
Phase II study of biweekly cetuximab plus mFOLFOX6 or mFOLFIRI as second-line treatment for metastatic colorectal cancer and exploratory analysis of associations between DNA methylation status and the efficacy of the anti-EGFR antibody: T-CORE1201
Highlight box
Key findings
• Biweekly cetuximab plus m FOLFOX6 or mFOLFIRI is confirmed as a useful second-line therapy for mCRC.
What is known and what is new?
• This is the first prospective study to evaluate the efficacy and safety of biweekly cetuximab plus mFOLFOX6 or mFOLFIRI in second-line therapy for mCRC.
• The mPFS and mOS of the treatment was 5.1 (95% CI, 3.8–7.6) and 12.7 (95% CI, 7.5–15.3) months, respectively.
• Grade 3 or higher neutropenia was observed in more than half of the patients, suggesting that it occurs more frequently in Japanese patients than in Western patients.
• DNA methylation status may be associated with treatment response from anti-EGFR treatment in RAS/BRAF wild-type mCRC.
What is the implication, and what should change now?
• DNA methylation status warrants further exploration as a predictive biomarker for anti-EGFR efficacy in mCRC.
Introduction
Recent advances in chemotherapy have increased the median overall survival (mOS) for metastatic colorectal cancer (mCRC) to >30 months (1-3). The role of anti-epidermal growth factor receptor (EGFR) therapies in chemotherapy for mCRC is extremely significant. RAS mutations predict the efficacy of anti-EGFR antibody therapy, which has become a standard treatment for patients with RAS wild-type (4-6). The primary site of colorectal cancer also predicts the efficacy of anti-EGFR antibodies; right-sided colon cancer is refractory to anti-EGFR antibodies as it is a RAS mutant cancer. Therefore, the use of anti-EGFR antibodies has been recommended for left-sided colon or rectal cancer with wild-type RAS as the first line of treatment (7).
Cetuximab, an anti-EGFR antibody, is an immunoglobulin G1 (IgG1) human-mouse chimeric monoclonal antibody and is indicated as a single agent or combined with chemotherapy for RAS wild-type mCRC. Several large randomized phase III trials have compared bevacizumab and anti-EGFR antibody with chemotherapy as the first-line treatment (6,8). However, there have been no randomized phase III trials to compare bevacizumab and anti-EGFR antibody therapy as the second-line treatment, and the evidence is limited to randomized phase II trials (9-11). In Japan, only one randomized phase II trial (WJOG6210G) has compared panitumumab with bevacizumab combined with FOLFIRI in wild-type KRAS exon 2 mCRC (11). Oxaliplatin combination regimens are widely used as the first-line treatment for mCRC, and little is known about the combined use of anti-EGFR antibody and FOLFOX therapy as the second-line treatment.
Cetuximab was initially administered as a weekly regimen, but in recent years it has also been administered biweekly. Prospective studies of biweekly cetuximab treatment have been conducted mainly in the first-line (12-15) or third or later-line setting (16,17), and the evidence of its use in the second-line setting is sparse. There was only one phase II study of biweekly cetuximab with chemotherapy for mCRC which contained second-line patients. Martín-Martorell et al. reported a phase II study of biweekly cetuximab with irinotecan after at least one previous line of chemotherapy (18). In that report, the efficacy and toxicity of biweekly cetuximab with irinotecan were similar to those of weekly cetuximab with irinotecan. However, this study was conducted in single institute and contained only 21 patients in undergoing second-line treatment. Therefore, to the best of our knowledge no prospective study of biweekly cetuximab with FOLFOX or FOLFIRI as the second-line therapy has been conducted.
In addition to RAS mutations, the DNA methylation status predicts treatment response to anti-EGFR antibody therapy (19-21). In a genome-wide DNA methylation analysis in RAS wild-type mCRC, Ouchi et al. reported that patients with highly-methylated colorectal cancer (HMCC) are resistant to the anti-EGFR antibody treatment regardless of the primary tumor site (22). Ouchi et al. developed a simple method for diagnosing DNA methylation status using the modified MethyLight assay (22). However, there are no reports examining the predictability of DNA methylation status on the efficacy of the anti-EGFR antibody in prospective clinical study cohort.
Therefore, the present study was designed to evaluate the efficacy and safety of biweekly cetuximab plus mFOLFOX6 or mFOLFIRI as the second-line treatment for KRAS exon 2 wild-type mCRC refractory or intolerant to the first-line chemotherapy. We also investigated the predictability of DNA methylation status on the efficacy of the anti-EGFR antibody-containing treatment. We present the following article in accordance with the TREND reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-862/rc).
Methods
Study design
This single-arm, phase II trial enrolled patients from eight institutions affiliated with the Tohoku Clinical Oncology Research and Education Society (T-CORE) in Japan. The present study evaluated the progression-free survival (PFS) of biweekly cetuximab in combination with mFOLFOX6 or mFOLFIRI as the second-line therapy for patients with advanced or recurrent unresectable colorectal cancer refractory or intolerant to the first-line chemotherapy. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Japanese Guidelines for the Ethics of Clinical Research and was approved by the Institutional review board of each participating institution. All participating hospitals/institutions were informed and agreed the study. Written informed consent was obtained from all patients before enrollment. This study was registered as UMIN000008298 with UMIN-CTR (https://www.umin.ac.jp/ctr/) on June 6th, 2012, and jRCTs021180014 with jRCT (https://jrct.niph.go.jp) on June 6th, 2012.
Participants
The main inclusion criteria were histologically confirmed colon or rectal cancer, surgical or biopsy specimen of the primary colorectal cancer submitted, confirmation of KRAS exon 2 (codon 12 or 13) wild-type using primary tumor or metastatic tumor specimen, age ≥20 years, Eastern Cooperative Oncology Group performance status of 0–2, presence of at least one radiographically measurable target lesion using Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1, patients refractory or intolerant to oxaliplatin-based or irinotecan-based therapy as the first-line treatment (postoperative adjuvant chemotherapy was not considered the first-line therapy if recurrence was not confirmed within 180 days after completion of adjuvant chemotherapy), and adequate organ function. The main exclusion criteria were severe pulmonary disease (e.g., interstitial pneumonia, pulmonary fibrosis, and severe emphysema), ascites, pleural, or pericardial effusion requiring drainage, and previous treatment with epidermal growth factor (EGF) signaling inhibitors or drugs targeting EGFR.
Procedures
Patients received either cetuximab (500 mg/kg) followed by mFOLFOX6 [oxaliplatin (85 mg/m2), l-leucovorin (200 mg/m2), intravenous bolus of fluorouracil (400 mg/m2), and continuous infusion of fluorouracil (2,400 mg/m2)], or cetuximab (500 mg/kg) followed by mFOLFIRI [irinotecan (150 mg/m2) instead of oxaliplatin in mFOLFOX6] on day 1 of each 2-week cycle. Treatment was continued until discontinuation of cetuximab, disease progression, or intolerable toxicity.
