
Hepatopancreatobiliary malignancies: time to treatment matters
Highlight box
Key findings
• Longer time to treatment initiation is associated with worse overall survival in patients with stage I–III extrahepatic bile duct cancer and stage I–II pancreatic cancer.
• Black and Hispanic patients with hepatopancreatobiliary cancer experience greater delays in care than white patients.
What is known and what is new?
• Time to treatment initiation is known to be a contributing factor to patient outcome for some aggressive malignancies.
• Our data identify early-stage extrahepatic bile duct and pancreatic cancer as malignancies for which time to treatment is associated with survival and suggest racial disparities in time to treatment exist.
What is the implication, and what should change now?
• The results suggest that initiating treatment less than 30 days from diagnosis is associated with longer patient survival for patients with early-stage extrahepatic bile duct or pancreatic cancer.
• Care for all patients with these malignancies should be expedited.
Introduction
The increasing complexity of cancer care is driving a national trend towards increasing time from diagnosis to treatment initiation for many cancers (1-3). Moreover, resource constraints due to the COVID-19 pandemic have exacerbated existing delays by forcing many institutions to systematically postpone cancer treatment further (4-7). On a case-by-case basis, due to the complexity, cost, and duration of care, treatment for an individual may be further delayed for a multitude of patient-, provider-, or facility-driven reasons. However, the impact of delays in oncologic care on patient outcomes, particularly for hepatopancreatobiliary (HPB) cancers, is unclear; in the case of biliary cancer specifically, evidence is absent.
The association between time to treatment initiation (TTI) and survival has been investigated in multiple cancer types previously. Studies in head and neck cancer and some gastrointestinal (GI) cancers have concluded that there is no association between treatment delay and survival (8-14), while investigations in renal, endometrial, bladder, and other GI cancers have suggested that survival decreases with longer TTI (1,11,15-18). For some cancers, including breast, prostate, testicular, and non-small cell lung cancer, the existing literature is conflicting (11,15,19-22).
These diverse results may reflect not only differences in study design and power, but also tumor biology and response to therapy. Cancers with favorable tumor biology (such as prostate cancer) or for which highly efficacious treatments exist (such as testicular cancer) may have outcomes that sometimes but do not reproducibly worsen with lengthening TTI, as these diseases are either unlikely to progress over a few months or are likely to be adequately treated despite any progression (23,24). On the other hand, disease with an attenuated response to current therapy (such as late stage pancreatic cancer) might fail to demonstrate an association with TTI, since therapy is often ineffective regardless of relative tumor burden (25). In a third category, cancers with aggressive tumor biology but relatively effective treatment for some stages of disease (such as early stage gastric cancer) might be found to have increased mortality with delays in care.
HPB cancers have 5-year survival rates on the order of 30–40% for localized disease, but only 2–3% for metastatic disease—among the lowest of all malignancies (26,27). It follows that outcomes of these aggressive cancers may be meaningfully compromised by even short periods of unchecked progression; however, data on the association between TTI and survival in HPB cancers is extremely limited and derived from relatively small cohorts (9,10,18).
In the current study, we evaluate the association between TTI and overall survival in a national cohort of patients with liver, pancreas, and intrahepatic or extrahepatic bile duct (EHBD) cancers of all stages, and determine clinical and demographic factors associated with increased risk of delayed treatment. A secondary aim was to describe national trends in TTI related to HPB cancers. We present the following study in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1067/rc).
Methods
Database
This study was a retrospective analysis of the patients and variables reported in the National Cancer Database (NCDB). The NCDB is a clinical oncology database that collects demographic variables and treatment details for all patients with a cancer diagnosis treated at a Commission on Cancer-accredited facility (28). Data are extracted from medical records by trained tumor registrars.
The NCDB reports TTI for each case, defined as “the number of days between Date of Initial Diagnosis (NAACCR Item #390) and the Date of First Course of Treatment [surgery, radiation, systemic, or other therapy] (NAACCR Item #1270) of the patient began at any facility” (29). The date of initial diagnosis is recorded as the date of earliest confirmation of the tumor, whether clinically or histologically, as documented in the medical record by a treating physician.
As the NCDB is a deidentified database, this research study did not qualify as human subjects research and did not meet criteria for review following processes outlined by our Institutional Review Board. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study population
The NCDB Participant User Files for cancers of the pancreas, liver, and intrahepatic and EHBDs were acquired for patients diagnosed between 2004 and 2017. Inclusion criteria were histology demonstrating the most common malignancies for each organ, as detailed in Table S1. Neuroendocrine tumors were excluded. Only patients 18 years or older were included. Sample size was determined by number of cases reported in the NCDB.
Patients with no TTI data were excluded. Patients with TTI less than 3 days were also excluded, as these patients evidently did not follow a conventional treatment pathway, diagnostic surgeries may have been misinterpreted as curative resections, and a patient’s treatment on the day of or days following cancer diagnosis may have been initiated in a non-elective setting. Patients with TTI greater than 1 year (365 days) were also excluded, as were patients with unknown staging.
Statistical analysis
The primary outcome was overall survival. The primary predictor variable was TTI. TTI was divided into four categories: 3–30, 31–60, 61–90, and 91–365 days. These categories were chosen as clinically relevant and easily interpretable end points.
Demographic and clinical characteristics were reported for each TTI group, with differences in prevalence of variables across the groups assessed using chi-squared (χ2) tests or one-way analysis of variance (ANOVA). Post-hoc z-tests for categorical variables and Tukey’s Honestly Significant Difference (HSD) for the continuous variables were used to make pairwise comparisons between TTI groups with a significant omnibus test; α (alpha) of 0.05 corrected with Bonferroni adjustment was utilized as an adjusted significance threshold.
Temporal TTI trends were examined across years stratified by a variety of demographic and treatment variables, with medians compared using a one-way ANOVA test followed by Tukey’s HSD for pairwise comparisons, with Bonferroni adjustment applied.
