
The predictive impact of dual somatostatin receptor/fluorodeoxyglucose (FDG) positron emission tomography (PET) in metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs): review of literature and a single institution experience
Introduction
Neuroendocrine tumors (NETs) are heterogeneous neoplasms that develop from secretory cells of the diffuse endocrine system and can originate in numerous organs, including the gastrointestinal tract (1). These tumors have been increasing in incidence and gastro-enteropancreatic NETs (GEP-NETs) account for a majority of these increasing cases (2). The treatment of GEP-NETS can be complicated and varies according to many factors, including the tumor histological differentiation, grade, somatostatin receptor (SSTR) expression, and tumor burden. In 2018, the US Food and Drug Administration (FDA) approved lutetium177 (177Lu)-DOTATATE as a new treatment modality for metastatic midgut and pancreatic NETs. This peptide receptor radionuclide therapy (PRRT) demonstrated improvement of progression-free survival (PFS) compared with high dose octreotide long acting release (LAR) 60 mg in a randomized phase 3 NETTER-1 trial {PFS at the 20th month, 65.2% [95% confidence interval (CI): 50.0–76.8%] vs. 10.8% (95% CI: 3.5–23.0%)} (3). Both tumor grade and increased expression of SSTRs in metastatic lesions have significant predictive roles for PRRT stratification (3,4). However, there is subgroup of patients with low/intermediate grade (G1/2) tumors, and intense SSTR expression who progress during or shortly after PRRT treatment, highlighting a need for other potential predictive biomarkers.
18F-fluorodeoxyglucose (FDG) PET avidity reflects increased tumor metabolic activity; it has been associated with higher histological grade and significantly poorer survival in NETs (5,6). Current guidelines recommend FDG PET mainly for patients with higher grade (grade 3) and poorly differentiated neuroendocrine neoplasms and limit use for those with lower grade, well-differentiated tumors (grades 1&2) despite the fact that previous data have demonstrated the prognostic value of FDG positivity in lower grade GEP-NETs. Patients with grades 1&2 GEP-NETs with positive 18F-FDG and SSTR PET have significantly lower PFS and overall survival (OS) compared to those with negative 18F-FDG PET (6,7). These results have demonstrated the prognostic value of FDG positivity in GEP-NETs. The available data for predictive value of dual SSTR/FDG PET are limited (6,7). Therefore, the objective of this literature review and case series is to assess the predictive role of FDG PET combined with SSTR 68Ga-PET for better PRRT stratification in patients with low and intermediate grade GEP-NETs.
Methods
We performed a review of the literature using PubMed, Cochrane CENTRAL, Embase, Medline, the National Institutes of Health trial registry, and published proceedings from major oncologic and gastrointestinal cancer meetings include [The North American Neuroendocrine Tumor Society (NANETs), The European Neuroendocrine Tumor Society (ENETS), Society of Nuclear Medicine and Molecular Imaging (SNMMI), The American Society of Clinical Oncology (ASCO), The Gastrointestinal Cancers Symposium (GI-ASCO), and European Society for Medical Oncology (ESMO)]. A professional medical research librarian searched from 2010 through December 2021 for retrospective and prospective data related to the predictive value of dual PET scan for PRRT in NETs. PRRT was FDA approved in the United States in 2018, however, it has been used off label in Europe before that time. The following combination of terms for search included: predictive value, dual SSTR/FDG PET, 68Ga-PET, FDG PET, neuroendocrine tumor, gastroenteropancreatic neuroendocrine tumor, pancreatic neuroendocrine tumor, 177Lu-DOTATATE, and related variants of these terms. Publications were limited to clinical trials and data published in English. We included all the available data up-to-date that evaluated the predictive value of adding FDG PET to SSTR imaging for patients with GEP-NETs who were treated with PRRT and reported clinical outcomes of PFS and OS. The results were imported into EndNote, and duplicate references were eliminated. The first author is a medical oncologist (A Mohamed) who independently reviewed all search results and determined publications that met the criteria for study inclusion. Studies that did not include GEP-NETs, did not report predictive value of FDG PET, or did not include PRRT were excluded. After determining which articles were relevant, the same author independently extracted all the clinical data, reviewed and summarized it accordingly. Then summarized data were reviewed by three other authors (A Kardan, SL Asa, and Z Lee). Additionally, we summarized the outcome of eight patients with progressed advanced metastatic well-differentiated GEP-NETs who received PRRT treatment at our institution [University Hospitals Seidman Cancer Center (UH SCC), Cleveland, Ohio]. All these patients had baseline 68Ga-DOTATATE and FDG PET-computed tomography (CT) scan either at baseline or at the time of progression. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board of UH SCC, Case Western Reserve University (UH IRB, No. STUDY20191593) and informed consent was taken from all the patients.