Tumor evaluations were performed every 2 months from the start of treatment, and tumor response was determined by each investigator using the RECIST version 1.1 criteria. Confirmation after 4 weeks was mandatory in cases of partial response. Adverse events (AEs) were evaluated by each investigator according to the Common Terminology Criteria for Adverse Events version 4.0.
Outcomes
The primary endpoint was PFS, defined as the time from the date of enrollment to the date of documented progression or death from any cause, whichever occurred first. The secondary endpoints were response rate and AEs. The clinical research forms were used to collect all required data.
Mutation analysis
Formalin-fixed paraffin-embedded tissues from surgical or biopsy specimens of the primary tumor were collected for KRAS (codons 59, 61, 117, and 146), NRAS (codons 12, 13, 59, 61, 117, and 146), and BRAF (codon 600) mutation analysis using direct DNA sequencing (23).
DNA methylation analysis
DNA methylation status was determined as previously described (22). A modified MethyLight assay was performed on 16 CpG sites to determine if the tumors were low-methylated colorectal cancer (LMCC) or HMCC. Cases positive for methylation at ≥8 cytosine-guanine dinucleotide (CpG) sites were classed as HMCC, whereas those positive for methylation at <8 CpG sites were classed as LMCC.
Statistical analysis
Since the PFS for FOLFOX and FOLFIRI in the second-line treatment has been reported to span 2.5–4.2 months (24), the threshold for mPFS was set at 3 months and the expected PFS was set at 4 months. A test of the null hypothesis that “true median PFS (mPFS) is less than or equal to the threshold of 3 months” was performed for all eligible patients based on their observed PFS. The estimated 95% confidence interval (CI) for PFS was calculated using the Kaplan-Meier method. The required sample size was estimated to be 94, assuming an enrollment period of 2 years, a follow-up period of 1 year (from the last case enrollment), a significance level of 2.5% on one side, and a power of 80%. The target number of patients was set at 100, including dropouts.
Two-sided Fisher’s exact test and Wilcoxon rank-sum test (or Kruskal-Wallis test) were used to analyze patient background. In uni- and multivariate analysis for PFS and OS, hazard ratios (HRs) and their CIs in the Cox proportional hazards model were calculated. P values <0.05 were considered statistically significant. Statistical analysis was performed using JMP pro, version 16.0.0 (SAS, Cary, NC, USA).
Results
Efficacy
We initially aimed to enroll 100 cases for 2 years from June 2012; however, we extended the enrollment period until December 2016 due to poor accrual. Finally, the study closed prematurely due to poor accrual and a total of 66 cases were enrolled. One patient who did not meet the eligibility criteria was excluded, and 65 patients were included in the study. In addition, one patient who withdrew consent was excluded from PFS and response analyses. The follow-up was completed on November 30, 2018.
The patient characteristics (Table 1) were as follows: 40 (61.5%) were males, 41 (63.1%) and 24 (36.9%) had PS0 and PS1, respectively, the median age was 66 years, and 46 (70.8%) were ≥65 years old. Minor mutations in the RAS gene, including NRAS, were found in 9 (13.6%) of the enrolled cases. BRAF gene mutations were observed in 8 (12.3%) patients. DNA methylation analysis revealed 46 (70.8%) and 17 (26.2%) cases of LMCC and HMCC, respectively. Cetuximab combined with mFOLFOX6 and mFOLFIRI were used in 21 (32.3%) and 44 (67.7%) patients, respectively.
Table 1
| Characteristics | All eligible patients (n=65) | Cetuximab + mFOLFOX6 (n=21) | Cetuximab + mFOLFIRI (n=44) | P value1 | LMCC (n=46) | HMCC (n=17) | P value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||||||
| Sex | 0.60 | 0.55 | |||||||||||||
| Male | 40 | 61.5 | 14 | 66.7 | 26 | 59.1 | 30 | 65.2 | 9 | 52.9 | |||||
| Female | 25 | 38.5 | 7 | 33.3 | 18 | 40.9 | 16 | 34.8 | 8 | 47.1 | |||||
| PS (ECOG) | 1.00 | 0.62 | |||||||||||||
| 0 | 41 | 63.1 | 13 | 61.9 | 28 | 63.6 | 29 | 63.0 | 10 | 58.8 | |||||
| 1 | 24 | 36.9 | 8 | 38.1 | 16 | 36.4 | 17 | 37.0 | 7 | 41.2 | |||||
| Age | 0.037 | 0.062 | |||||||||||||
| <65 years | 19 | 29.2 | 3 | 14.3 | 16 | 36.4 | 15 | 32.6 | 3 | 17.6 | |||||
| ≥65 years | 46 | 70.8 | 18 | 85.7 | 28 | 63.6 | 31 | 67.4 | 14 | 82.4 | |||||
| Age (years), median [range] | 66 [40–84] | 69 [49–84] | 66 [40–78] | 0.036 | 66 [40–84] | 68 [48–78] | 0.21 | ||||||||
| Primary tumor location | 0.091 | 0.30 | |||||||||||||
| Right-sided | 21 | 32.3 | 10 | 47.6 | 11 | 25.0 | 13 | 28.3 | 8 | 47.1 | |||||
| Left-sided | 44 | 67.7 | 11 | 52.4 | 33 | 75.0 | 33 | 71.7 | 9 | 52.9 | |||||
| Primary tumor resection | 1.00 | 0.22 | |||||||||||||
| Yes | 42 | 64.6 | 14 | 66.7 | 28 | 63.6 | 30 | 65.2 | 12 | 70.6 | |||||
| No | 23 | 35.4 | 7 | 33.3 | 16 | 36.4 | 16 | 34.8 | 5 | 29.4 | |||||
| Metastatic organs | |||||||||||||||
| Liver | 46 | 70.8 | 16 | 76.2 | 30 | 68.2 | 0.57 | 34 | 73.9 | 10 | 58.8 | 0.41 | |||
| Lung | 23 | 35.4 | 5 | 23.8 | 17 | 38.6 | 0.28 | 15 | 32.6 | 6 | 35.3 | 1.00 | |||
| Lymph node | 22 | 33.8 | 4 | 19.0 | 18 | 40.9 | 0.10 | 13 | 28.3 | 7 | 41.2 | 0.088 | |||
| Peritoneum | 16 | 24.6 | 6 | 28.6 | 10 | 22.7 | 0.76 | 9 | 19.6 | 7 | 41.2 | 0.15 | |||
| Bone | 2 | 3.1 | 1 | 4.8 | 1 | 2.3 | 0.55 | 0 | 0.0 | 2 | 11.8 | 0.13 | |||
| Others | 2 | 3.1 | 1 | 4.8 | 1 | 2.3 | 0.55 | 2 | 4.3 | 0 | 0.0 | 1.00 | |||
| Differentiation assessed by histology | 0.52 | 0.26 | |||||||||||||
| Well or moderate | 51 | 78.5 | 18 | 85.7 | 33 | 75.0 | 38 | 82.6 | 11 | 64.7 | |||||
| Poorly | 14 | 21.5 | 3 | 14.3 | 11 | 25.0 | 8 | 17.4 | 6 | 35.3 | |||||
| RAS mutation | 1.00 | 0.33 | |||||||||||||
| Wild-type | 56 | 86.2 | 18 | 85.7 | 38 | 86.4 | 40 | 87.0 | 15 | 88.2 | |||||
| Mutant | 9 | 13.8 | 3 | 14.3 | 6 | 13.6 | 6 | 13.0 | 2 | 11.8 | |||||
| BRAF mutation | 0.74 | <0.0001 | |||||||||||||
| Wild-type | 54 | 83.1 | 18 | 85.7 | 36 | 81.8 | 44 | 95.7 | 8 | 47.1 | |||||
| Mutant | 8 | 12.3 | 3 | 14.3 | 5 | 11.4 | 0 | 0.0 | 8 | 47.1 | |||||
| Not definable | 3 | 4.6 | 0 | 0.0 | 3 | 6.8 | 2 | 4.3 | 1 | 5.9 | |||||
| DNA methylation status | 1.00 | ||||||||||||||
| LMCC | 46 | 70.8 | 15 | 71.4 | 31 | 70.5 | |||||||||
| HMCC | 17 | 26.2 | 5 | 23.8 | 12 | 27.3 | |||||||||
| Not definable | 2 | 3.1 | 1 | 4.8 | 1 | 2.3 | |||||||||
1, cetuximab + mFOLFOX6 vs. cetuximab + mFOLFIRI; 2, LMCC vs. HMCC. LMCC, low-methylated colorectal cancer; HMCC, highly-methylated colorectal cancer; PS, performance status; ECOG, Eastern Cooperative Oncology Group.