Kaplan-Meier survival analysis and log-rank testing were performed to compare survival between patients in the prespecified TTI time frames for every stage of each cancer type. Patients diagnosed in 2017 were excluded from survival analyses, as survival information was not available for these patients at the time of the primary analysis. Patients lost to follow up were censored at time lost. P<0.05 was considered significant. Multivariable Cox hazards regressions adjusting for age (<65 vs. ≥65), sex, year of diagnosis (before or after 2013), race, Hispanic ethnicity, geographic region, facility urbanicity, patient insurance, facility type, and Charlson-Deyo score were performed to estimate hazard ratios associated with each TTI time frame for every stage of all cancer types.
These factors were chosen as covariates because each is either a known prognostic factor for HPB cancer or has been shown to be related to health or healthcare disparities for these tumors (30-34). Age and year of diagnosis were categorized as binary variables. An age cutoff of 65 was chosen as previous studies have demonstrated risk for adverse outcome after treatment of HPB cancers increases beginning at age ≥65 (35-37). Year of diagnosis was included to adjust for evolutions in management and healthcare delivery over time, with the median year of diagnosis [2013] used as a split point.
Sensitivity analyses were conducted to assess the influence of covariate adjustment and TTI categorization on the results of the survival analyses. Results of Cox regressions with progressive covariate adjustment are reported, and the fully-adjusted Cox regression was additionally performed with TTI as a binary variable with split point at TTI of 30 days and again with split point at 60 days.
Linear regression adjusting for the same covariates and additionally for treatment at more than one facility, treatment modality, and cancer type was performed with TTI in days as the continuous dependent variable to identify factors associated with TTI.
For all analyses, cases with missing data were excluded by listwise deletion on an analysis-by-analysis basis, as most variables had fewer than 5% of values missing. Data were analyzed using IBM SPSS Statistics, version 26 (IBM Corporation, Armonk, New York) and RStudio, version 1.4.1717 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population characteristics
During the study period, there were 737,400 HPB cancer cases. After exclusion criteria were applied (Figure S1), 318,931 patients were left for analysis: 23,934 with EHBD cholangiocarcinoma or adenocarcinoma, 180,714 with pancreatic adenocarcinoma or ductal carcinoma, 98,515 with hepatocellular cancer (HCC), and 15,768 with intrahepatic bile duct (IHBD) cholangiocarcinoma or adenocarcinoma.
Patient and treatment facility characteristics of each TTI cohort are reported in Table 1. In the study population, 48.6% of patients were treated within 3 to 30 days, 31.6% in 31 to 60 days, 10.9% in 61 to 90 days, and 8.8% in >90 days. The proportions treated in each time frame varied by cancer type (Table S2). Median TTI was 31 days (IQR, 18–49 days) for EHBD cancer, 26 days (IQR, 16–40 days) for pancreatic cancer, 48 days (IQR, 28–79 days) for HCC, and 37 days (IQR, 22–58 days) for IHBD cancer. Mean follow up for the cohort was 21.6 months, and was longer for patients with longer TTI.
Table 1
| Characteristics | Time to treatment initiation (days) | Total (n=318,931) | |||
|---|---|---|---|---|---|
| 3–30 (n=155,126) | 31–60 (n=100,849) | 61–90 (n=34,788) | 91+ (n=28,168) | ||
| Age (years), mean ± SD | 65.2±11.0a | 65.9±10.8b | 65.6±10.7c | 63.8±10.3d | 65.3±10.9 |
| Sex (male), n [%] | 88,657 [57]a | 60,015 [60]b | 22,565 [65]c | 19,415 [69]d | 190,652 [60] |
| Race, n [%] | |||||
| White | 128,875 [84]a | 81,348 [82]b | 27,081 [79]c | 20,992 [75]d | 258,296 [82] |
| Black | 16,838 [11]a | 12,671 [13]b | 4,946 [14]c | 4,578 [16]d | 39,033 [12] |
| Other | 7,913 [5]a | 5,849 [6]b | 2,423 [7]c | 2,287 [8]d | 18,472 [6] |
| Unknown | 1,500 [1] | 981 [1] | 338 [1] | 311 [1] | 3,130 [1] |
| Ethnicity, n [%] | |||||
| Hispanic | 9,039 [6]a | 7,036 [7]b | 3,206 [10]c | 3,534 [13]d | 22,815 [7] |
| Non-Hispanic | 139,194 [94]a | 89,987 [93]b | 30,338 [90]c | 23,694 [87]d | 283,213 [93] |
| Unknown | 6,893 [4] | 3,826 [4] | 1,244 [4] | 940 [3] | 12,903 [4] |
| Facility location, n [%] | |||||
| Northeast | 33,199 [22]a | 23,713 [24]b | 7,783 [23]c | 6,337 [23]c | 71,032 [23] |
| South | 56,429 [37]a | 35,757 [36]b | 12,614 [37]a,b | 10,049 [36]b | 114,849 [37] |
| Midwest | 41,928 [27]a | 24,222 [24]b | 7,620 [22]c | 5,221 [19]d | 78,991 [25] |
| West | 21,323 [14]a | 15,939 [16]b | 6,431 [19]c | 6,286 [23]d | 49,979 [16] |