Results
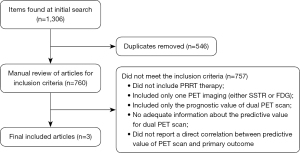
A total of 1,306 articles were identified, of which 546 were eliminated because they were duplicates. We retrieved these articles and reviewed them for preset inclusion criteria. In total, 757 papers did not meet the inclusion criteria and were removed. The most frequent reasons for exclusion were no PRRT therapy, studies that included only one PET imaging (either SSTR or FDG), and studies which included only the prognostic value without adequate information about the predictive value for dual PET scan. Only three articles met the inclusion criteria and were included in this review article (Figure 1). All of the included data were exclusively retrospective there was no available prospective data identified. We summarized the collected data for evaluation of the predictive value of dual PET scan and we summarized the case series from our single institution experience (UH SCC).
Predictive value of combined PET-CT using 68Ga-DOTATATE and 18F-FDG
Only three studies analyzed the predictive impact of combined FDG and DOTATATE PET-CT in 204 patients with metastatic GEP-NETs (8-10). These studies correlated the uptake on FDG PET with response to PRRT in patients with well-differentiated GEP-NETs.
A study by Grain et al., demonstrated that FDG PET avidity shows excellent predictive values for early tumor progression (within 6 months) in patients with low grade, well-differentiated NETs (8). The study prospectively analyzed the predictive value of FDG PET in 38 patients, 34 of which had G1 or G2 NETs. FDG PET results were positive in 15/38 (39%), and negative in 23/38 (61%) patients. Fourteen of 15 patients with positive FDG PET had early progressive disease, while 21 of 23 patients with negative FDG PET had stable disease (P<0.001). When patients with high grade and poorly differentiated tumors were excluded and the analysis solely considered low and intermediate grade tumors, the outcome was the same; 6 of the 7 patients with positive FDG PET but only 2 of the 23 with negative FDG PET had early disease progression (P<0.001). Multivariate analysis confirmed that FDG PET results were independently predictive of PFS. SSTR imaging was also useful in predicting early progressive disease, as only 3/23 patients who were SSTR-positive and 5/7 who were SSTR-negative had early progressive disease (P<0.001). This prospective analysis indicated that both FDG and SSTR PET scans can be used to recognize patients with lower grade NETs who may develop rapidly progressive disease compared to those with relatively stable disease. Therefore, FDG PET could be proposed as an extension of the assessment of low and intermediate grade metastatic GEP-NETs, and should not be reserved exclusively for those with higher grade and poorly differentiated neoplasms.
