
A more radical perspective on surgical approach and outcomes in pancreatic cancer—a narrative review
Introduction
Time has proven there is no simple answer to such a complex problem as pancreatic cancer. Despite rising concern regarding this highly lethal disease, the scientific community still lacks a state-of-art approach that translates into better outcomes to pancreatic ductal adenocarcinoma (PDAC).
Since 1990, both its incidence and mortality has more than doubled worldwide (1). In the United States, pancreatic cancer is already the fourth cause of cancer-related deaths both in men and women while in Europe it is projected to rank third soon (2,3). By 2030, it will likely surpass colorectal, prostate and breast cancer, becoming the second most common cause of cancer-related fatalities (4). The alarming scenario is that despite all current advances, mortality might double once more until 2060 (5).
Long-term survival is poor. When all stages of disease are combined, 5-year survival is still around 11% (2). Although modest, real-world data from the Surveillance, Epidemiology and End Results program demonstrated some progress, as long-term survivors have risen from 1.5% in 1975 to 17.4% in 2011 in surgically resected patients. When no surgical treatment was offered survival remained below 1% (6).
Unfortunately, delayed diagnosis is the rule and only a small percentage of patients are offered surgical resection (7-9). There are two main ways to face this harsh reality. The first is to blame the tumor’s aggressive biology and focus on systemic therapy with the risk of overselection and preclude curative-intent treatment for a set number of patients. Second is to rely solely on the technical feasibility of more radical resections and therefore increase morbidity in an already fragile recipient without clear oncologic benefit.
As medical science usually converges, neither approach shall be an incontestable truth. The key answer is to better coordinate both into a robust multimodal strategy (8,9). Historically, pancreatectomy is an underperformed procedure even for earlier stage disease in the absence of formal contraindications (10). As Fergus et al. pointed in a retrospective study, this appears to be a current issue with 36%, or 8,594 of 23,842, patients with T1–T2N0M0 pancreas cancer not receiving surgery as part of their treatment plan with no identifiable reason. Older age and African American race were commonly associated factors (11).
It has been shown that pancreatic surgery evolved to become a safe procedure and general mortality is below 5%, as long as performed in the adequate setting of high-volume specialized centers (8,10,12). Elderly patients have comparable 90-day mortality and perioperative results to younger ones, proving that age should not be the only factor to exclude patients from curative-intent surgical resection (13,14).
The purpose of this review is to summarize the different surgical aspects, perioperative management and hopefully contribute to a more successful treatment chain for pancreatic cancer. We present this article in accordance with the Narrative Review reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-763/rc).
Methods
We conducted an extensive literature review through PubMed, prioritizing papers published in the last 5 years, but older emblematic papers were also included. We included articles that explored the treatment of pancreatic adenocarcinoma, with focus on the surgical aspect and strategies to improve outcomes. References of selected articles were also reviewed to identify any missed studies. Only papers in English were included. We used keywords such as “pancreatic ductal adenocarcinoma”, “pancreatic cancer”, “surgical treatment”, “technical advances”, “pancreaticoduodenectomy”, “lymphadenectomy”, “outcomes”, but free term search was also used (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | April–September, 2022 |
| Databases and other sources searched | PubMed |
| Search terms used | “Pancreatic ductal adenocarcinoma”, “pancreatic cancer”, “surgical treatment”, “technical advances”, “pancreaticoduodenectomy”, “lymphadenectomy”, “outcomes” |
| Timeframe | Articles published until September 2022 |
| Inclusion and exclusion criteria | Articles in English. Relevant articles were screened by their abstract. Priority was given to more recently published papers (5 years) with a few exceptions. No specific exclusion criteria |
| Selection process | The articles were independently selected by the first three authors, Eduardo de Souza M. Fernandes, Felipe Pedreira T. de Mello, Eduardo Pinho Braga, and included in the review after discussion with the co-authors |
Staging—who to operate?
Clinical staging of PDAC was last revised by eighth edition of the American Joint Committee on Cancer (AJCC) and is based on the tumor-node-metastasis (TNM) (15). Nonetheless, the tumor’s relationship with neighboring vessels better determine resectability and guide management (Figure 1A-1C).