| Unknown | 2,247 [1] | 1,218 [1] | 340 [1] | 275 [1] | 4,080 [1] |
| Facility county, n [%] | |||||
| Metropolitan | 126,856 [85]a | 82,894 [85]b | 28,880 [86]c | 23,681 [87]d | 262,311 [85] |
| Urban | 20,254 [14]a | 12,827 [13]a | 4,215 [13]b | 3,146 [12]c | 40,442 [13] |
| Rural | 2,689 [2]a | 1,553 [2]b | 485 [1]b,c | 336 [1]c | 5,063 [2] |
| Unknown | 5,327 [3] | 3,575 [4] | 1,208 [4] | 1,005 [4] | 11,115 [4] |
| Median income, n [%] | |||||
| <$38,000 | 24,676 [17]a | 17,073 [18]b | 6,518 [20]c | 5,761 [22]d | 54,028 [18] |
| $38,000–$47,999 | 32,950 [23]a | 21,931 [23]b | 7,796 [24]c | 6,148 [24]b,c | 68,825 [23] |
| $48,000–$62,999 | 39,060 [27]a | 25,190 [27]a | 8,529 [27]a | 6,831 [26]a | 79,610 [27] |
| ≥$63,000 | 49,832 [34]a | 29,732 [32]b | 9,330 [29]c | 7,143 [28]d | 96,037 [32] |
| Unknown | 8,608 [6] | 6,923 [7] | 2,615 [8] | 2,285 [8] | 20,431 [6] |
| No high school degree, n [%] | |||||
| >21.0% | 23,980 [16]a | 16,836 [18]b | 6,822 [21]c | 6,363 [25]d | 54,001 [18] |
| 13.0–20.9% | 36,420 [25]a | 24,704 [26]b | 8,763 [27]c | 7,277 [28]c | 77,164 [26] |
| 7.0–12.9% | 47,744 [33]a | 30,801 [33]a | 10,123 [32]b | 7,598 [29]c | 96,266 [32] |
| <7.0% | 38,455 [26]a | 21,626 [23]b | 6,479 [20]c | 4,661 [18]d | 71,221 [24] |
| Unknown | 8,527 [6] | 6,882 [7] | 2,601 [8] | 2,269 [8] | 20,279 [6] |
| Insurance, n [%] | |||||
| None | 4,558 [3]a | 2,653 [3]b | 1,095 [3]a | 1,065 [4]c | 9,371 [3] |
| Private | 58,399 [38]a | 34,108 [35]b | 10,862 [32]c | 8,894 [32]c | 112,263 [36] |
| Government | 89,014 [59]a | 61,804 [63]b | 22,105 [65]c | 17,659 [64]c | 190,582 [61] |
| Unknown | 3,155 [2] | 2,284 [2] | 726 [2] | 550 [2] | 6,715 [2] |
| Facility type, n [%] | |||||
| Community | 8,745 [6]a | 5,038 [5]b | 1,486 [4]c | 981 [4]d | 16,250 [5] |
| Comprehensive | 50,035 [33]a | 27,334 [27]b | 8,432 [25]c | 5,508 [20]d | 91,309 [29] |
| Academic | 73,146 [48]a | 54,531 [55]b | 20,415 [59]c | 18,320 [66]d | 166,412 [53] |
| Network | 20,953 [14]a | 12,728 [13]b | 4,115 [12]c | 3,084 [11]d | 40,880 [13] |
| Unknown | 2,247 [1] | 1,218 [1] | 340 [1] | 275 [1] | 4,080 [1] |
| Year of diagnosis, n [%] | |||||
| 2004–2012 | 81,361 [52]a | 48,403 [48]b | 16,551 [48]b | 13,372 [48]b | 159,687 [50] |
| 2013–2017 | 73,765 [48]a | 52,446 [52]b | 18,237 [52]b | 14,796 [53]b | 159,244 [50] |
| Charlson-Deyo score, n [%] | |||||
| 0 | 101,645 [66]a | 63,187 [63]b | 20,213 [58]c | 15,473 [55]d | 200,518 [63] |
| 1 | 36,650 [24]a | 23,813 [24]a | 8,280 [24]a | 6,592 [23]a | 75,335 [24] |
| 2 | 9,336 [6]a | 6,989 [7]b | 2,900 [8]c | 2,472 [9]c | 21,697 [7] |
| 3+ | 7,495 [5]a | 6,860 [7]b | 3,395 [10]c | 3,631 [13]d | 21,281 [7] |
| Treatment at >1 CoC facility, n [%] | |||||
| Yes | 30,725 [20]a | 22,740 [23]b | 7,739 [22]b | 5,664 [20]a | 66,868 [21] |
| No | 124,401 [80]a | 78,109 [77]b | 27,049 [78]b | 22,504 [80]a | 252,063 [79] |
| Primary surgery only, n [%] | |||||
| Yes | 16,706 [11]a | 14,022 [14]b | 5,832 [17]c | 5,829 [21]d | 42,389 [13] |
| No | 135,820 [89]a | 85,489 [86]b | 28,579 [83]c | 22,020 [79]d | 271,908 [87] |
| Unknown | 2,600 [2] | 1,338 [1] | 377 [1] | 319 [1] | 4,634 [2] |
| Radiation only, n [%] | |||||
| Yes | 4,335 [3]a | 5,424 [6]b | 3,648 [11]c | 3,478 [13]d | 16,885 [5] |
| No | 147,731 [97]a | 93,678 [94]b | 30,552 [89]c | 24,247 [87]d | 296,208 [95] |
| Unknown | 3,060 [2] | 1,747 [2] | 588 [2] | 443 [2] | 5,838 [2] |
| Chemotherapy only, n [%] | |||||
| Yes | 75,900 [50]a | 46,871 [48]b | 15,205 [45]c | 11,779 [43]d | 149,755 [48] |
| No | 75,574 [50]b | 51,825 [52]b | 18,869 [55]c | 15,784 [57]d | 162,052 [52] |
| Unknown | 3,652 [2] | 2,153 [2] | 714 [2] | 605 [2] | 7,124 [2] |
| 3–30 (n=155,126) | 31–60 (n=100,849) | 61–90 (n=34,788) | 91+ (n=28,168) | ||
| Combination therapye, n [%] | |||||
| Yes | 51,585 [33]a | 30,964 [31]b | 8,776 [25]c | 5,967 [21]d | 97,292 [31] |
| No | 102,803 [67]a | 69,544 [69]b | 25,920 [75]c | 22,135 [79]d | 220,402 [69] |
| Unknown | 738 (0.5) | 341 (0.3) | 92 (0.3) | 66 (0.2) | 1,237 (0.4) |
| Follow-up (months), mean ± SD | 18.8±24.0a | 21.9±25.0b | 25.4±26.4c | 31.4±29.3d | 21.6±25.4 |
All variables had a significant omnibus test, indicating at least one TTI group had a different distribution for every variable. Percentages represent valid percentages (i.e., denominators exclude cases with unknown values). a,b,c,d, results of pairwise comparisons; proportions in the same row with the same superscript are not statistically different from one another at alpha =0.05 corrected for multiple comparisons with Bonferroni adjustment; e, combination therapy = utilization of more than one of the following: surgery, radiation, chemotherapy. SD, standard deviation; CoC, Commission on Cancer.