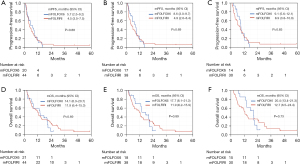
The mPFS for all eligible patients (n=64), which was the primary endpoint, was 5.1 (95% CI, 3.8–7.6) months, and the lower limit of the 95% CI was above the predefined threshold (3 months), validating the benefit of the treatment (Figure 1A). In patients with RAS wild-type (n=56) and both RAS and BRAF (RAS/BRAF) wild-type (n=44), mPFS were 5.7 (95% CI, 3.7–8.5) and 7.5 (95% CI, 4.2–9.2) months, respectively (Figure 1B,1C). The mOS for all eligible patients (n=65) were 12.7 (95% CI, 7.5–15.3) months. In patients with RAS wild-type (n=57) and RAS/BRAF wild-type (n=45), mOS were 12.8 (95% CI, 7.5–17.7), and 14.2 (95% CI, 11.6–21.0) months, respectively (Figure 1D-1F). The response rate for all eligible patients (n=64), which was the secondary endpoint, was 23.4%, and those for patients with RAS wild-type (n=56) and RAS/BRAF wild-type (n=44) were 26.8% and 34.1%, respectively (Table 2). A comparison of efficacy between the mFOLFOX6 and mFOLFIRI groups is shown in Figure 2 and Table 2. A comparison of patient characteristics revealed a significantly higher proportion of elderly patients in the mFOLFOX6 group than in the mFOLFIRI group. No significant differences were observed between the two groups in terms of the other factors (Table 1). There was no statistically significant difference in mPFS between the mFOLFOX6 and mFOLFIRI groups [5.7 (95% CI, 2.5–9.2) vs. 4.5 (95% CI, 3.5–7.5) months, respectively; P=0.88] (Figure 2A). The mPFS were also similar between the mFOLFOX6 and mFOLFIRI groups in RAS wild-type [8.6 (95% CI, 3.0–9.7) vs. 4.9 (95% CI, 2.6–8.4) months, respectively; P=0.69) and RAS/BRAF wild-type patients [9.1 (95% CI, 5.6–12.1) vs. 6.9 (95% CI, 3.8–10.9) months, respectively; P=0.83] (Figure 2B,2C). The mOS was 14.1 (95% CI, 6.3–20.7) and 11.9 (95% CI, 6.4–15.3) months in the mFOLFOX6 and mFOLFIRI groups, respectively (P=0.69) (Figure 2D). The mOS were also similar between the mFOLFOX6 and mFOLFIRI groups in RAS wild-type [17.7 (95% CI, 8.1–21.3) vs. 11.9 (95% CI, 6.2–15.9) months, respectively; P=0.89] and RAS/BRAF wild-type patients [20.4 (95% CI, 13.4–21.3) vs. 12.7 (95% CI, 6.5–24.4) months, respectively; P=0.73] (Figure 2E,2F). The objective response rates (ORRs) were not significantly different between the mFOLFOX6 and mFOLFIRI groups in all eligible patients (35.0% vs. 18.2%, P=0.20), as well as the RAS wild-type (41.2% vs. 20.5%, P=0.19) and RAS/BRAF wild-type patients (50.0% vs. 26.7%, P=0.18) (Table 2).
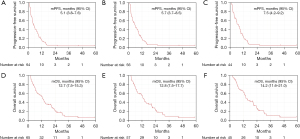
Table 2
| Response | All | RAS wild-type | RAS/BRAF wild-type | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n=64) | Cetuximab + mFOLFOX6 (n=20) | Cetuximab + mFOLFIRI (n=44) | P value | All (n=56) | Cetuximab + mFOLFOX6 (n=17) | Cetuximab + mFOLFIRI (n=39) | P value | All (n=44) | Cetuximab + mFOLFOX6 (n=14) | Cetuximab + mFOLFIRI (n=30) | P value | |||||||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |||||||||||||
| CR | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.49 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.46 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0.66 | |||||||||
| PR | 15 | 23.4 | 7 | 35.0 | 8 | 18.2 | 15 | 26.8 | 7 | 41.2 | 8 | 20.5 | 15 | 34.1 | 7 | 50.0 | 8 | 27 | ||||||||||||
| SD | 30 | 46.9 | 8 | 40.0 | 22 | 50.0 | 28 | 50.0 | 7 | 41.2 | 21 | 53.8 | 20 | 45.4 | 5 | 35.7 | 15 | 50.0 | ||||||||||||
| PD | 18 | 28.1 | 5 | 25.0 | 13 | 29.5 | 12 | 21.4 | 3 | 17.6 | 9 | 23.1 | 8 | 18.2 | 2 | 14.3 | 6 | 20.0 | ||||||||||||
| NE | 1 | 1.6 | 0 | 0.0 | 1 | 2.3 | 1 | 1.8 | 0 | 0.0 | 1 | 2.6 | 1 | 3.3 | 0 | 0.0 | 1 | 3.3 | ||||||||||||
| ORR | – | 23.4 | – | 35.0 | – | 18.2 | 0.20 | – | 26.8 | – | 41.2 | – | 20.5 | 0.19 | – | 34.1 | – | 50.0 | – | 26.7 | 0.18 | |||||||||
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, not evaluable; ORR, objective response rate.