Oh et al. examined the effect of PRRT on glucose metabolism assessed by FDG PET in 25 patients with metastatic well-differentiated NETs (9). The lesions were both SSTR and FDG positive in 58.6%, only SSTR positive in 28.6% and only FDG positive in 5.3%. The study showed a complex relationship between SSTR expression and glucose metabolism with only 56% of the lesions showing correlation between the receptor status and glucose metabolism, and with a much better match with NET grade. They observed that higher tumor remission rate was significantly correlated with a high-baseline maximum standard uptake value (SUVmax) on SSTR but not on the FDG PET-CT. Interestingly, nearly all the positive FDG lesions showed good response to PRRT even in higher grade tumors (Ki-67 >20%) provided that they express SSTRs as well. When they compared the effect of PRRT according to different tumor locations, the response to PRRT was indifferent to the degree of glucose metabolism in the primary tumor, liver lesions and lymph node metastases. However, hypermetabolic bone lesions did not show any significant response to PRRT. These results are consistent with previous studies (3,4) confirming that SSTR expression is the predictive biomarker for PRRT response, and positive FDG can be useful in predicting response to PRRT in bone lesions due to different biological properties of tumor cells in bone as compared to other metastatic lesions.
Another study by Nilica et al. compared the results of FDG PET-CT and SSTR PET in 66 patients treated with PRRT (10). The study analyzed the results of 68Ga-DOTA-TOC and FDG PET-CT in the initial assessment and at 3- and 6-month follow-up after PRRT. They also evaluated whether possible changes in tumor FDG uptake correlate with disease progression. The study included 46 GEP-NETs; all were well-differentiated, and most were low and intermediate grade (12 grade 1, and 47 grade 2). All patients had SSTR-positive tumor lesions. FDG PET was positive in 38 of the 66 patients (57.6%). The results indicated that the 24 patients (36.4%) who had disease progression in the study all had FDG-positive tumors initially and during follow-up. This study is in agreement with the findings of Garin et al. (8) who confirmed that FDG-positivity correlates strongly with a higher risk of progression and has predictive value for early tumor progression. In addition, three patients with higher SUVmax had shorter OS and died during the follow-up (SUVmax values increased 41–82% from the initial assessment). These results support the value of repeating FDG PET during surveillance, given that some patients develop FDG-positive lesions with increased SUVmax during follow-up. They concluded that patients with low and intermediate grade tumors may have FDG-positivity initially and during follow-up, and this feature should be considered when optimizing PRRT planning.
UH SCC institution experience for PRRT in GEP-NETs
From January 2020 through June 2021, eight patients (3 men and 5 women; mean age, 62 years; range, 55–70 years) who progressed during or within 1 year of PRRT were included retrospectively (Table 1). All of them had metastatic well-differentiated GEP-NETs. The site of the primary tumor was mainly in the small bowel (n=7), and pancreas (n=1). Metastases were found in the liver, lymph nodes, peritoneum, and bones. The World Health Organization (WHO) grade was intermediate grade (G2) in 5 patients, and high grade (G3) in 3 patients. None of the patients had poorly differentiated neuroendocrine carcinoma (NEC). Five patients progressed on somatostatin analogues (SSA), three of them progressed after the addition of everolimus, and three patients with G3 tumors progressed on chemotherapy before receiving PRRT. Six patients completed the 5 doses of PRRT; two with G3 tumors completed only 2 doses due to clinical progression confirmed with imaging and one patient with G2 disease received only 2 doses due to nephrotoxicity. At the time of progression, all patients had both anatomical scans [either CT or magnetic resonance imaging (MRI)] and FDG PET scan. Patients were in excellent general health [performance status, the Eastern Cooperative Oncology Group (ECOG) =0], and only one patient had minor symptoms related to functional disease.
Table 1
| Patient | Gender | Age (years) | Primary tumor | Grade | PRRT doses | Time to progression (months) |
|---|---|---|---|---|---|---|
| 1 | F | 67 | SB | G3 | 2 | 6 |
| 2 | F | 50 | SB | G3 | 4 | 7 |
| 3 | M | 53 | Pan | G3 | 2 | 2 |
| 4 | F | 70 | SB | G2 | 4 | 4 |
| 5 | M | 68 | SB | G2 | 4 | 11 |
| 6 | F | 62 | SB | G2 | 2 | 3 |
| 7 | M | 64 | SB | G2 | 4 | 5 |
| 8 | F | 68 | SB | G2 | 4 | 3 |
PRRT, peptide receptor radionuclide therapy; F, female; M, male; SB, small bowel; Pan, pancreas.