Some characteristics were observed to have stepwise lower representation with each increase in TTI (i.e., were overrepresented in the early treatment groups), such as White race (84%, 82%, 79%, 75%, respectively), treatment in a comprehensive (33%, 27%, 25%, 20%) or a network (14%, 13%, 12%, 11%) facility, Charlson-Deyo score 0 (66%, 63%, 58%, 55%), and treatment with chemotherapy only (50%, 48%, 45%, 43%) or with combination therapy (33%, 31%, 25%, 21%) (Table 1). In contrast, other characteristics had a stepwise higher representation with each increase in TTI (i.e., were overrepresented in the delayed treatment groups), including Hispanic ethnicity (6%, 7%, 10%, 13%), median income <$38,000 (17%, 18%, 20%, 22%), treatment at an academic facility (48%, 55%, 59%, 66%), Charlson-Deyo score of 3 or greater (5%, 7%, 10%, 13%), and treatment with surgery only (11%, 14%, 17%, 21%) or radiation only (3%, 6%, 11%, 13%). All pairwise differences in proportions between each TTI group for the above variables were statistically significant.
Trends over time
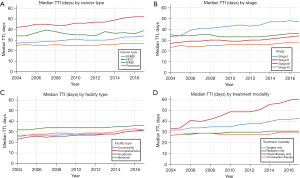
Temporal trends for TTI by cancer type, cancer stage, facility type, and treatment modality were examined in Figure 1. Median TTI was persistently longest over the years studied for liver cancer, stage I disease, treatment at an academic facility, and treatment with radiation only. A general trend of increasing median TTI from 2004 to 2017 for all patients was observed, and was most pronounced for liver cancer (median of 42 days in 2004 vs. 52 days in 2017, P<0.001), stage I cancer (35 vs. 47 days, P<0.001) and treatment with radiation only (33 vs. 61 days, P<0.001).
Survival
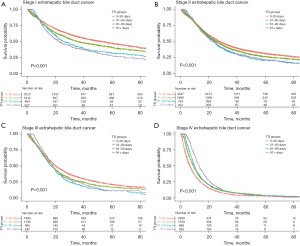
Kaplan-Meier survival analysis was performed for every stage of each cancer, stratified by the predefined TTI groups. For stage I EHBD cancer, there was a stepwise significant (pairwise log-rank P<0.005 for each step) decrease in survival associated with each increase in TTI for the first three TTI periods: median overall survivals for TTI 3–30, 31–60, 61–90, and >90 days were 51.5, 34.9, 25.4, and 22.0 months, respectively (Figure 2A). The decrease in survival between the third and fourth TTI periods did not reach statistical significance (P=0.191). A stepwise significant (P<0.05 for each step) decrease in survival was also observed for the first three TTI periods for stage II and III EHBD cancer (median survivals 27.4, 25.2, 23.6 months and 20.3, 18.1, 16.8 months, respectively) (Figure 2B,2C). There was a reversal of trend for stage IV EHBD cancer, with longer TTI associated with increased survival (Figure 2D).
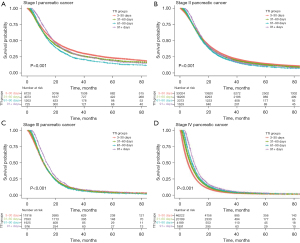
For stage I pancreatic cancer, median overall survival was significantly (P<0.01) longer for patients who were treated within 3–30 vs. 31–60 and 61–90 days (18.8, 16.6, and 15.2 months, respectively) (Figure 3A). This trend also held true for stage II pancreatic cancer, with median survival of the first three TTI groups of 16.9, 16.2, and 15.6 months, respectively. Survival differences between TTI groups for other stages of pancreatic cancer and for all stages of hepatocellular and IHBD cancer were not clinically significant, or there was an increase in survival with longer TTI (Figure 3, Figures S2,S3).
Multivariable Cox proportional hazards regressions were performed to determine the magnitude of association between TTI group and survival, adjusting for ten clinical and demographic factors (see Methods). TTI of 3–30 days was used as the reference group; results are reported in Table 2. Hazard ratios were significant and greater than one for all TTI groups of stages I–III EHBD cancer except for TTI >90 days for stage II, which was greater than one but did not reach significance (P=0.055). Hazard ratios were also significant and greater than one for stage I and II pancreatic cancer for TTI 31–60 and 61–90 days.