AEs
Table 3 summarizes all grades of AEs that were observed in >10% of patients. Any skin disorders such as rash acneiform, dry skin, paronychia, and pruritis were observed in 42–77% of patients, whereas grade 3 or higher skin disorders were generally observed in <15% of patients. Leukopenia, neutropenia, and anemia were frequently observed (71.2%, 69.7%, and 50.0%, respectively). Grade 3 or higher neutropenia was observed in 53.0% of patients. However, febrile neutropenia was observed in only 1 patient (1.5%). Moreover, 29 patients (44.6%) required dose reduction or withdrawal of cetuximab, and 53 patients (81.5%) required dose reduction or withdrawal of combined chemotherapy.
Table 3
| Parameters | Any grade | Grade ≥3 | Cetuximab + mFOLFOX6 | Cetuximab + mFOLFIRI | P value (any grade) | P value (grade ≥3) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | ||||||||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||||||
| Clinical findings | |||||||||||||||||||
| Rash acneiform | 51 | 77.3 | 7 | 10.6 | 16 | 76.2 | 5 | 23.8 | 34 | 79.1 | 2 | 4.7 | 1.00 | 0.034 | |||||
| Dry skin | 45 | 68.2 | 7 | 10.6 | 18 | 85.7 | 3 | 14.3 | 27 | 62.8 | 4 | 9.3 | 0.082 | 0.67 | |||||
| Paronychia | 38 | 57.6 | 10 | 15.2 | 14 | 66.7 | 3 | 14.3 | 24 | 55.8 | 7 | 16.3 | 0.43 | 1.00 | |||||
| Pruritus | 28 | 42.4 | 2 | 3.0 | 9 | 42.9 | 1 | 4.8 | 19 | 44.2 | 1 | 2.3 | 1.00 | 1.00 | |||||
| Fatigue | 49 | 74.2 | 8 | 12.1 | 17 | 81.0 | 2 | 9.5 | 31 | 72.1 | 6 | 14.0 | 0.55 | 1.00 | |||||
| Mucositis oral | 43 | 65.2 | 10 | 15.2 | 14 | 66.7 | 3 | 14.3 | 29 | 67.4 | 7 | 16.3 | 1.00 | 1.00 | |||||
| Diarrhea | 36 | 54.5 | 2 | 3.0 | 11 | 52.4 | 0 | 0.0 | 23 | 53.5 | 2 | 4.7 | 1.00 | 1.00 | |||||
| Anorexia | 25 | 37.9 | 9 | 13.6 | 9 | 42.9 | 4 | 19.0 | 16 | 37.2 | 5 | 11.6 | 0.79 | 0.46 | |||||
| Peripheral neuropathy | 22 | 33.3 | 2 | 3.0 | 13 | 61.9 | 2 | 9.5 | 9 | 20.9 | 0 | 0.0 | 0.0019 | 0.10 | |||||
| Nausea | 15 | 22.7 | 3 | 4.5 | 4 | 19.0 | 1 | 4.8 | 11 | 25.6 | 2 | 4.7 | 0.76 | 1.00 | |||||
| Vomiting | 13 | 19.7 | 2 | 3.0 | 6 | 28.6 | 1 | 4.8 | 7 | 16.3 | 1 | 2.3 | 0.32 | 1.00 | |||||
| Alopecia | 12 | 18.2 | 0 | 0.0 | 6 | 28.6 | 0 | 0.0 | 16 | 37.2 | 0 | 0.0 | 0.58 | – | |||||
| Constipation | 11 | 16.7 | 0 | 0.0 | 4 | 19.0 | 0 | 0.0 | 7 | 16.3 | 0 | 0.0 | 1.00 | – | |||||
| Dysgeusia | 11 | 16.7 | 1 | 1.5 | 4 | 19.0 | 1 | 4.8 | 7 | 16.3 | 0 | 0.0 | 1.00 | 0.33 | |||||
| Fever | 9 | 13.6 | 0 | 0.0 | 2 | 9.5 | 0 | 0.0 | 6 | 14.0 | 0 | 0.0 | 1.00 | – | |||||
| Infection | 8 | 12.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 8 | 18.6 | 0 | 0.0 | 0.045 | – | |||||
| Abdominal pain | 8 | 12.1 | 0 | 0.0 | 1 | 4.8 | 0 | 0.0 | 7 | 16.3 | 0 | 0.0 | 0.25 | – | |||||
| Laboratory findings | |||||||||||||||||||
| Leukopenia | 47 | 71.2 | 18 | 27.3 | 14 | 66.7 | 7 | 33.3 | 33 | 76.7 | 11 | 25.6 | 0.55 | 0.56 | |||||
| Neutropenia | 46 | 69.7 | 35 | 53.0 | 15 | 71.4 | 10 | 47.6 | 30 | 69.8 | 25 | 58.1 | 1.00 | 0.59 | |||||
| Anemia | 33 | 50.0 | 3 | 4.5 | 20 | 95.2 | 2 | 9.5 | 38 | 88.4 | 1 | 2.3 | 0.65 | 0.25 | |||||
| Thrombocytopenia | 26 | 39.4 | 4 | 6.1 | 14 | 66.7 | 2 | 9.5 | 23 | 53.5 | 2 | 4.7 | 0.42 | 0.59 | |||||
| Bilirubin | 11 | 16.7 | 3 | 4.5 | 4 | 19.0 | 0 | 0.0 | 10 | 23.3 | 3 | 7.0 | 1.00 | 0.54 | |||||
| AST | 19 | 28.8 | 2 | 3.0 | 13 | 61.9 | 1 | 4.8 | 29 | 67.4 | 1 | 2.3 | 0.78 | 1.00 | |||||
| ALT | 22 | 33.3 | 0 | 0.0 | 7 | 33.3 | 0 | 0.0 | 17 | 39.5 | 0 | 0.0 | 0.78 | – | |||||
| Creatinine | 10 | 15.2 | 3 | 4.5 | 2 | 9.5 | 0 | 0.0 | 11 | 25.6 | 3 | 7.0 | 0.19 | 0.54 | |||||
| Hypomagnesemia | 39 | 59.1 | 4 | 6.1 | 14 | 66.7 | 1 | 4.8 | 27 | 62.8 | 3 | 7.0 | 1.00 | 1.00 | |||||
| Hypernatremia | 8 | 12.1 | 0 | 0.0 | 4 | 19.0 | 0 | 0.0 | 4 | 9.3 | 0 | 0.0 | 0.42 | – | |||||
| Hyponatremia | 22 | 33.3 | 1 | 1.5 | 8 | 38.1 | 0 | 0.0 | 15 | 34.9 | 1 | 2.3 | 1.00 | 1.00 | |||||
| Hyperkalemia | 11 | 16.7 | 0 | 0.0 | 4 | 19.0 | 0 | 0.0 | 8 | 18.6 | 0 | 0.0 | 1.00 | – | |||||
| Hypokalemia | 15 | 22.7 | 3 | 4.5 | 4 | 19.0 | 2 | 9.5 | 11 | 25.6 | 1 | 2.3 | 0.76 | 0.25 | |||||
| Hypoalbuminemia | 16 | 24.2 | 0 | 0.0 | 6 | 28.6 | 0 | 0.0 | 11 | 25.6 | 0 | 0.0 | 1.00 | – | |||||
AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Rash acneiform was significantly more frequent in the mFOLFOX6 group than in the mFOLFIRI group (P=0.034). For AEs of any grade, peripheral neuropathy was more frequent in the mFOLFOX6 group than the mFOLFIRI group (P=0.0019), whereas infection was more frequent in the mFOLFIRI group than the mFOLFOX6 group (P=0.045).