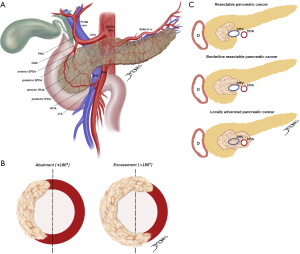
Resectable disease is defined as a tumor with no arterial contact and no or limited venous contact [superior mesenteric vein/portal vein (SMV/PV) with unilateral narrowing or <180° tumor invasion] (16,17). In contrast, several definitions for borderline resectable PDAC were proposed. They usually include limited arterial invasion (less than 180º) or tumors in which contact with SMV/PV are more extensive (more than 180º) while reconstruction is still technically feasible. Although somewhat imprecise, the rationale is to differentiate patients with higher risk of positive margin, non-R0 resection (17,18). As such, we understand that the anatomic definition of borderline resectable PDAC is directly linked to the center’s expertise and capabilities to perform more advanced procedures while maintaining true oncological resection.
More recently, other factors were taken in consideration as a localized tumor with favorable anatomy may be deemed borderline resectable due to tumor biology [carbohydrate antigen 19-9 (CA19-9) >500 U/mL or regional lymph node metastasis found on biopsy or positron emission tomography (PET)-computed tomography (CT)] or to patient’s condition [either poor performance status (≥2) or serious comorbidities] (17). CA19-9 levels have been recently validated as independent prognostic factors on OS in some retrospective studies (19). However, whether laboratorial parameters should limit the choice to undergo surgery is still debatable.
Finally, unresectable PDAC is further classified as locally advanced, when vascular contact exceeds what is considered borderline, and as metastatic, which include the presence of macroscopic para-aortic/extra abdominal lymph nodes metastasis (17).
Neoadjuvant therapy—who can benefit?
The rationale behind neoadjuvant chemotherapy is to increase R0 resections, better select tumor biology and prevent or at least discover occult metastatic disease (20). A compelling argument is that up to 50% of patients receiving upfront surgery may not complete adjuvant therapy (21). The Dutch Randomized PREOPANC Phase III trial that compared initial surgery and adjuvant gemcitabine with neoadjuvant gemcitabine plus radiotherapy and posterior resection initially reported negative results (22). On the other hand, recent analysis of long-term results deemed positive the survival impact in both resectable and borderline resectable patients who underwent neoadjuvant treatment (23).
However, neoadjuvant therapy can be demanding as it frequently requires biopsy, preoperative stent placement and complex multidisciplinary care. This contributes to a risk of missing the brief window of opportunity for curative-intent surgery, specially in resectable patients (20). Moreover, successful completion of adjuvant therapy has been reported as high as 72.8% in a cohort of 932 patients with resectable and borderline PDAC patients submitted to upfront surgery, which might reflect the reality in more specialized centers (24). On top of that, survival rates from the ESPAC-4 and the PRODIGE 24/CCTG PA.6 trials with initial surgery and adjuvant folfirinox or gemcitabine/capecitabine were significantly higher than those reported in the Dutch trial, reinforcing that the standard management can be successful for these patients (23,25,26).
An upcoming trend is to plan a surgical strategy after systemic therapy to those deemed unresectable at first. In probably the largest study regarding this topic, the Heidelberg group depicted resectability rates around 60% for locally advanced PDAC after systemic therapy, maintaining standard postoperative mortality. A major challenge still is how to properly select who might benefit from surgery. Imaging appears to overestimate disease as fibrosis is poorly differentiated from active cancer tissue and CA19-9 levels are most useful only when initially elevated, thus not for all cases. Possibly the best approach is aggressive surgical treatment to those who did not exhibit disease progression (27). Another option is evaluation of metabolic response estimated by post-chemotherapy PET [PET/CT or PET/magnetic resonance imaging (MRI)], understanding that those patients with complete metabolic response might benefit more from conversion surgery (28).
Therefore, we believe that current evidence supports neoadjuvant treatments pathways to borderline and locally advanced tumors, but it is still not enough to change the standard management of resectable PDAC outside the context of clinical trials. Moreover, it is always necessary to consider the patient’s expectations regarding treatment options, keeping in mind that the best treatment is also a function of adequate follow up and compliance.
Anatomy-first approach
Regardless of the approach chosen by the surgeon, preemptive procedure planning is crucial and knowledge of vascular variability with 3D reconstruction of multidetector row CT scans is paramount in making dissection safer (29).