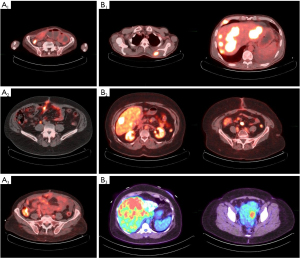
The baseline 68Ga-PET results were positive in all patients with Krenning score of 3 (100%), and they were confirmed to have an isointense homogenous SSTR expression in all lesions. The three patients with G3 tumors were also confirmed to have well-differentiated SST-avid lesions on the 68Ga-PET. The median time to progression was 4 months (range, 3–11 months). At the time of progression, FDG PET results were positive in seven patients (G2 =4 patients, G3 =3 patients) (87.5%) and negative in one patients (G2) (12.5%) (Figure 2). SUV ranged from 3.6 to 14.3 SUV (median 8.16 SUV).
Discussion
This is one of very few articles that summarized the current literature for using dual PET scan in management of GEP-NETs with description of a case series. We highlighted the potential predictive value of dual PET scan for PRRT benefit in metastatic GEP-NETs. PRRT using 177Lu-DOTATATE was approved by the FDA for treatment of advanced metastatic well-differentiated GEP-NETs based on the phase III randomized prospective NETTER-1 trial, which showed 79% reduction in risk of progression or death compared to placebo (3). The biomarkers that identify patients who will benefit from PRRT remain to be clarified. Positive SSTR imaging is the only current predictive biomarker for PRRT, however some patients with low tumor grade and SSTR positivity have early progression, indicating there are other factors that may impact PRRT outcome.
FDG PET scan is used to assess glycolytic metabolism, and higher uptake of 18F-FDG has been found to be associated with higher tumor grade and aggressiveness (5,6). Although high FDG uptake has prognostic impact and correlates with short survival in well-differentiated NETs, the data on its predictive value for PRRT are very limited. In this review, we identified only three publications that reported the predictive role of FDG PET scan in PRRT. The results confirm the association between FDG avidity and tumor aggressiveness even among lower grade NETs (G1&G2). Additionally, low or negative SSTR expression and high FDG SUVmax correlated strongly with a higher risk of early progression in low and intermediate grade GEP-NETs. These data and our series endorse the predictive power of FDG PET in PRRT planning for patients with all grades of well-differentiated GEP-NETs and not exclusively for grade 3 tumors.
In our case series, patients with low-, intermediate- and high-grade, well-differentiated GEP-NETs who had early progression (within the first year of PRRT) had FDG uptake in all the lesions that progressed; this suggests the possibility of dedifferentiation with loss of SSTR expression, given that 177Lu-PRRT delivers a therapeutic radionuclide to cells with SSTRs that facilitate uptake. It is thought that FDG-avid lesions with gain in glucose utilization represent less differentiated and more aggressive tumors that would not be targeted effectively by PRRT.
Current guidelines recommend re-biopsy for patients with grade 1 or 2 NET who have rapid progression, but do not recommend FDG PET routinely during follow-up (11,12). In our case series, we confirmed that patients with grade 2 GEP-NET who had early progression also have FDG-positive tumors during follow-up. This is concerning, as sampled tumor tissue might not be representative of all tumor lesions, and re-biopsy may not be truly representative of disease heterogeneity. Therefore, baseline and follow-up dual SSTR-FDG PET imaging should be considered for individualized PRRT planning.