Predictive significance of the DNA methylation status on treatment efficacy
A significantly higher proportion of patients had BRAF mutations in the HMCC group compared with the mutation rate in the LMCC group (P<0.0001). No other factors were significantly different between the two groups (Table 1).
Univariate analysis of PFS in all eligible cases showed that RAS gene mutation, BRAF gene mutation, and DNA methylation status were significantly associated with PFS (Table 4). Multivariate analysis of these three factors showed that RAS and BRAF mutations were significantly associated with PFS. Univariate analysis of OS showed that primary tumor resection, presence of poorly differentiated component, BRAF mutation, and DNA methylation status were significantly associated with OS, whereas multivariate analysis of these four factors showed that primary tumor resection and BRAF mutation were significantly associated with OS.
Table 4
| Parameters | PFS (n=64) | OS (n=65) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Univariate | Multivariate | N | Univariate | Multivariate | ||||||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||||
| Gender | 0.75 | 0.74 | |||||||||||||||
| Male | 40 | 1 | 40 | 1 | |||||||||||||
| Female | 24 | 0.92 | 0.54–1.56 | 25 | 0.91 | 0.52–1.59 | |||||||||||
| Age | 0.54 | 0.33 | |||||||||||||||
| <65 years | 19 | 1 | 20 | 1 | |||||||||||||
| ≥65 years | 45 | 1.19 | 0.68–2.11 | 45 | 1.35 | 0.73–2.48 | |||||||||||
| PS (ECOG) | 0.57 | 0.55 | |||||||||||||||
| 0 | 40 | 1 | 41 | 1 | |||||||||||||
| 1 | 24 | 1.17 | 0.68–1.99 | 24 | 1.18 | 0.68–2.08 | |||||||||||
| Resection for primary tumor | 0.23 | 0.041 | 0.0087 | ||||||||||||||
| Yes | 42 | 1 | 42 | 1 | 1 | ||||||||||||
| No | 22 | 1.40 | 0.81–2.41 | 23 | 1.80 | 1.02–3.16 | 2.31 | 1.24–4.32 | |||||||||
| Poorly differentiated component | 0.094 | 0.027 | 0.14 | ||||||||||||||
| − | 50 | 1 | 51 | 1 | 1 | ||||||||||||
| + | 14 | 1.74 | 0.91–3.32 | 14 | 2.10 | 1.09–4.04 | 1.71 | 0.83–3.53 | |||||||||
| Primary site | 0.29 | 0.27 | |||||||||||||||
| Right colon | 21 | 1 | 21 | 1 | |||||||||||||
| Left colon and rectum | 43 | 0.75 | 0.44–1.28 | 44 | 0.73 | 0.41–1.28 | |||||||||||
| Liver metastasis | 0.12 | 0.12 | |||||||||||||||
| − | 19 | 1 | 19 | 1 | |||||||||||||
| + | 45 | 1.59 | 0.89–2.84 | 46 | 1.64 | 0.87–3.09 | |||||||||||
| Lung metastasis | 0.50 | 0.42 | |||||||||||||||
| − | 43 | 1 | 43 | 1 | |||||||||||||
| + | 21 | 0.83 | 0.48–1.43 | 22 | 0.79 | 0.44–1.41 | |||||||||||
| Peritoneal metastasis | 0.94 | 0.13 | |||||||||||||||
| − | 48 | 1 | 49 | 1 | |||||||||||||
| + | 16 | 1.03 | 0.56–1.88 | 16 | 1.57 | 0.82–3.00 | |||||||||||
| Combined regimen | 0.88 | 0.93 | |||||||||||||||
| FOLFOX | 20 | 1 | 21 | 1 | |||||||||||||
| FOLFIRI | 44 | 1.04 | 0.60–1.81 | 44 | 0.87 | 0.47–1.59 | |||||||||||
| RAS | 0.023 | 0.0023 | 0.30 | ||||||||||||||
| Wild type | 55 | 1 | 1 | 56 | 1 | ||||||||||||
| Mutant type | 9 | 2.39 | 1.13–5.08 | 3.71 | 1.60–8.60 | 9 | 1.50 | 0.70–1.43 | |||||||||
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | ||||||
| BRAF | <0.0001 | 0.0017 | <0.0001 | 0.0095 | |||||||||||||
| Wild type | 53 | 1 | 1 | 54 | 1 | 1 | |||||||||||
| Mutant type | 8 | 6.27 | 2.62–15.00 | 6.33 | 2.00–20.04 | 8 | 5.14 | 2.26–11.67 | 3.57 | 1.16–10.98 | |||||||
| Not definable | 3 | 3 | |||||||||||||||
| DNA methylation | 0.0057 | 0.39 | 0.0013 | 0.086 | |||||||||||||
| LMCC | 46 | 1 | 1 | 46 | 1 | 1 | |||||||||||
| HMCC | 17 | 2.30 | 1.28–4.16 | 1.43 | 0.63–3.22 | 17 | 2.72 | 1.48–5.01 | 2.13 | 0.90–5.04 | |||||||
| Not definable | 1 | 2 | |||||||||||||||
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; PS, performance status; ECOG, Eastern Cooperative Oncology Group; LMCC, low-methylated colorectal cancer; HMCC, highly-methylated colorectal cancer.