Table 2
| Cancer type and stage | Time to treatment initiation (days) | |||
|---|---|---|---|---|
| 3–30 | 31–60 | 61–90 | 91+ | |
| EHBD | ||||
| Stage I | REF | 1.17 (1.07–1.29)* | 1.39 (1.21–1.59)* | 1.63 (1.40–1.90)* |
| Stage II | REF | 1.07 (1.00–1.14)* | 1.17 (1.06–1.29)* | 1.14 (1.00–1.29) |
| Stage III | REF | 1.12 (1.02–1.22)* | 1.37 (1.20–1.57)* | 1.21 (1.03–1.42)* |
| Stage IV | REF | 0.79 (0.74–0.84)* | 0.74 (0.67–0.81)* | 0.61 (0.54–0.68)* |
| Pancreas | ||||
| Stage I | REF | 1.08 (1.03–1.13)* | 1.19 (1.11–1.28)* | 0.99 (0.90–1.09) |
| Stage II | REF | 1.05 (1.02–1.07)* | 1.09 (1.04–1.13)* | 1.02 (0.97–1.09) |
| Stage III | REF | 0.98 (0.94–1.01) | 0.98 (0.92–1.03) | 0.81 (0.75–0.87) |
| Stage IV | REF | 0.81 (0.79–0.82)* | 0.70 (0.67–0.72)* | 0.60 (0.57–0.63)* |
| Liver | ||||
| Stage I | REF | 0.93 (0.90–0.97)* | 0.94 (0.90–0.99)* | 0.89 (0.85–0.93)* |
| Stage II | REF | 0.92 (0.88–0.96)* | 0.91 (0.86–0.95)* | 0.82 (0.78–0.86)* |
| Stage III | REF | 0.81 (0.78–0.84)* | 0.68 (0.65–0.72)* | 0.58 (0.55–0.61)* |
| Stage IV | REF | 0.69 (0.66–0.73)* | 0.55 (0.52–0.59)* | 0.45 (0.42–0.49)* |
| IHBD | ||||
| Stage I | REF | 0.94 (0.81–1.09) | 0.94 (0.89–1.12) | 1.18 (0.98–1.41) |
| Stage II | REF | 0.78 (0.69–0.89)* | 0.84 (0.72–0.98)* | 0.68 (0.56–0.81)* |
| Stage III | REF | 0.88 (0.77–1.00)* | 0.98 (0.82–1.16) | 0.67 (0.54–0.83)* |
| Stage IV | REF | 0.77 (0.73–0.82)* | 0.68 (0.62–0.74)* | 0.64 (0.56–0.72)* |
*, P value <0.05. Covariates included in hazard regression: age (<65 vs. ≥65), sex, year of diagnosis (before or after 2013), race, Hispanic ethnicity, geographic region, facility urbanicity, patient insurance, facility type, and Charlson-Deyo score. REF, reference group; TTI, time to treatment initiation; IHBD, intrahepatic bile duct; EHBD, extrahepatic bile duct.
The robustness of these survival associations was confirmed by sensitivity analyses (Tables S3-S7), which had similar results. In some models, the hazard ratio associated with TTI >90 days for stage II EHBD cancer was statistically significant. Results were also similar when TTI was considered as a binary variable, whether delayed treatment was defined as >30 or >60 days (Table S7).
Predictors of TTI
Multivariable linear regression was performed against TTI in days for all cancer types in order to determine clinical and demographic predictors of longer TTI (Table 3). Regression coefficient estimates were largest in magnitude for cancer type, with liver cancer associated with the longest TTI (β=+22.2 days vs. pancreatic cancer). Treatment modality was the next strongest predictor, with treatment by radiation only associated with the greatest increase in TTI (β=+13.9 days vs. treatment with a non-radiation modality or combination therapy). Other factors with significant impacts on TTI were stage IV disease (β=–13.7 days vs. stage I), Black race (β=+4.6 days vs. White race), Hispanic ethnicity (β=+4.3 days), and treatment at more than one facility (β=+4.1 days).
Table 3
| Patient or facility characteristic | B coefficient (days) | 95% CI | P value |
|---|---|---|---|
| Age, ≥65 | –2.9 | –3.2 to –2.6 | <0.001 |
| Sex, female | –0.3 | –0.5 to 0.02 | 0.074 |
| Year of diagnosis, 2013–2017 | +0.7 | 0.4 to 1.0 | <0.001 |
| Race | |||
| White | REF | REF | REF |
| Black | +4.6 | 4.2 to 5.1 | <0.001 |
| Other | –1.5 | –2.1 to –0.9 | <0.001 |
| Hispanic ethnicity | +4.3 | 3.8 to 4.8 | <0.001 |
| Location | |||
| Northeast | REF | REF | REF |
| South | –1.7 | –2.0 to –1.3 | <0.001 |
| Midwest | –3.2 | –3.6 to –2.8 | <0.001 |
| West | +4.0 | 3.6 to 4.5 | <0.001 |
| Urbanicity | |||
| Metropolitan | REF | REF | REF |
| Urban | +0.2 | –0.2 to 0.6 | 0.252 |
| Rural | –0.2 | –1.3 to 0.8 | 0.672 |
| Insurance | |||
| None | REF | REF | REF |
| Private | –1.8 | –2.6 to –1.0 | <0.001 |
| Government | +1.9 | 1.1 to 2.7 | <0.001 |
| Facility | |||
| Community | REF | REF | REF |
| Comprehensive | –1.7 | –2.3 to –1.1 | <0.001 |
| Academic | +3.1 | 2.5 to 3.7 | <0.001 |
| Network | –1.3 | –2.0 to –0.6 | <0.001 |
| Charlson-Deyo score | |||
| 0 | REF | REF | REF |
| 1 | +0.3 | –0.0 to 0.6 | 0.052 |
| 2 | +1.7 | 1.2 to 2.2 | <0.001 |
| 3+ | +2.9 | 2.4 to 3.5 | <0.001 |
| Stage | |||
| I | REF | REF | REF |
| II | –5.7 | –6.1 to –5.3 | <0.001 |
| III | –8.6 | –9.1 to –8.2 | <0.001 |
| IV | –13.7 | –14.2 to –13.2 | <0.001 |
| Cancer type | |||
| Pancreas | REF | REF | REF |
| Liver | +22.2 | 21.8 to 22.6 | <0.001 |
| IHBD | +12.6 | 11.9 to 13.2 | <0.001 |
| EHBD | +5.5 | 4.9 to 6.0 | <0.001 |
| Treatment >1 CoC facility | +4.1 | 3.7 to 4.4 | <0.001 |
| Primary surgery only | +1.0 | –0.1 to 2.2 | 0.075 |
| Radiation only | +13.9 | 12.7 to 15.1 | <0.001 |
| Chemotherapy only | +3.7 | 2.6 to 4.8 | <0.001 |
| Combination therapya | –1.4 | –2.5 to –0.3 | 0.014 |
a, combination therapy = utilization of more than one of the following: surgery, radiation, chemotherapy. REF, reference group; CI, confidence interval; IHBD, intrahepatic bile duct; EHBD, extrahepatic bile duct; CoC, Commission on Cancer.