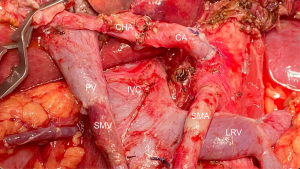
One of the reasons artery-first approaches have been successful in reducing intraoperative bleeding is probably the early identification of superior mesenteric artery (SMA) and SMV branches and tributaries. In other words, surgical maneuvers that unravel the “consistently inconsistent” inferior pancreatic vascular anatomy are known to be extremely helpful (30). Therefore, preoperative radiologic study can also be a powerful tool in the identification of the inferior pancreaticoduodenal artery and vein (IPDA and IPDV), the first jejunal artery and vein (J1A and J1V) and other eventual but relevant vascular anomalies present (Figure 2A-2D).
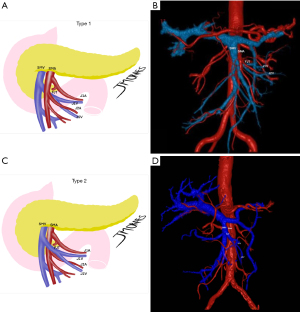
Ishikawa et al. (29) brilliantly illustrated the main vascular patterns in 155 patients. IPDA and J1A formed a common trunk in 66% of cases, while IPDA originated from the SMA directly in 33% and more rarely, from a replaced right hepatic artery (RHA). The even more complex venous drainage was categorized into 3 types with further subgrouping. The first jejunal trunk (FJT) includes both the J1V and J2V and was present in 84% of the patients. When present, it could run dorsal (type 1: 63%) or ventral to the SMA (type 2: 21%). In the minority of cases when J1V and J2V drained separately to the SMV was classified as type 3 and could be further subdivided regarding each’s relationship with the SMA. This is useful because ventral FJT tends to be larger and could obscure IPDA/J1A origins in the artery-first approach. Even more dangerous in this fine dissection is the risk of IPDV laceration when it drains to the FJT and not to the SMV directly. Partial preservation of the FJT, in the case of extensive drainage encompassing the territory of multiple jejunal arteries, can prevent small bowel congestion (29).
Other landmarks useful to know beforehand are the gastrocolic trunk of Henle, the SMV groove and its relationship with the SMA (31,32). In parallel, accurate study of the splenic-mesenteric confluence, certifying the absence of portal thrombosis and verifying patency of SMV and tributaries is necessary in order to properly plan more complex vascular reconstructions (33). Furthermore, preoperative identification of a replaced RHA and its course or the existence of a hepatic mesenteric trunk can prevent accidental injuries (34).
An often neglected problem is celiac artery stenosis (CAS), which can be an important risk factor for worse outcomes such as postoperative pancreatic fistula (POPF), biliary leakage, liver perfusion failure and gastric complications. Preoperative diagnosis might be helpful in triggering surgical planned division of the median arcuate ligament or other perioperative treatments (35).
Moreover, it is interesting to outline that the pancreatic uncinate process is a structure that lacks precise anatomical and surgical landmarks and at the same time pose as one of the most common sites of margin positivity (36,37). Its resection can be challenging because of variability in shape, diameter and relationship with the superior mesenteric vessels (36). Risk of bleeding and inadvertent injury to the SMA is especially important when the uncinate process’s leftward projection reaches or extends beyond this vessel, as classified by Zhu et al. as type III or IV. In these cases, the adoption of uncinate-first or other artery-first approaches can aid in more complete dissection and R0 resection rate (37).
In the setting of distal pancreatectomies, preoperative imaging is crucial from the beginning, as to define the plane of dissection. A deeper retroperitoneal invasion should prompt the surgeon for the removal of the adrenal gland, following the posterior radical antegrade modular pancreatosplenectomy (RAMPS) technique (38).
Here, knowledge of the peripancreatic vascular anatomy can also help predict intraoperative challenges. As an example, when pancreatic parenchyma surrounds the splenic artery’s root, this can translate into greater risk of bleeding. Splenic vein tumoral contact should also be investigated. Precise understanding of this anatomy appears to be specially important for minimally invasive distal pancreatectomies (39). Preoperative assessment of the pattern of anatomical variant is even more crucial for classification and planning of celiac arterial resections. For instance, when a tumor spares the proper hepatic and the gastroduodenal arteries, the celiac artery (CA) resection may dismiss the need for revascularization (40).