The mPFS in the LMCC group was significantly better than that in the HMCC group [7.5 (95% CI, 4.9–9.1) vs. 2.3 (95% CI, 1.6–3.8) months, P=0.0044] (Figure 3A). The mOS of the LMCC group was also significantly better than that of the mOS in the HMCC group [15.3 (95% CI, 12.7–21.3) vs. 4.7 (95% CI, 3.0–7.5), P=0.0008] (Figure 3B). The PFS and OS according to methylation status combined with RAS/BRAF mutation status are shown in Figure 3C,3D, respectively. In RAS/BRAF wild-type patients, the mPFS and mOS were not significantly different in the LMCC and HMCC groups [mPFS: 8.5 (95% CI, 6.1–10.9) vs. 3.3 (95% CI, 1.2–not reached) months, P=0.79; mOS: 15.3 (95% CI, 11.9–23.5) vs. 6.5 (95% CI, 3.1–not reached) months, P=0.53], but the differences were not small (ΔmPFS, 5.2 months; ΔmOS, 8.8 months). In patients with the RAS mutation, the mPFS in the LMCC group was significantly better than those in the HMCC group [mPFS: 4.4 (95% CI, 1.4–7.6) vs. 2.5 (95% CI, 1.2–3.8) months, P=0.032]. The mOSs in the LMCC and HMCC groups were not significantly different, although the mOS for the LMCC group tended to be longer than the mOS in the HMCC group [12.7 (95% CI, 2.4–not reached) vs. 4.7 (95% CI, 3.1–6.3) months, P=0.19]. In the LMCC group, PFS in patients with RAS/BRAF wild-type was significantly better than PFS in patients with the RAS mutation (P=0.0038) (Figure 3C). In contrast, OS was similar between patients with RAS/BRAF wild-type mutations with RAS mutations (P=0.48) (Figure 3D). In the HMCC group, PFS and OS were similar between patients with RAS/BRAF wild-type and those with the RAS mutation (P=0.27 and P=0.37, respectively). The PFS and the OS were worst in patients with the BRAF mutation [mPFS: 2.2 (95% CI, 1.6–3.0) months; mOS: 3.5 (95% CI, 2.4–8.1) months, respectively] among all subgroups.
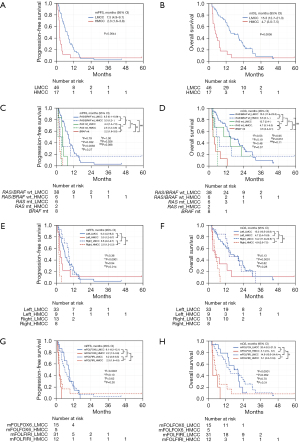
Comparisons of PFS and OS according to the methylation status combined with the primary tumor site are shown in Figure 3E,3F. In patients with left-sided tumors, the mPFS and mOS in the LMCC group were not significantly better than the mPFS and mOS in the HMCC group [mPFS: 6.2 (95% CI, 4.2–9.2) vs. 3.0 (95% CI, 1.2–9.7) months, P=0.36; mOS: 15.3 (95% CI, 10.5–23.8) vs. 4.7 (95% CI, 2.4–15.9) months, P=0.13]. In patients with right-sided tumors, the mPFS and mOS in the LMCC group were significantly better than the mPFS and mOS in the HMCC group [mPFS: 8.6 (95% CI, 4.2–9.7) vs. 2.0 (95% CI, 1.2–2.3) months, P<0.0001; mOS: 14.2 (95% CI, 11.9–22.6) vs. 4.9 (95% CI, 2.6–7.5) months, P=0.0001]. Conversely, in the LMCC group, the mPFS and mOS were not different in patients with left-sided vs. right-sided tumors (P=0.94 and P=0.82, respectively). In the HMCC group, the mPFS in patients with left-sided tumors was significantly better than the mPFS in patients with right-sided tumors (P=0.014), but the difference was only 1.0 M. In the HMCC group, the mOSs in patients with left- and right-sided tumors were not significantly different (P=0.28).
Comparisons of PFS and OS according to the methylation status combined with treatment regimens are shown in Figure 3G,3H. In the LMCC group, no differences in the mPFS and mOS in the mFOLFOX6 and mFOLFIRI groups were detected [mPFS: 9.1 (95% CI, 4.2–12.1) vs. 6.2 (95% CI, 3.8–8.5) months, P=0.62; mOS: 20.4 (95% CI, 8.2–21.3) vs. 14.9 (95% CI, 10.5–24.4) months, P=0.70]. In the HMCC group, the mPFS and mOS were also not significantly different in the mFOLFOX6 and mFOLFIRI groups [mPFS: 2.2 (95% CI, 1.2–3.8) vs. 2.3 (95% CI, 1.6–4.5) months, respectively, P=0.30; mOS: 3.1 (95% CI, 2.6–8.1) vs. 5.1 (95% CI, 2.6–12.9) months, respectively, P=0.31].
Discussion
We conducted this prospective study to evaluate the efficacy and safety of biweekly cetuximab in combination with mFOLFOX6 or mFOLFIRI as the second-line treatment for KRAS exon 2 wild-type mCRC refractory or intolerant to the first-line chemotherapy. mPFS, which was the primary endpoint, was 5.1 (95% CI, 3.8–7.6) months, confirming that the mPFS of the treatment was >3 months. The mOS and ORR were 12.7 (95% CI, 7.5–15.3) months and 23.4% (95% CI, 13.1–33.8%), respectively, indicating the efficacy of the study treatment.
The present study was initiated when only KRAS exon 2 mutations were considered biomarkers for anti-EGFR antibodies; therefore, we analyzed mutations in exon 3 and 4 in KRAS, exon 2–4 in NRAS, and codon 600 in BRAF. As a result, nine cases with RAS mutation and eight with BRAF mutation were found. The mPFS, mOS, and ORR were most favorable in patients with RAS/BRAF wild-type consistently, suggesting the importance of RAS and BRAF mutations as biomarkers for anti-EGFR antibodies.
Because oxaliplatin combination chemotherapy is frequently used as the first-line treatment, there is little evidence showing the efficacy of cetuximab plus mFOLFOX as the second-line treatment. Furthermore, no prospective studies have been conducted to evaluate the efficacy of biweekly cetuximab with FOLFOX or FOLFIRI as the second-line treatment. Therefore, the efficacy and safety data reported in the present study are important. In Japan, the only prospective study on anti-EGFR antibody as the second-line treatment is WJOG6210G, wherein mPFS after panitumumab plus FOLFIRI in patients with KRAS exon 2 wild-type mCRC was reported to be 6 months (11). In western countries, the prospective studies on anti-EGFR antibody plus FOLFOX or FOLFIRI in KRAS exon 2 wild-type mCRC patients as the second-line treatment reported an mPFS of 5.6–7.7 months (5,9,10,25). The PFS of the present study was comparable to that of the previous studies.
In the present study, the frequency of neutropenia was very high, and grade 3 or higher neutropenia was observed in >50% of patients. In clinical trials of anti-EGFR antibodies with FOLFIRI or FOLFOX for the second-line treatment for mCRC in Western countries, neutropenia of grade 3 or higher was reported to be around 10–20% (5,9,10,25). In contrast, in a study conducted in Japan (WJOG6210G), grade 3 or higher neutropenia was reported in 49.2% of patients, which was as high as that in the present study (11). It should be noted that anti-EGFR antibody plus mFOLFOX or mFOLFIRI is associated with a high degree of neutropenia in Japanese patients. Other AEs were comparable to those previously reported and were confirmed to be well tolerated. Withdrawal or dose reduction of cetuximab was necessary for 44.6% of patients, suggesting the importance of management of cetuximab-specific AEs, such as skin disorders (e.g., rash acneiform, dry skin, and paronychia).
