | |||
| Low differentiation | 29 | 6 | 23 | |||
| Low-medium differentiation | 10 | 3 | 7 | |||
| pT | 1.157–3.400 | 1.983 | 0.013 | |||
| 1 | 6 | 0 | 6 | |||
| 2 | 65 | 6 | 59 | |||
| 3 | 609 | 167 | 442 | |||
| 4 | 14 | 1 | 13 | |||
| pN | 1.234–2.727 | 1.835 | 0.003 | |||
| N1 | 470 | 134 | 336 | |||
| N2 | 224 | 40 | 184 | |||
| Clinical stage | 0.756–1.569 | 1.089 | 0.645 | |||
| IIIA | 55 | 5 | 50 | |||
| IIIB | 538 | 150 | 388 | |||
| IIIC | 101 | 19 | 82 | |||
| Relationship between the lower part of the tumor and peritoneal reflection | 0.815–1.278 | 1.021 | 0.860 | |||
| Under the peritoneal reflection | 228 | 52 | 176 | |||
| Across the peritoneal reflection | 288 | 81 | 207 | |||
| On the peritoneal reflection | 178 | 41 | 137 | |||
| mrEMVI | 0.522–1.041 | 0.737 | 0.084 | |||
| + | 296 | 84 | 212 | |||
| − | 398 | 90 | 308 | |||
| MRF | 0.502–1.163 | 0.764 | 0.209 | |||
| + | 133 | 39 | 94 | |||
| − | 561 | 135 | 426 | |||
| LNM | 13.685–55.387 | 27.531 | <0.001 | |||
| + | 623 | 113 | 510 | |||
| − | 71 | 61 | 10 | |||
| Nerve invasion | 0.643–1.376 | 0.941 | 0.753 | |||
| + | 193 | 50 | 143 | |||
| − | 501 | 124 | 377 | |||
| LVI | 0.367–1.317 | 0.695 | 0.264 | |||
| + | 47 | 15 | 32 | |||
| − | 647 | 159 | 488 | |||
| Postoperative distant metastasis | 0.493–1.411 | 0.834 | 0.498 | |||
| + | 78 | 22 | 56 | |||
| − | 616 | 152 | 464 | |||
| Neoadjuvant therapy | 0.309–0.682 | 0.459 | <0.001 | |||
| + | 140 | 53 | 87 | |||
| − | 554 | 121 | 433 | |||
| Postoperative adjuvant therapy | 0.422–0.963 | 0.637 | 0.033 | |||
| + | 507 | 138 | 369 | |||
| − | 187 | 36 | 151 | |||
CI, confidence interval; LNM, lymph node metastasis; LVI, lymph vascular invasion; mrEMVI, magnetic resonance imaging-detected extramural vascular invasion; MRF, mesorectal fascia; OR, odds ratio; pN, pathological N stage; pT, pathological T stage; TDs, tumor deposits.
Among the 694 included patients, there were 178 cases in the on the peritoneal reflection group, 288 cases in the across the peritoneal reflection group, and 228 cases in the under the peritoneal reflection group (mrEMVI+TDs−: 212 cases; mrEMVI−TDs−: 308 cases; mrEMVI+TDs+: 84 cases; and mrEMVI−TDs+: 90 cases). Among the non-neoadjuvant subgroup (554 cases), there were 156 cases in the on the peritoneal reflection group, 228 cases in the across the peritoneal reflection group, and 170 cases in the under the peritoneal reflection group (mrEMVI+TDs−: 162 cases; mrEMVI−TDs−: 271 cases; mrEMVI+TDs+: 57 cases; and mrEMVI−TDs+: 64 cases). Among the mrEMVI and LVI matching subgroup, negative or positive for both mrEMVI and LVI (347 cases), there were 85 cases in the on the peritoneal reflection group, 136 cases in the across the peritoneal reflection group, and 126 cases in the under the peritoneal reflection group (mrEMVI+TDs−: 17 cases; mrEMVI−TDs−: 262 cases; mrEMVI+TDs+: 8 cases; and mrEMVI−TDs+: 60 cases).