Discussion
This study in a national cohort investigating the association of TTI with outcomes of patients with HPB cancers found a significant negative association of longer TTI with overall survival for stages I–III EHBD cancer and stages I and II pancreatic cancer. Underscoring this finding, patients with stage I EHBD cancer who started treatment within 3 to 30 days had a median survival that was more than twice as long as those treated within 61 to 90 days (51.5 vs. 25.4 months, P<0.001). Moreover, TTI and survival exhibited a dose-response relationship for all stages I–III EHBD cancer. Even after adjusting for differences in the populations treated within each time frame, the hazard ratios for mortality associated with longer TTI remained significant for early stage EBHD and pancreatic cancer, and increased with each additional delay.
To our knowledge, this is the first study to describe the association of delay in treatment with worse survival for EHBD cancer patients. As we hypothesized, this may be attributable to the aggressiveness of the cancer’s biology, such that even a month’s delay in care leads to progression of disease and worsening of outcomes. It is also possible that a subset of poor prognosis patients, such as those with refractory obstructive jaundice, see longer treatment delays due to prolonged efforts to optimize the patient prior to surgery. For example, patients with complex disease extending into the intrahepatic ducts may require repeated endoscopic and percutaneous biliary tract interventions and referral to an academic center. Data from this study support coordination of complex care as a contributing factor to delayed treatment for at least the subset of patients treated between 31 and 90 days, given that this group had a higher rate of treatment at more than one facility compared to those treated within 30 days or after 90 days. These patients can also be expected to be more surgically challenging and may be less likely to have an R0 resection, more likely to suffer a complication of treatment, and more likely to die. Moreover, any progression in these patients with complex disease is likely to have an outsized impact on their outcome, and may make a negative margin resection even more challenging.
With respect to the current literature, our EHBD cancer results stand in contrast to a study in a related population, which retrospectively reviewed 355 patients with periampullary adenocarcinoma and found that timing of resection was not associated with survival (9). Regarding pancreatic adenocarcinoma, the results of our study corroborate existing evidence that prompt treatment of early stage disease is associated with slightly improved survival, on the order of 1–3 months (1,38), while timing of treatment for late-stage disease is of lesser or no importance (1,10,12). This finding is possibly attributable to low overall survival rates for patients with late-stage pancreatic cancer regardless of therapy (25).
Notably, for metastatic EHBD and pancreatic cancer—in addition to all stages of HCC and IHBD cancer—longer TTI was largely associated with longer survival. This seemingly paradoxical finding of lower mortality with longer delay in treatment has been retrospectively demonstrated before in non-small cell lung cancer (39,40). This observation is likely due to a selection bias amongst clinicians favoring expedited treatment for patients with features of more aggressive disease, such as high symptom burden, such that patients triaged for early treatment may be those with the most concerning presentations and worst prognoses. In this setting, any negative survival impact due to a delay in care must necessarily overcome the survival disparity introduced by clinician triage in order to be retrospectively observed. Although more detailed clinical information would be helpful in further clarifying the impact of TTI on survival in HCC and IHBD cancer, the results of this study demonstrate that clinicians currently triage these patients effectively such that those treated in a delayed fashion have outcomes comparable to patients with similar disease burden treated more expeditiously. This also appears to be the case for metastatic EHBD and pancreatic cancer—but for earlier stages of these two diseases, the patients who are treated first have the best outcomes; thus, TTI in early stages of these cancers may have a sufficiently strong impact on survival to overcome the effect of clinician triage. This finding warrants further investigation.
With respect to the current literature, these findings are novel. The association of treatment timing with outcomes has not been previously studied in IHBD cancer. In liver cancer, a single retrospective analysis of 267 patients found a TTI of greater than 3 months to be associated with worse survival (18).
Trends and predictors of TTI
Our study also draws attention to national trends in TTI and to factors associated with longer TTI for patients with HPB cancer. One major finding from our study is that time to treatment differs significantly by patient race and ethnicity, with Black race and Hispanic ethnicity associated with delays in care. In fact, Hispanic patients represented more than 1 in 8 patients starting treatment after 90 days, compared to less than 1 in 16 patients starting treatment within 30 days. This finding reinforces previous observations of racial and ethnic disparities in the presentation, treatment, and survival of patients with HPB cancer (41-43), and identifies a possible target for interventions aimed at combating outcomes disparities in cancer care. Cancer treatment centers should work to study and address obstacles to expedient care that disproportionately affect the minority patient populations seeking treatment at their institution.
Early stage disease was also associated with significantly longer TTI than late stage disease. This finding is intuitively explained by the typical treatment pathways for these populations: work up and management decisions for early stage disease often require multidisciplinary evaluation and planning, whereas treatment options for late stage disease tend to be limited and management directed primarily by one specialist. Furthermore, patients with later stage disease may be more symptomatic, thereby adding a level of urgency to their therapy.
To a lesser degree, insurance status and facility type were also found to be predictors of TTI. Private insurance was found to be associated with significantly shorter TTI than not having insurance. This reflects previously described insurance-related disparities in quality of cancer care (44,45), and represents another possible target population for access interventions. Last, treatment at an academic center was associated with longer TTI, but this is likely attributable to referral patterns and differences in patient population: academic institutions may serve as the treating facility for patients diagnosed at other hospitals, particularly the most complex patients, given the highly specialized care required for the treatment of many HPB tumors.
In examining national trends, one noteworthy finding of our study is that time to initiation of radiation monotherapy increased by nearly 80% between 2004 and 2017. This finding may represent a deprioritization of radiation in the management of HPB cancers over time, as gemcitabine- and FOLFIRINOX-based chemotherapy regimens have increased response rates to systemic therapy and taken center stage. Given the lesser role of radiation in the curative treatment of HPB cancers, this observation may also represent a trend related to longer postponements of palliative intervention.