The importance of oncological resection
Favorable subgroups of pN0R0 patients submitted to upfront resection plus adjuvant chemotherapy can present impressive 5-year survival rates above 50% (24). Standardized radical dissection and systematic lymphadenectomy is paramount to achieve this kind of outcome.
As previously addressed, different R-status assessments resulted in high variability of reported R0 resection rates, declining from 70–80% to less than 24% when more strict protocols were used (41). Tumor free circumferential margins of 1 mm evaluated by axial slicing pathological assessment is the most current R0 definition proposed and appears to be a key prognostic indicator (42). This revisited concept was further validated as an independent predictor of survival in a recently published meta-analysis, although some doubts remain such as whether wider margins might be beneficial (43). Specimens should be examined by an experienced surgical pathologist following strict protocols such as the Royal College of Pathologists, which enforces the evaluation of seven radial margins (44).
Pancreaticoduodenectomy (PD): mesopancreas management
In this pursuit of state-of-art negative margin resections, proper mesopancreas dissection might be the answer for tumors at the head of the pancreas. Mesopancreas, mesopancreatoduodenum, retroportal lamina or pancreas-major arteries (P-A) ligament were different terms used to describe the lymph vascular structures, lymph nodes and nerve plexus behind the pancreatic head and uncinate process up to the extent of third and fourth duodenal portions and the proximal jejunum mesentery. Pancreatic cancer is known for invading these perineural structures and local recurrence might often be due to insufficient dissection in this area. Central vascular ligation with the objective of true oncological resections, a concept borrowed from others gastrointestinal cancers, was translated into total mesopancreas excision (Figure 3) (45).
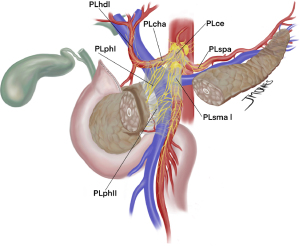
Inoue et al. (46) went a step further and described the different levels of mesopancreas dissection in PD. Level 1 does not include lymphadenectomy and is standard to more benign pathologies. In level 2, mesopancreas is excised en bloc, while ligation at the root of IPDA and J1A facilitates proper lymphadenectomy and soft tissue removal. This might be the choice for tumors far from the SMA and patients with poor performance status, when a less radical surgery might be fit. Regarding PDAC, what appears to be more appropriate as standard dissection was described as level 3, which includes removal of the nerve plexus of the pancreas head (both PLphI and PLphII), and is further characterized by hemicircumferential removal of the right and posterior nerve plexus around the SMA (PLsma) (46).
In fact, neurovascular invasion is so dreaded that complete circumferential removal of PLsma was proposed to achieve R0 resections, what was later classified as extended-level 3 dissection. An important drawback of this approach is the increased rates of postoperative diarrhea, a reason many Japanese surgeons advocate for partial preservation of PLsma. However, when properly treated with opioids-based medication most cases appear to be well controlled and do not preclude adjuvant therapy completion rate (47). The triangle operation described by Hackert et al. shares this concept of total mesopancreas excision with arterial skeletonization (48). In fact, removal of all soft tissue around even the CA and hepatic artery (HA) can be safe and without significant increase of postoperative morbidity or mortality. Nonetheless, further evaluation is needed to achieve optimal balance between radicality and true oncological outcomes (49).
PD: artery-first approach
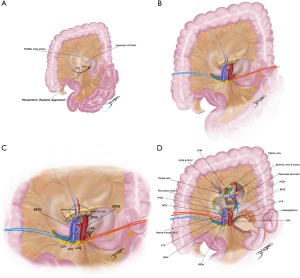
The several artery-first approaches focus on early control of the SMA, but vary in the initial mode of dissection (Figure 4) (50). Proposed advantages are less blood loss and operative time, more complete lymphadenectomy and increased R0 resections. Even further, these techniques allow early identification of a replaced RHA or even visualization of a non-resectable status before irreversible steps are taken. To date, it is not known any real difference in outcomes between the at least six different artery-first procedures, although when compared to standard pancreatectomy, artery-first approach has shown improved postoperative results and even survival benefits as confirmed in recent meta-analysis (51,52).