The median OS was 22 months in the whole study population, 20.4 months in the non-neoadjuvant subgroup, and 22 months in the mrEMVI and LVI compatible subgroup. The median DFS was 20.5, 19.7, and 21.4 months in these three cohorts, respectively.
Outcomes
Effects of the presence or absence of TDs on postoperative distant metastasis and long-term survival in the entire study population and the non-neoadjuvant population
We analyzed the effects of the presence or absence of TDs on postoperative distant metastasis and long-term survival in the whole population and in the non-neoadjuvant population using Kaplan-Meier curves. TDs+ was negatively associated with postoperative distant metastasis and long-term survival in the across and under the peritoneal reflection groups (Figures 2,3 and Figure S1).
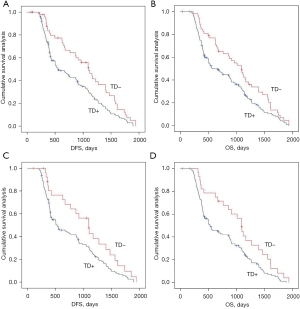
Effects of the presence or absence of TDs on postoperative distant metastasis and long-term survival in the mrEMVI and LVI matching population
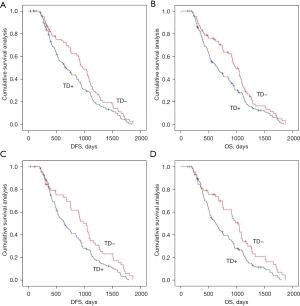
We analyzed the effects of the presence or absence of TDs on postoperative distant metastasis and long-term survival in the mrEMVI and LVI matching population using Kaplan-Meier curves. TDs+ was negatively associated with postoperative distant metastasis and long-term survival in the across the peritoneal reflection group (Figure 4 and Figure S1).
Effects of the new categories combining mrEMVI and TDs definition on postoperative distant metastasis and long-term survival in the entire study population
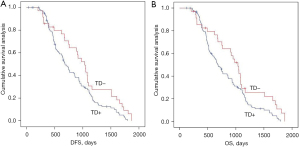
We analyzed the effects of the new categories combining mrEMVI and TDs definition on postoperative distant metastasis and long-term survival using Kaplan-Meier curves in the entire study population. In the under the peritoneal reflection group, the new categories were negatively associated with postoperative distant metastasis and long-term survival, and the groups were comparable. Among them, mrEMVI+TDs+ had the worst survival period (Figure 5).
Univariate and multivariate analyses of the clinicopathological factors associated with the presence or absence of TDs
Univariate analyses were performed to assess the effects of relevant variables on the presence or absence of TDs in rectal cancer, and results were as follows: pT (P=0.013), pN (P=0.003), LNM (P<0.001), neoadjuvant therapy (P<0.001), and postoperative adjuvant therapy (P=0.003) were correlated with the presence or absence of TDs in rectal cancer. Multivariate analysis showed that LNM (P<0.001) and neoadjuvant therapy (P=0.023) were independent risk factors for the presence or absence of TDs after rectal cancer surgery (Table 1 and Table S1).
Univariate and multivariate analyses of clinicopathological variables associated with distant metastasis and long-term survival after radical resection of rectal cancer in the entire study population
Univariate and multivariate analyses of the clinicopathological variables associated with distant metastasis after radical resection of rectal cancer in the entire study population
Univariate analysis was performed to evaluate the effects of related variables on distant metastasis after radical resection of rectal cancer, and the results were as follows: mrEMVI (P=0.019) and neoadjuvant therapy (P=0.001) were negatively correlated with distant metastasis after radical resection of rectal cancer. Multivariate analysis showed that neoadjuvant therapy (P=0.003) was negatively correlated with distant metastasis after radical resection of rectal cancer (Table 2).