Limitations
Our study has several limitations. First, this was a retrospective study, and the timeline for initiation of definitive oncologic care is influenced by a wide variety of individual patient and facility factors, many of which were not possible to control for in this analysis. For example, a delay in treatment initiation may be facility-driven due to limited resources such as operating room availability; provider-driven to allow time for optimization of patient comorbidities; or patient-driven due to difficulty with transportation, receiving time off work, low health literacy, scheduling conflicts, or other health circumstances. Some of these factors affecting timing of care might also be independent predictors of outcome and therefore bias our results. Intrinsic limitations to the NCDB also apply to our study, including inability to assess for non-reported outcomes (e.g., cancer-specific survival) and control for non-reported variables (e.g., patient symptoms, prior surgery, serum albumin). The NCDB also does not capture all cancer diagnoses in the United States, but does capture more than two-thirds, allowing for inclusion in this study of men and women and a wide range of ages, geographic location, income, and types of treatment facilities, increasing the external validity of these results. Last, the data analyzed in this study are from prior to the COVID-19 pandemic, and therefore conclusions about delays in treatment in this cohort may not be generalizable to delays in treatment due to the COVID-19 pandemic.
Conclusions
Longer time to initiation of definitive therapy is associated with increased mortality in stage I–III EHBD and stage I and II pancreatic cancer. Some patients, including those with early stage disease, Blacks, and Hispanics, are more likely to experience delayed care. Efforts should be made to better understand these disparities and mitigate them through targeted outreach to those at highest risk for treatment delay. While this retrospective study is unable to support a causative association, the strong and clinically-significant risk-adjusted relationship between delay in care and increased mortality in EHBD cancer compels further investigation. Overall, the findings underscore the importance of timely multidisciplinary evaluation and coordination of care for patients with these high-risk malignancies, and propose TTI as a possible quality metric for HPB cancer centers.
Acknowledgments
An earlier version of the abstract for this manuscript was presented at the Society of Surgical Oncology International Conference on Surgical Cancer Care in Dallas, Texas, USA in March 2022. The abstract was published in the meeting’s Abstract Book as an electronic supplement to the Annals of Surgical Oncology (PubMed ID 35469114). The authors thank Dr. Marina Horiates Kerekes for her help with manuscript editing.
Funding: This work was supported by the National Institutes of Health Clinical and Translational Science Awards (No. UL1 TR001863), and Lampman Yale Surgical Oncology funding.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1067/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1067/coif). BFB reports funding from National Institute of Diabetes and Digestive and Kidney Diseases (No. T35DK104689). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khorana AA, Tullio K, Elson P, et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS One 2019;14:e0213209. [Crossref] [PubMed]
- Murphy CT, Galloway TJ, Handorf EA, et al. Increasing time to treatment initiation for head and neck cancer: an analysis of the National Cancer Database. Cancer 2015;121:1204-13. [Crossref] [PubMed]
- Lin NU, Bichkoff H, Hassett MJ. Increasing Burden of Prior Authorizations in the Delivery of Oncology Care in the United States. J Oncol Pract 2018;14:525-8. [Crossref] [PubMed]
- Neumann E. Overwhelmed With COVID Patients, Oregon Hospitals Postpone Surgeries And Cancer Care. NPR [Internet]. 2021 Sep 16 [cited 2021 Oct 29]; Available online: https://www.npr.org/sections/health-shots/2021/09/16/1037621883/overwhelmed-with-covid-patients-oregon-hospitals-postpone-surgeries-and-cancer-c
- Neumann E, Radio JP. Covid-Overwhelmed Hospitals Postpone Cancer Care and Other Treatment [Internet]. Kaiser Health News. 2021 [cited 2021 Oct 29]. Available online: https://khn.org/news/article/covid-overwhelmed-hospitals-postpone-cancer-care-and-other-treatment/
- Boutros M, Moujaess E, Kourie HR. Cancer management during the COVID-19 pandemic: Choosing between the devil and the deep blue sea. Crit Rev Oncol Hematol 2021;167:103273. [Crossref] [PubMed]
- The Lancet Oncology. Valuing all lives equally: cancer surgery, COVID-19, and the NHS in crisis. Lancet Oncol 2021;22:155. [Crossref] [PubMed]
- Pruitt SL, Harzke AJ, Davidson NO, et al. Do diagnostic and treatment delays for colorectal cancer increase risk of death? Cancer Causes Control 2013;24:961-77. [Crossref] [PubMed]
- McLean SR, Karsanji D, Wilson J, et al. The effect of wait times on oncological outcomes from periampullary adenocarcinomas. J Surg Oncol 2013;107:853-8. [Crossref] [PubMed]
- Kruger S, Schirle K, Haas M, et al. Prolonged time to treatment initiation in advanced pancreatic cancer patients has no major effect on treatment outcome: a retrospective cohort study controlled for lead time bias and waiting time paradox. J Cancer Res Clin Oncol 2020;146:391-9. [Crossref] [PubMed]
- Cone EB, Marchese M, Paciotti M, et al. Assessment of Time-to-Treatment Initiation and Survival in a Cohort of Patients With Common Cancers. JAMA Netw Open 2020;3:e2030072. [Crossref] [PubMed]
- Gbolahan O, Hashemi-Sadraei N, Yash S, et al. Time to treatment initiation and its impact on real-world survival in metastatic colorectal cancer and pancreatic cancer. Cancer Med 2023;12:3488-98. [Crossref] [PubMed]