The data we present have some limitations. All three previously published studies were exclusively retrospective, and some of them did not include a detailed distinction between G3 NET and poorly-differentiated NEC. Garin et al. (8) used scintigraphy with 111In instead of gallium PET to assess positivity at SSTRs. In addition, these three studies included GEP-NETs of different grades that may impacted prognosis and overall outcome. In our institution experience, the study population was small, and there was no baseline FDG PET for these patients to compare with post-treatment FDG PET. Also, there was no post-treatment 68Ga-PET or biopsy to confirm tumor heterogeneity. Therefore, a final conclusion cannot be made without a prospective trial.
Conclusions
In conclusion, our results show that 68Ga-PET and initial biopsy may not reflect disease heterogeneity in all grades of well-differentiated NETs. We show that FDG PET can be used to recognize patients with low grade NETs who will have rapid progression despite PRRT. Our findings show a discordance between histopathological grading and dual tracer PET-CT (68Ga-DOTATATE and FDG) which may have prognosticating and predictive value in GEP-NETs. This highlights the importance of including FDG PET as a complementary tool to SSTR imaging to personalize PRRT planning in patients with well-differentiated GEP-NETs. Therefore, the optimum planning for PRRT therapy should include more comprehensive evaluation with dual SSTR and FDG PET imaging before starting treatment and during follow-up. Our results need to be confirmed in a future prospective trial to evaluate the predictive value of FDG PET in detecting early progression for PRRT, particularly in patients with both histologically low and intermediate grade NETs, and to stratify pancreatic and small bowel tumors.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1011/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pearse AG, Polak JM. Neural crest origin of the endocrine polypeptide (APUD) cells of the gastrointestinal tract and pancreas. Gut 1971;12:783-8. [Crossref] [PubMed]
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Zhang J, Kulkarni HR, Singh A, et al. Peptide Receptor Radionuclide Therapy in Grade 3 Neuroendocrine Neoplasms: Safety and Survival Analysis in 69 Patients. J Nucl Med 2019;60:377-85. [Crossref] [PubMed]
- Bahri H, Laurence L, Edeline J, et al. High prognostic value of 18F-FDG PET for metastatic gastroenteropancreatic neuroendocrine tumors: a long-term evaluation. J Nucl Med 2014;55:1786-90. [Crossref] [PubMed]
- Zhang J, Liu Q, Singh A, et al. Prognostic Value of (18)F-FDG PET/CT in a Large Cohort of Patients with Advanced Metastatic Neuroendocrine Neoplasms Treated with Peptide Receptor Radionuclide Therapy. J Nucl Med 2020;61:1560-9. [Crossref] [PubMed]
- Han S, Lee HS, Woo S, et al. Prognostic Value of 18F-FDG PET in Neuroendocrine Neoplasm: A Systematic Review and Meta-analysis. Clin Nucl Med 2021;46:723-31. [Crossref] [PubMed]
- Garin E, Le Jeune F, Devillers A, et al. Predictive value of 18F-FDG PET and somatostatin receptor scintigraphy in patients with metastatic endocrine tumors. J Nucl Med 2009;50:858-64. [Crossref] [PubMed]
- Oh S, Prasad V, Lee DS, et al. Effect of Peptide Receptor Radionuclide Therapy on Somatostatin Receptor Status and Glucose Metabolism in Neuroendocrine Tumors: Intraindividual Comparison of Ga-68 DOTANOC PET/CT and F-18 FDG PET/CT. Int J Mol Imaging 2011;2011:524130. [Crossref] [PubMed]
- Nilica B, Waitz D, Stevanovic V, et al. Direct comparison of (68)Ga-DOTA-TOC and (18)F-FDG PET/CT in the follow-up of patients with neuroendocrine tumour treated with the first full peptide receptor radionuclide therapy cycle. Eur J Nucl Med Mol Imaging 2016;43:1585-92. [Crossref] [PubMed]
- Halfdanarson TR, Strosberg JR, Tang L, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020;49:863-81. [Crossref] [PubMed]
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 2017;46:707-14. [Crossref] [PubMed]