Table 2
| Factors | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| 95% CI | OR | P value | 95% CI | OR | P value | ||
| Sex (male/female) | 0.517–1.374 | 0.843 | 0.493 | – | – | – | |
| Age (years) | 0.961–1.005 | 0.983 | 0.137 | – | – | – | |
| Tumor length to diameter (cm) | 0.837–1.144 | 0.979 | 0.788 | – | – | – | |
| Degree of tumor differentiation | 0.872–1.361 | 1.090 | 0.449 | – | – | – | |
| pT | 0.709–2.839 | 1.419 | 0.323 | ||||
| pN | 0.480–1.277 | 0.783 | 0.326 | ||||
| Clinical stage | 0.889–2.391 | 1.457 | 0.136 | – | – | – | |
| Relationship between the lower part of the tumor and peritoneal reflection | 0.727–1.350 | 0.991 | 0.952 | – | – | – | |
| mrEMVI (+/−) | 0.353–0.911 | 0.567 | 0.019 | 0.382–1.001 | 0.619 | 0.050 | |
| MRF (+/−) | 0.405–1.229 | 0.705 | 0.218 | – | – | – | |
| LNM (+/−) | 0.461–2.181 | 1.003 | 0.994 | – | – | – | |
| Nerve invasion (+/−) | 0.423–1.148 | 0.697 | 0.156 | – | – | – | |
| LVI (+/−) | 0.266–1.316 | 0.591 | 0.198 | – | – | – | |
| Neoadjuvant therapy (+/−) | 0.255–0.706 | 0.424 | 0.001 | 0.272–0.761 | 0.455 | 0.003 | |
| Postoperative adjuvant therapy (+/−) | 0.451–1.287 | 0.731 | 0.278 | – | – | – | |
| TDs (+/−) | 0.709–2.029 | 1.199 | 0.498 | – | – | – | |
| CEA (ng/mL) | 0.996–1.005 | 1.000 | 0.823 | – | – | – | |
| CA19-9 (U/mL) | 0.997–1.006 | 1.002 | 0.480 | – | – | – | |
CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; LNM, lymph node metastasis; LVI, lymph vascular invasion; mrEMVI, magnetic resonance imaging-detected extramural vascular invasion; MRF, mesorectal fascia; OR, odds ratio; pN, pathological N stage; pT, pathological T stage; TDs, tumor deposits.
Univariate and multivariate analyses of the clinicopathological variables associated with long-term survival after radical resection of rectal cancer in the entire study population
Univariate analysis was performed to evaluate the effects of related variables associated with long-term survival after radical resection of rectal cancer, and the results were as follows: MRF (P=0.026), neoadjuvant therapy (P=0.006), postoperative distant metastasis (P<0.001), and TDs (P<0.001) were negatively correlated with long-term survival after radical resection of rectal cancer. Multivariate analysis showed that MRF (P=0.024), postoperative distant metastasis (P<0.001), and TDs (P<0.001) were independent risk factors for long-term survival after rectal cancer surgery (Table 3).
Table 3
| Factors | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| 95% CI | HR | P value | 95% CI | HR | P value | ||
| Sex (male/female) | 0.877–1.225 | 1.037 | 0.671 | – | – | – | |
| Age (years) | 0.990–1.006 | 0.998 | 0.679 | – | – | – | |
| Tumor length to diameter (cm) | 0.956–1.060 | 1.007 | 0.801 | – | – | – | |
| Degree of tumor differentiation | 0.908–1.031 | 0.967 | 0.304 | – | – | – | |
| pT | 0.833–1.285 | 1.035 | 0.757 | – | – | – | |
| pN | 0.855–1.200 | 1.013 | 0.884 | – | – | – | |
| Clinical stage | 0.927–1.318 | 1.106 | 0.263 | – | – | – | |
| Relationship between the lower part of the tumor and peritoneal reflection | 0.972–1.193 | 1.077 | 0.157 | – | – | – | |
| mrEMVI (+/−) | 0.751–1.035 | 0.881 | 0.124 | – | – | – | |
| MRF (+/−) | 0.650–0.973 | 0.795 | 0.026 | 0.647–0.970 | 0.792 | 0.024 | |
| LNM (+/−) | 0.885–1.481 | 1.145 | 0.302 | – | – | – | |
| Nerve invasion (+/−) | 0.869–1.236 | 1.036 | 0.692 | – | – | – | |
| LVI (+/−) | 0.841–1.651 | 1.178 | 0.340 | – | – | – | |
| Neoadjuvant therapy (+/−) | 0.614–0.921 | 0.752 | 0.006 | 0.689–1.041 | 0.847 | 0.115 | |
| Postoperative adjuvant therapy (+/−) | 0.799–1.150 | 0.959 | 0.648 | – | – | – | |
| Postoperative distant metastasis (+/−) | 0.345–0.631 | 0.467 | <0.001 | 0.353–0.649 | 0.478 | <0.001 | |
| TDs (+/−) | 0.586–0.849 | 0.705 | <0.001 | 0.592–0.862 | 0.714 | <0.001 | |
| CEA (ng/mL) | 1.000–1.003 | 1.001 | 0.058 | – | – | – | |
| CA19-9 (U/mL) | 0.999–1.003 | 1.001 | 0.434 | – | – | – | |
CA19-9, carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; LNM, lymph node metastasis; LVI, lymph vascular invasion; mrEMVI, magnetic resonance imaging-detected extramural vascular invasion; MRF, mesorectal fascia; pN, pathological N stage; pT, pathological T stage; TDs, tumor deposits.