Several studies have reported predictors of therapeutic efficacy of anti-EGFR antibody drugs; however, no biomarkers other than RAS mutations have been introduced into clinical practice (19,20,26-28). Ouchi et al. recently reported that DNA methylation status is a predictor of therapeutic response to anti-EGFR antibodies, and a novel diagnostic method of DNA methylation status was developed using a modified MethyLight assay (20,22). Results of the present study indicated that DNA methylation status was also significantly associated with PFS and OS in the univariate analysis, and HMCC group showed a significantly poorer treatment outcome than LMCC group, but not in multivariate analysis. DNA methylation status is known to be associated with BRAF and RAS gene mutations (29,30), and all cases with BRAF mutation (n=8) and two cases with RAS mutations were classed as HMCC. Among a total of 15 HMCC cases, 67% (10 cases) had RAS/BRAF mutations, which may have eliminated the significance of DNA methylation status on PFS and OS. In addition, DNA methylation status has been reported to be associated with sensitivity to anti-EGFR antibodies (20-22,31), but not to chemotherapy (32). Therefore, in the case of HMCC tumors that are sensitive to chemotherapy, therapeutic benefit can be achieved from the chemotherapy with anti-EGFR antibodies. This may have attenuated the predictive power of DNA methylation status for the treatment response form anti-EGFR antibodies. Studies are required to examine the significance of DNA methylation status as predictors of treatment response to anti-EGFR antibodies in a larger number of patients without RAS/BRAF mutations. In the present study, the mPFS and mOS in the LMCC group were significantly better than those in the HMCC group (Figure 3A,3B). In RAS/BRAF wild-type patients, no significant differences in mPFS and mOS between the LMCC and HMCC groups were detected, although the numerical differences were not small (ΔmPFS, 5.2 months; ΔmOS, 8.8 months). In patients with the RAS mutation, the mPFS in the LMCC group was significantly longer than the PFS in the HMCC group (Figure 3C). The mOS in the LMCC group was numerically better than the mOS in the HMCC group (ΔmOS, 8.0 months), but the difference was not statistically significant (Figure 3D). These results suggest that patients with LMCC may potentially benefit from anti-EGFR antibody treatment even in the presence of RAS gene mutations. The PFS and OS in patients with BRAF mutations were worse than the PFS and OS in other patients (Figure 3C,3D). All patients with BRAF mutations had HMCC. Therefore, the resistance to anti-EGFR antibody treatment in patients with BRAF mutations may be because of not only the BRAF mutation but also HMCC.
Ouchi et al. and Okita et al. also demonstrated that the predictive significance of DNA methylation status on the efficacy of anti-EGFR antibody treatment was superior to the predictive ability of primary-tumor sidedness (21,22). In the present study, the mPFS and mOS in the LMCC group were significantly or numerically better than the mPFS and mOS in the HMCC group with both left- and right-sided tumors (Figure 3E,3F). In patients with left-sided tumors, no significant differences in PFS and OS were detected between the LMCC and HMCC groups, although the differences were not small (ΔmPFS, 3.2 months; ΔmOS 10.6 months). In contrast, in both the LMCC and HMCC groups, mPFS and mOS were similar in patients with left- and right-sided tumors. These results were consistent with previous reports (21,22). Therefore, DNA methylation status could be a biomarker to predict the efficacy of anti-EGFR treatment. On the other hand, DNA methylation status is not associated with the therapeutic effects of oxaliplatin and irinotecan, according to the results of the translational research of TRICOLORE, randomized phase III trial in untreated mCRC patients (32-34). Our results confirm these previous results (Figure 3G,3H).
The limitations of this study were that it was a Japanese, single-arm, phase II trial and that the number of enrolled patients did not reach the planned sample size. Therefore, in some analyses, the study was underpowered, and the DNA methylation status as a biomarker for anti-EGFR antibody therapy could not be confirmed. However, our results prospectively show similar results to previous reports concerning DNA methylation status as a biomarker of anti-EGFR antibody effectiveness; all previous reports about DNA methylation were obtained using retrospective cohorts.
Conclusions
In conclusion, the mPFS of cetuximab plus mFOLFOX or mFOLFIRI was 5.1 M, and the results indicate that cetuximab plus mFOLFOX6 or mFOLFIRI could be a useful regimen for mCRC as the second-line therapy. DNA methylation status warrants further exploration as a predictive biomarker for anti-EGFR efficacy in mCRC.
Acknowledgments
This study was supported by the Tohoku Clinical Research and Education Society (T-CORE). We thank our patients and medical and technical staff, especially Ms. Hiromi Nakano.
Funding: This study was funded by Merck Biopharma.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-862/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-862/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-862/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-862/coif). ST reports personal fees from Taiho, Chugai, Asahi Kasei, Bristol-Myers Squibb, Bayer, Japan blood products organization, Medicon, Takeda, Yakult, and Daiichi-Sankyo, Eli Lilly, Eisai, and grants and personal fees from Merck Biopharma and Ono, outside the submitted work. TM reports a grant from Repertoire Genesis and stock in Ono Pharmaceutical Co., Ltd. and Japan Post. Masanobu Takahashi reports personal fees from Daiichi-Sankyo, Bristol-Myers Squibb, and grants and personal fees from Chugai and Ono. HO reports personal fees from Yakult, Merck Biopharma, and Kyowa Kirin. Masahiro Takahashi reports grants from Ono and Incyte Bioscience. TY reports personal fees Nipro and grants from AC Medical, A2 Healthcare, EP Croit, ClinChoice, Japan Tabacco, Japan Media, Medidata Solutions, Ono, Kyowa Kirin, Tsumura, Daiichi-Sankyo, Otsuka, Eisai, Asahi Intec, 3H Clinical Trial, Medrio, Nipro, Intellim, Welby, 3H Medi Solution, Baseconnect, Nobori, Puravida Technologies, and Hemp Kitchen, and consulting fees from EP Croit, Japan Tabacco, Medidata Solutions, Ono, Kowa, Chugai, Tsumura, Daiichi-Sankyo, Eisai, Asahi Intec Asahi Kasei Pharma, 3H Clinical Trial, Intellim, Takeda, AstraZeneca, Sonire Therapeutics, and Seikagaku, and participates on a data safety monitoring board of Incyte Biosciences. CI reports grants from Shionogi, Nippon Kayaku, Eisai, Sanofi, Taiho, Chugai, Kyowa Kirin, Asahi Kasei Pharma, Daiichi-Sankyo, Eli Lilly, Yakult, Ono, Takeda, Tsumura, and Bayer, and honoraria from Chugai. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and the Japanese Guidelines for the Ethics of Clinical Research. The study was approved by the institutional review board of each participating institution. All participating hospitals/institutions were informed and agreed the study. Written informed consent to participate in this study and to publish the data of this study was obtained from all patients before enrollment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yamada Y, Takahari D, Matsumoto H, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 2013;14:1278-86. [Crossref] [PubMed]