Among them, Nakao’s mesenteric approach is a sophisticated technique to perform isolated “no touch” PD. It takes advantage of the root of the transverse mesocolon to gradually expose SMA branches, facilitating systematic mesopancreas excision in a safe manner and has become increasingly popular in Japan (Figure 5A-5D) (53,54). Prospective evidence of whether this approach is superior to conventional PD starting with Kocher’s maneuver is still under investigation by the prospective MAPLE-PD trial (55).
PD: lymphadenectomy
Although once unclear, standard lymphadenectomy for PD was established by the latest international consensus as resection of pyloric nodes (Nos. 5 and 6), nodes around the common hepatic (No. 8a), hepatoduodenal ligament lymph nodes (Nos. 12b, 12c recently all merged in 12b), the SMA proximal nodes (Nos. 14a and 14b recently updated as 14p) and, naturally, the anterior and posterior pancreaticoduodenal nodes (Nos. 17a, 17b, 13a, and 13b), following the Japan Pancreas Society nomenclature (56,57). Further dissection through more distant stations of the common hepatic and celiac arteries nodes (Nos. 8p and 9), hepatoduodenal ligament nodes (Nos. 12a and 12p) and nodes distal of the SMA (No. 14 d). remained without clear recommendation, although some of these stations are often described as routinely dissected in several centers, including ours (45,46,53,56,58).
More controversial is the extended lymphadenectomy including No. 16 para-aortic lymph nodes stations, which are even considered by the latest staging system as distant metastasis (M1), when afflicted by disease. In general, most randomized controlled trials (RCTs) to date didn’t show any survival benefit supporting extended lymphadenectomy. It might even offer greater risk of postoperative morbidity, therefore, it is not recommended by most guidelines (59). However, discrepancies in lymphadenectomy classification, center-related technical issues and, even further, reasonably favorable outcomes reported for patients with para-aortic lymph node metastasis continue to make this subject a gray area (59,60).
In parallel, insufficient lymph node retrieval might lead to understaging. What once was defined as the minimum of 12 or 15 lymph nodes is being questioned (56). More adequate staging is obtained when at least 20 lymph nodes are examined (61).
In our institution, the preferred approach tries to approximate the western and eastern visions in treating pancreatic cancer. To ensure proper lymphadenectomy and negative margins, we often start dissection by the artery-first mesenteric approach. At the same time, we pursue total mesopancreas excision with a clear Heidelberg’s triangle visualization and entire circumferential clearance of PLsma (Figure 6A,6B).
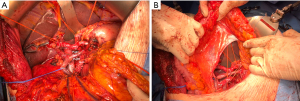
Extended pancreatoduodenectomy—venous management
Recent advances have made extended pancreatectomy more often performed. In fact, a recent proposed classification of 4 different types of PD appears more adequate yet still simple to use than the last International Study Group for Pancreatic Surgery (ISGPS) extended PD definition (Table 2) (62,63). In high-volume centers, type 2 PD with venous resection of the portomesenteric veins is expected to account for at least 15% of pancreatic resections (63). Recent meta-analysis reinforces that venous resection is associated with worse short-term outcomes, such as increased blood loss, perioperative morbidity and higher positive margins as well as lower long-term survival (64,65). Although high heterogeneity and different levels of center experience will always be an inherent challenge in this type of comparison. Nonetheless, it is well accepted that it is a feasible procedure with reproducible outcomes and latest recommendations are that minimal portomesenteric involvement should prompt venous resection, specially in good general condition patients and when R0 resections are possible (66,67).
Table 2
| ISGPS definition for extended pancreatoduodenectomy—standard PD plus resection of any of the following (62): |
| More than the antrum or distal half of the stomach |
| Colon and/or mesocolon with relevant vascular structures of the transverse mesocolon (ileocolic, right, or middle colic vessels) |
| Small bowel beyond the first segment of jejunum |
| Portal, superior mesenteric, and/or inferior mesenteric vein; HA, celiac trunk, and/or SMA; inferior vena cava |
| Right adrenal gland; right kidney and/or its vasculature; liver; diaphragmatic crura |
| Mihaljevic et al. definition for different pancreaticoduodenectomies (63) |
| Type 1: standard PD (without vascular or adjacent organ resection) |
| Type 2: PD with PV/SMV resection |
| Type 3: PD with multivisceral resection (includes ISGPS definition) |
| Type 4: PD with arterial resection |
ISGPS, International Study Group for Pancreatic Surgery; HA, hepatic artery; SMA, superior mesenteric artery; PD, pancreaticoduodenal; PV, portal vein; SMV, superior mesenteric vein.