Univariate analyses of the clinicopathological variables associated with long-term survival after radical resection of rectal cancer in the non-neoadjuvant population
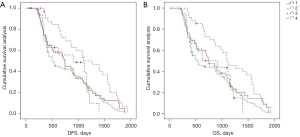
Univariate analysis was performed to assess the effects of related variables on long-term survival after radical resection of rectal cancer, and the results were as follows: postoperative distant metastasis (P<0.001) was considered to be an independent risk factor for long-term survival after radical resection of rectal cancer (Figure 6 and Figure S2).
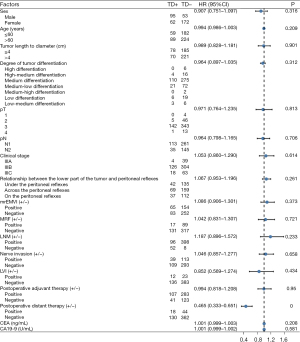
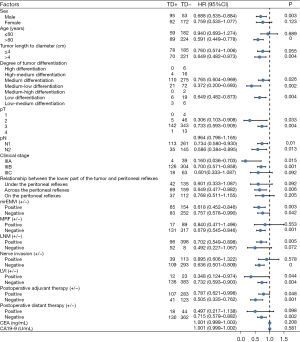
Univariate analysis of the combined clinicopathological factors and TDs on long-term survival after radical resection of rectal cancer in the non-neoadjuvant population
By combining the clinicopathological factors and TDs, we analyzed its impact on long-term survival after radical resection of rectal cancer through univariate analysis, and the results were as follows: male (P=0.003), age >60 years (P<0.001), tumor length to diameter >4 cm (P=0.004), medium differentiation (P=0.026), medium-low differentiation (P=0.002), low differentiation (P=0.004), pT2 (P=0.033), pT3 (P=0.004), IIIA (P=0.015), IIIB (P=0.001), the relationship between the lower part of the tumor and peritoneal reflection is across the peritoneal reflection (P=0.006), MRF− (P=0.001), LNM+ (P=0.005), negative nerve invasion (P<0.001), and no postoperative distant metastasis (P=0.002) were more likely to affect the long-term survival of TDs. However, when the clinicopathological factors were pN (pN1/pN2), mrEMVI (+/−), LVI (+/−), and whether postoperative neoadjuvant, the effects of TDs on long-term survival after surgery was consistent (Figure 7 and Figure S3).
Discussion
This study investigated 694 rectal cancer patients treated at a single center over a 5-year period, and we found that 25% of patients had TDs+. The AJCC TNM staging system is an important factor determining the treatment and prognosis of rectal cancer, especially for TDs. The AJCC fifth edition TNM staging system defines TDs according to the maximum diameter: 3 mm is the boundary, and nodules <3 mm are considered TDs (10). The AJCC sixth edition TNM staging system defines TDs based on contour: nodules with irregular contour are considered TDs. It was not until the seventh edition of AJCC TNM staging that TDs were included in the TNM staging and defined as pN1c, which is still used today (11). As the definition of TDs has become clearer, their prognostic role in rectal cancer has become more widely known, especially in stage III rectal cancer. It has become a consensus that TDs+ is an independent risk factor for poor prognosis in rectal cancer (2,4,12). Some scholars believe that rectal cancer patients with TDs+ are more prone to metastasis, their metastasis risk is comparable to that of pN2, and their prognosis is similar to that of stage IV (4,12), which may lead to changes in adjuvant therapy.