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609-18. [Crossref] [PubMed]
- Yamazaki K, Nagase M, Tamagawa H, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 2016;27:1539-46. [Crossref] [PubMed]
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [Crossref] [PubMed]
- Peeters M, Oliner KS, Price TJ, et al. Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin Cancer Res 2015;21:5469-79. [Crossref] [PubMed]
- Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016;17:1426-34. [Crossref] [PubMed]
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713-29. [Crossref] [PubMed]
- Venook AP, Niedzwiecki D, Lenz HJ, et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017;317:2392-401. [Crossref] [PubMed]
- Bennouna J, Hiret S, Bertaut A, et al. Continuation of Bevacizumab vs Cetuximab Plus Chemotherapy After First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER PRODIGE18 Randomized Clinical Trial. JAMA Oncol 2019;5:83-90. [Crossref] [PubMed]
- Hecht JR, Cohn A, Dakhil S, et al. SPIRITT: A Randomized, Multicenter, Phase II Study of Panitumumab with FOLFIRI and Bevacizumab with FOLFIRI as Second-Line Treatment in Patients with Unresectable Wild Type KRAS Metastatic Colorectal Cancer. Clin Colorectal Cancer 2015;14:72-80. [Crossref] [PubMed]
- Shitara K, Yonesaka K, Denda T, et al. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci 2016;107:1843-50. [Crossref] [PubMed]
- Brodowicz T, Ciuleanu TE, Radosavljevic D, et al. FOLFOX4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with KRAS wild-type metastatic colorectal cancer: a randomized phase II CECOG study. Ann Oncol 2013;24:1769-77. [Crossref] [PubMed]
- Fernandez-Plana J, Pericay C, Quintero G, et al. Biweekly cetuximab in combination with FOLFOX-4 in the first-line treatment of wild-type KRAS metastatic colorectal cancer: final results of a phase II, open-label, clinical trial (OPTIMIX-ACROSS Study). BMC Cancer 2014;14:865. [Crossref] [PubMed]
- Personeni N, Rimassa L, Verusio C, et al. FOLFIRI and Cetuximab Every Second Week for First-Line Treatment of KRAS Wild-Type Metastatic Colorectal Cancer According to Phosphatase and Tensin Homolog Expression: A Phase II Study. Clin Colorectal Cancer 2015;14:162-9. [Crossref] [PubMed]
- Kotake M, Aoyama T, Munemoto Y, et al. Multicenter phase II study of infusional 5-fluorouracil (5-FU), leucovorin, and oxaliplatin, plus biweekly cetuximab as first-line treatment in patients with metastatic colorectal cancer (CELINE trial). Oncol Lett 2017;13:747-53. [Crossref] [PubMed]
- Pfeiffer P, Nielsen D, Bjerregaard J, et al. Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol 2008;19:1141-5. [Crossref] [PubMed]
- Shitara K, Yuki S, Yoshida M, et al. Phase II study of combination chemotherapy with biweekly cetuximab and irinotecan for wild-type KRAS metastatic colorectal cancer refractory to irinotecan, oxaliplatin, and fluoropyrimidines. Invest New Drugs 2012;30:787-93. [Crossref] [PubMed]
- Martín-Martorell P, Roselló S, Rodríguez-Braun E, et al. Biweekly cetuximab and irinotecan in advanced colorectal cancer patients progressing after at least one previous line of chemotherapy: results of a phase II single institution trial. Br J Cancer 2008;99:455-8. [Crossref] [PubMed]
- Lee MS, McGuffey EJ, Morris JS, et al. Association of CpG island methylator phenotype and EREG/AREG methylation and expression in colorectal cancer. Br J Cancer 2016;114:1352-61. [Crossref] [PubMed]
- Ouchi K, Takahashi S, Yamada Y, et al. DNA methylation status as a biomarker of anti-epidermal growth factor receptor treatment for metastatic colorectal cancer. Cancer Sci 2015;106:1722-9. [Crossref] [PubMed]
- Okita A, Takahashi S, Ouchi K, et al. Consensus molecular subtypes classification of colorectal cancer as a predictive factor for chemotherapeutic efficacy against metastatic colorectal cancer. Oncotarget 2018;9:18698-711. [Crossref] [PubMed]
- Ouchi K, Takahashi S, Okita A, et al. A modified MethyLight assay predicts the clinical outcomes of anti-epidermal growth factor receptor treatment in metastatic colorectal cancer. Cancer Sci 2022;113:1057-68. [Crossref] [PubMed]
- Soeda H, Shimodaira H, Watanabe M, et al. KRAS mutation in patients with metastatic colorectal cancer does not preclude benefit from oxaliplatin-or irinotecan-based treatment. Mol Clin Oncol 2014;2:356-62. [Crossref] [PubMed]
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37. [Crossref] [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [Crossref] [PubMed]
- Lenz HJ, Ou FS, Venook AP, et al. Impact of Consensus Molecular Subtype on Survival in Patients With Metastatic Colorectal Cancer: Results From CALGB/SWOG 80405 (Alliance). J Clin Oncol 2019;37:1876-85. [Crossref] [PubMed]
- Schütte M, Risch T, Abdavi-Azar N, et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat Commun 2017;8:14262. [Crossref] [PubMed]
- Jacobs B, De Roock W, Piessevaux H, et al. Amphiregulin and epiregulin mRNA expression in primary tumors predicts outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2009;27:5068-74. [Crossref] [PubMed]
- Yagi K, Akagi K, Hayashi H, et al. Three DNA methylation epigenotypes in human colorectal cancer. Clin Cancer Res 2010;16:21-33. [Crossref] [PubMed]
- Kaneda A, Yagi K. Two groups of DNA methylation markers to classify colorectal cancer into three epigenotypes. Cancer Sci 2011;102:18-24. [Crossref] [PubMed]
- Osumi H, Ouchi K, Shinozaki E, et al. Effect of DNA methylation status on first-line anti-epidermal growth factor receptor treatment in patients with metastatic colorectal cancer. Int J Colorectal Dis 2022;37:1439-47. [Crossref] [PubMed]
- Takahashi S, Sakamoto Y, Denda T, et al. Advanced colorectal cancer subtypes (aCRCS) help select oxaliplatin-based or irinotecan-based therapy for colorectal cancer. Cancer Sci 2021;112:1567-78. [Crossref] [PubMed]
- Yamada Y, Denda T, Gamoh M, et al. S-1 and irinotecan plus bevacizumab versus mFOLFOX6 or CapeOX plus bevacizumab as first-line treatment in patients with metastatic colorectal cancer (TRICOLORE): a randomized, open-label, phase III, noninferiority trial. Ann Oncol 2018;29:624-31. [Crossref] [PubMed]
- Denda T, Takashima A, Gamoh M, et al. Combination therapy of bevacizumab with either S-1 and irinotecan or mFOLFOX6/CapeOX as first-line treatment of metastatic colorectal cancer (TRICOLORE): Exploratory analysis of RAS status and primary tumour location in a randomised, open-label, phase III, non-inferiority trial. Eur J Cancer 2021;154:296-306. [Crossref] [PubMed]