- Kouka M, Engelhardt M, Wittig A, et al. No impact of time to treatment initiation for head and neck cancer in a tertiary university center in 2003, 2008 and 2013. Eur Arch Otorhinolaryngol 2022;279:4549-60. [Crossref] [PubMed]
- Edwards GC, Gamboa AC, Feng MP, et al. What's the magic number? Impact of time to initiation of treatment for rectal cancer. Surgery 2022;171:1185-92. [Crossref] [PubMed]
- Bleicher RJ, Ruth K, Sigurdson ER, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA Oncol 2016;2:330-9. [Crossref] [PubMed]
- Dolly D, Mihai A, Rimel BJ, et al. A Delay from Diagnosis to Treatment Is Associated with a Decreased Overall Survival for Patients with Endometrial Cancer. Front Oncol 2016;6:31. [Crossref] [PubMed]
- Fahmy NM, Mahmud S, Aprikian AG. Delay in the surgical treatment of bladder cancer and survival: systematic review of the literature. Eur Urol 2006;50:1176-82. [Crossref] [PubMed]
- Singal AG, Waljee AK, Patel N, et al. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J Natl Compr Canc Netw 2013;11:1101-8. [Crossref] [PubMed]
- Redaniel MT, Martin RM, Cawthorn S, et al. The association of waiting times from diagnosis to surgery with survival in women with localised breast cancer in England. Br J Cancer 2013;109:42-9. [Crossref] [PubMed]
- Bell D, Morash C, Dranitsaris G, et al. Does prolonging the time to testicular cancer surgery impact long-term cancer control: a systematic review of the literature. Can J Urol 2006;13:30-6. [PubMed]
- Brazda A, Estroff J, Euhus D, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol 2010;17:291-6. [Crossref] [PubMed]
- Öztürk Ç, Fleer J, Hoekstra HJ, et al. Delay in Diagnosis of Testicular Cancer; A Need for Awareness Programs. PLoS One 2015;10:e0141244. [Crossref] [PubMed]
- Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med 2016;375:1415-24. [Crossref] [PubMed]
- Hanna NH, Einhorn LH. Testicular cancer--discoveries and updates. N Engl J Med 2014;371:2005-16. Erratum in: N Engl J Med 2014;371:2342. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Jang JY, Kim SW, Park DJ, et al. Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg 2005;241:77-84. [Crossref] [PubMed]
- National Cancer Database [Internet]. American College of Surgeons. [cited 2021 Oct 27]. Available online: http://www.facs.org/quality-programs/cancer/ncdb
- The American College of Surgeons Commission on Cancer. National Cancer Database Participant User File 2018 Data Dictionary. National Cancer Database; 2020.
- Shapiro M, Chen Q, Huang Q, et al. Associations of Socioeconomic Variables With Resection, Stage, and Survival in Patients With Early-Stage Pancreatic Cancer. JAMA Surg 2016;151:338-45. [Crossref] [PubMed]
- Chang YS, Huang JS, Yen CL, et al. The Charlson Comorbidity Index is an Independent Prognostic Factor for Treatment-Naïve Hepatocellular Carcinoma Patients with Extrahepatic Metastases. Hepatogastroenterology 2015;62:1011-5. [PubMed]
- Chu QD, Zhou M, Peddi P, et al. Influence of facility type on survival outcomes after pancreatectomy for pancreatic adenocarcinoma. HPB (Oxford) 2017;19:1046-57. [Crossref] [PubMed]
- Sutton TL, Walker BS, Nabavizadeh N, et al. Geographic Disparities in Referral Rates and Oncologic Outcomes of Intrahepatic Cholangiocarcinoma: A Population-Based Study. Ann Surg Oncol 2021;28:8152-9. [Crossref] [PubMed]
- Kirkegård J, Ladekarl M, Fristrup CW, et al. Urban versus rural residency and pancreatic cancer survival: A Danish nationwide population-based cohort study. PLoS One 2018;13:e0202486. [Crossref] [PubMed]
- Chen YT, Ma FH, Wang CF, et al. Elderly patients had more severe postoperative complications after pancreatic resection: A retrospective analysis of 727 patients. World J Gastroenterol 2018;24:844-51. [Crossref] [PubMed]
- Yin L, Zhao S, Zhu H, et al. Primary tumor resection improves survival in patients with multifocal intrahepatic cholangiocarcinoma based on a population study. Sci Rep 2021;11:12166. [Crossref] [PubMed]
- Ruzzenente A, Conci S, Ciangherotti A, et al. Impact of age on short-term outcomes of liver surgery: Lessons learned in 10-years' experience in a tertiary referral hepato-pancreato-biliary center. Medicine (Baltimore) 2017;96:e6955. [Crossref] [PubMed]
- Gobbi PG, Bergonzi M, Comelli M, et al. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol 2013;37:186-90. [Crossref] [PubMed]
- Anggondowati T, Ganti AK, Islam KMM. Impact of time-to-treatment on overall survival of non-small cell lung cancer patients-an analysis of the national cancer database. Transl Lung Cancer Res 2020;9:1202-11. [Crossref] [PubMed]
- Diaconescu R, Lafond C, Whittom R. Treatment delays in non-small cell lung cancer and their prognostic implications. J Thorac Oncol 2011;6:1254-9. [Crossref] [PubMed]
- de Geus SW, Sachs TE, Ng SCW, et al. Racial/ethnic disparities in the use of high-volume centers for hepatobiliary and pancreatic cancer surgery. J Clin Oncol 2019;37:abstr 457.
- Mathur AK, Osborne NH, Lynch RJ, et al. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg 2010;145:1158-63. [Crossref] [PubMed]
- Xu L, Kim Y, Spolverato G, et al. Racial disparities in treatment and survival of patients with hepatocellular carcinoma in the United States. Hepatobiliary Surg Nutr 2016;5:43-52. [PubMed]
- Parikh-Patel A, Morris CR, Kizer KW. Disparities in quality of cancer care: The role of health insurance and population demographics. Medicine (Baltimore) 2017;96:e9125. [Crossref] [PubMed]
- Yabroff KR, Reeder-Hayes K, Zhao J, et al. Health Insurance Coverage Disruptions and Cancer Care and Outcomes: Systematic Review of Published Research. J Natl Cancer Inst 2020;112:671-87. [Crossref] [PubMed]