Moreover, TDs have important guiding significance for the prognosis of rectal cancer patients who have received neoadjuvant chemoradiotherapy. Xu et al. believed that TDs+ was associated with poor prognosis in patients with locally advanced rectal cancer after neoadjuvant chemoradiotherapy, and TDs+ patients seemed to benefit more from adjuvant chemotherapy (13). Yu et al. (14) agreed with them and believed that adding TDs to LNM might provide more accurate risk stratification. Another study argued that although TDs are an independent predictor of poor prognosis in rectal cancer patients after neoadjuvant chemoradiotherapy, this is only applicable to ypN0 stage patients (15). Although these studies have demonstrated that the presence of TDs may lead to poor prognosis in rectal cancer from different perspectives, the researchers only focused on the variable of the presence or absence of TDs, which obviously makes their research content insufficient.
In recent years, the lymph node ratio (LNR) has attracted increasing attention as a strong predictor of long-term survival in CRC patients after radical resection (16-18), and many researchers have also proposed calculating TDs as positive lymph nodes (19), so as to further explore the impact of the new LNR on the long-term survival of rectal cancer patients after radical resection. Pu et al. proposed that the new LNR was better than others in predicting long-term outcomes, and calculated the optimal intercept value of this new LNR (20). Another retrospective study based on the Chinese population showed that it was necessary to combine the number of TDs with the number of positive lymph nodes. After the number of TDs was reclassified into the number of positive lymph nodes, the recurrence risk of patients with previous stage pN1 was similar to that of patients with pN2 (21). Yet, the notion of TDs as positive lymph nodes remains controversial, and scientists have reservations.
On the basis of retaining previous studies, we grouped patients after radical surgery in a new way, according to the relationship between the lower part of the tumor and peritoneal reflection. In addition, we combined mrEMVI and TDs to define the new category. To make this experiment more convincing, the researchers also conducted subgroup analyses of the non-neoadjuvant population and the mrEMVI and LVI matching population, which offered a fresh perspective to our study and drew the following conclusions: in both the whole study population and the non-neoadjuvant population, TDs+ was negatively correlated with distant metastasis and long-term survival in the under and across the peritoneal reflection groups. In the mrEMVI and LVI matching subgroup, TDs+ was negatively correlated with postoperative distant metastasis and long-term survival in the across the peritoneal reflection groups. Moreover, in the overall study population, the new category defined by combining mrEMVI and TDs was negatively associated with postoperative distant metastasis and long-term survival in the under the peritoneal reflection group, with mrEMVI+TDs+ exhibiting the worst survival.
The presence of peritoneal reflection makes the rectum more special than any other organ in the body, and different positional relationships have different venous return and lymphatic drainage system. The relationship between the lower tumor segment and peritoneal reflection is an indispensable factor in determining the surgical method and prognosis of patients. However, no studies have grouped patients on this basis or linked them to TDs to further explore their prognosis. mrEMVI was first proposed in 2008 and is a filamentous extension of tumor signals with vascular structure, which leads to venous dilatation through this special tumor signal. Compared with EMVI detected by pathology, mrEMVI seems to have more advantages (22). Furthermore, a study believes that MRI is also important in the assessment of TDs (23). Lord et al. showed that mrEMVI and mrTDs had higher prognostic accuracy in patients undergoing radical resection (23); however, the author believed that TDs detected by pathology were more convincing than mrTDs.
In the present study, we first included the factor that is a key concern among researchers, namely TDs, and further verified the effects of TDs on the prognosis of patients after radical resection of rectal cancer on the basis of retaining the results of previous studies. It is worth noting that in our study, patients were grouped by anatomical characteristics for the first time, which may avoid experimental errors derived from anatomical characteristics to a certain extent and is one of the highlights of this experiment. Inspired by Lord et al. (23), we combined mrEMVI and TDs into a new category, effectively constructing a bridge between imaging examination and pathological results, which not only confirmed the important role of mrEMVI in the prognosis of rectal cancer patients but also eliminated the error caused by mrTDs to a certain extent. This provides a new direction and fresh ideas for future researchers and clinical staff. Since the status of mrEMVI and TDs may change before and after neoadjuvant therapy, subgroup analysis of the non-neoadjuvant population was also conducted to reduce the research error caused by this. When tumor cells invade small lymphatic vessels or blood vessels, LVI appears. LVI is a well-known pathological risk factor for poor prognosis in rectal cancer. Considering the similarities between LVI and mrEMVI, we also performed a subgroup analysis of the mrEMVI and LVI matching population, which greatly enhances the content of our research and experiment. As for the research results, this approach significantly improves the accuracy of the experimental results and also enhances the persuasiveness of the research conclusions to a certain extent. Above all, the new grouping criteria, the subgroup analysis of multiple populations, and the different inclusion factors made our study more unique.
However, this study still has some noteworthy limitations. Firstly, this was a retrospective study and was designed by a single institution. Next, our study had a small sample size. To confirm our hypothesis, two clinicopathological variables, TDs and mrEMVI, were included in the grouping based on the relationship between the lower end of the tumor and peritoneal reflection, and a new association was established between the two variables to further explore their impact on distant metastasis and long-term survival after radical resection of rectal cancer. The application of new grouping methods and the establishment of the new categories provide more diversified reference data for later researchers, and also offer a richer perspective for clinical staff. To enhance the strength of our study, the researchers also performed subgroup analyses of multiple populations. In this study, it was easy to obtain the relationship between the lower end of the tumor and peritoneal reflection through surgical records, and both mrEMVI and TDs are easy to obtain in clinical work. Therefore, stratifying the risk of patients after surgery through the above methods is simple and feasible. The results of this study cannot be ignored in the management of postoperative patients and the selection of individualized treatment plans. The authors believe that after applying these results to clinical work, by combining existing guidelines, patients’ own conditions, physical conditions, family conditions, and their own wishes, new clinical options will gradually emerge. This approach also greatly improves the accuracy of postoperative risk stratification of these patients. For postoperative adjuvant therapy, both therapeutic methods and treatment plans will be more diversified and individualized, which can extend the lives of patients, improve their quality of life, and reduce the social burden on their families.
Conclusions
In the under and across the peritoneal reflection groups, TD+ may be an independent predictor of postoperative distant metastasis and long-term survival. In the under the peritoneal reflection group, the combination of mrEMVI and TDs seems to play a certain guiding role in predicting distant metastasis and long-term survival after rectal cancer surgery.
Acknowledgments
We thank the medical imaging department of our hospital for their strong support and technical guidance in image acquisition and analysis.
Funding: This work was supported by the Wu Jieping Medical Foundation (No. 320.6750.2020-12-6), the Beijing Medical Award Foundation (No. YXJL-2020-0785-0185), and the Beijing Science and Innovation Medical Development Foundation (No. KC2021-JX-0186-113).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-222/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-222/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-222/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-222/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Review Board of Harbin Medical University (No. KY2022-19). All patients signed the informed consent for the second use of medical history data/biospecimens in the process of diagnosis and treatment and agreed to provide diagnosis and treatment information for scientific research. The privacy and identity information of patients was protected.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sánchez-Alcoholado L, Ramos-Molina B, Otero A, et al. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers (Basel) 2020;12:1406. [Crossref] [PubMed]
- Zheng H, Zhang J, Liu Y, et al. Prognostic value of tumor deposits in locally advanced rectal cancer: a retrospective study with propensity score matching. Int J Clin Oncol 2021;26:1109-19. [Crossref] [PubMed]
- Gallo G. The Emerging Role of Tumor Deposits as an Independent Prognostic Factor in Advanced Colorectal Cancer. J Invest Surg 2022;35:860-1. [Crossref] [PubMed]
- Benoit O, Svrcek M, Creavin B, et al. Prognostic value of tumor deposits in rectal cancer: A monocentric series of 505 patients. J Surg Oncol 2020;122:1481-9. [Crossref] [PubMed]
- Lino-Silva LS, Anchondo-Núñez P, Chit-Huerta A, et al. Stage I-III colon cancer patients with tumor deposits behave similarly to stage IV patients. Cross-section analysis of 392 patients. J Surg Oncol 2019;120:300-7. [Crossref] [PubMed]
- Zhang S, Chen F, Ma X, et al. MRI-based nomogram analysis: recognition of anterior peritoneal reflection and its relationship to rectal cancers. BMC Med Imaging 2021;21:50. [Crossref] [PubMed]
- Park JS, Sakai Y, Simon NSM, et al. Long-Term Survival and Local Relapse Following Surgery Without Radiotherapy for Locally Advanced Upper Rectal Cancer: An International Multi-Institutional Study. Medicine (Baltimore) 2016;95:e2990. [Crossref] [PubMed]
- Nijkamp J, Kusters M, Beets-Tan RG, et al. Three-dimensional analysis of recurrence patterns in rectal cancer: the cranial border in hypofractionated preoperative radiotherapy can be lowered. Int J Radiat Oncol Biol Phys 2011;80:103-10. [Crossref] [PubMed]
- Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997;80:1803-4.
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Wu W, Zeng S, Zhang X, et al. The value of tumor deposits in evaluating colorectal cancer survival and metastasis: a population-based retrospective cohort study. World J Surg Oncol 2022;20:41. [Crossref] [PubMed]
- Zhang LN, Xiao WW, Xi SY, et al. Tumor deposits: markers of poor prognosis in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Oncotarget 2016;7:6335-44. [Crossref] [PubMed]
- Xu T, Yu Z, Zhang Q, et al. Prognostic and staging value of tumor deposits in patients with rectal cancer after neoadjuvant chemoradiotherapy. Transl Cancer Res 2021;10:5028-39. [Crossref] [PubMed]
- Yu L, Xu T, Zhang L, et al. Tumor Deposits Should Not Be Ignored in the AJCC TNM Staging System for ypN(+) Stage Rectal Cancer with Neoadjuvant Chemoradiotherapy. J Gastrointest Surg 2020;24:2298-301. [Crossref] [PubMed]
- Zekri J, Ahmad I, Fawzy E, et al. Lymph node ratio may predict relapse free survival and overall survival in patients with stage II & III colorectal carcinoma. Hepatogastroenterology 2015;62:291-4. [PubMed]
- Ozawa T, Ishihara S, Nishikawa T, et al. Prognostic significance of the lymph node ratio in stage IV colorectal cancer patients who have undergone curative resection. Ann Surg Oncol 2015;22:1513-9. [Crossref] [PubMed]
- Zhang MR, Xie TH, Chi JL, et al. Prognostic role of the lymph node ratio in node positive colorectal cancer: a meta-analysis. Oncotarget 2016;7:72898-907. [Crossref] [PubMed]
- Nagtegaal ID, Knijn N, Hugen N, et al. Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis. J Clin Oncol 2017;35:1119-27. [Crossref] [PubMed]
- Yang J, Xing S, Li J, et al. Novel lymph node ratio predicts prognosis of colorectal cancer patients after radical surgery when tumor deposits are counted as positive lymph nodes: a retrospective multicenter study. Oncotarget 2016;7:73865-75. [Crossref] [PubMed]
- Pu H, Pang X, Fu J, et al. Significance of tumor deposits combined with lymph node metastasis in stage III colorectal cancer patients: a retrospective multi-center cohort study from China. Int J Colorectal Dis 2022;37:1411-20. [Crossref] [PubMed]
- van den Broek JJ, van der Wolf FSW, Heijnen LA, et al. The prognostic importance of MRI detected extramural vascular invasion (mrEMVI) in locally advanced rectal cancer. Int J Colorectal Dis 2020;35:1849-54. [Crossref] [PubMed]
- Fernandes MC, Gollub MJ, Brown G. The importance of MRI for rectal cancer evaluation. Surg Oncol 2022;43:101739. [Crossref] [PubMed]
- Lord AC, D'Souza N, Shaw A, et al. MRI-Diagnosed Tumor Deposits and EMVI Status Have Superior Prognostic Accuracy to Current Clinical TNM Staging in Rectal Cancer. Ann Surg 2022;276:334-44. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