Techniques vary in function of patients’ venous anatomy, tumor’s size, location, surgeon`s expertise and are classified into four different types by the ISGPS (67). Minor tangential venous resections are usually reconstructed by direct suture or using a patch, respectively types 1 and 2. Segmental resections are more demanding and require right hemicolon and mesenteric root mobilization to ensure tension-free anastomosis, either type 3 primary end-to-end venous reconstruction or type 4 with an interposition graft (Figure 7) (18,53).
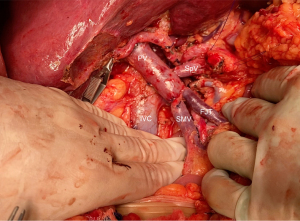
Recent discussion by Oba et al. hypothesized that the modified regional pancreatectomy, originally described by Fortner and coworkers, could achieve more oncological resection of en bloc soft tissue around the PV when segmental resections are planned (68,69). Diversion of portal flow is described as a form to mitigate portal hypertension and reduce the risk of major bleeding, an important approach specially in more extensive portomesenteric axis occlusion or in patients with cirrhosis, due to important collateral development. Mesocaval shunt, the use of an antithrombogenic catheter or the venous bypass graft-first technique are all operative strategies that a pancreatic surgeon should be familiar with (33,53,70).
Aside from challenges in the resection, venous involvement of the portomesenteric axis should be accounted for careful and planned reconstruction. Consequences of not maintaining splenic vein drainage can be severe and lead to segmental left sided portal hypertension, esophageal varices, splenomegaly and gastric congestion (66). When feasible, reinsertion of the splenic vein into the PV with an end-to-side anastomosis is both straightforward and effective (Figure 8).
Extended pancreatoduodenectomy—arterial management
Arterial resection, or type 4 PD, is much more rarely performed and comprises around 1% of PD even in high-volume centers (63). Recommendations are still controversial and not to be routinely proposed, thus depending strongly on patient selection (66,67). In spite of this, few centers’ reports made clear that it is technically feasible and reproducible and with 5-year survival greater than 11%, emphasizing that a learning curve plays a major role in achieving acceptable postoperative mortality (71,72). The most relevant remarks so far are the impact of neoadjuvant treatment, multidisciplinary discussion, patient informed decision and surgeons’ experience. In these highly selected cases, arterial resection appears a better option than palliation alone (73).
Arterial invasion when talking about pancreatic head tumors mainly implicate the SMA or HA. Reconstruction can be performed as end-to-end anastomosis or through the use of conduits, such as interposition grafts (74). Particularly useful is the splenic artery that can serve both for interposition or transposition in several complex reconstruction strategies (75). Common adopted strategies are shown (Figure 7).
Following the understanding that CT images often overestimate tumor burden after neoadjuvant therapy, an artery-sparing approach was recently described. Despite artery encasement on preoperative imaging, no viable tumor was confirmed in frozen specimens from arterial sites, allowing artery clearance without resection (76,77). Although promising, the periarterial divestment technique still requires further validation as it challenges the standard oncological R0 principles and poses risks such as arterial wall weakening (78).
Distal or left pancreatectomy
Regarding body and tail tumors, ample resection is also accepted as the way to ensure negative margins (18). Similarly to the adaptations that occurred in the Kausch-Whipple procedure, left pancreatectomy’s original left to right mode of dissection was subverted to a medial to lateral approach with early vessel-oriented dissection (38). RAMPS procedure achieves desired oncological goals by dissection through the anterior renal fascia (anterior RAMPS) or even removal of the left adrenal gland (posterior RAMPS), ensuring proper retroperitoneal margins (Figure 9). Recent meta-analysis proved this technique successful not only in ensuring R0 resection rates but also less bleeding volume and improving overall survival (79).
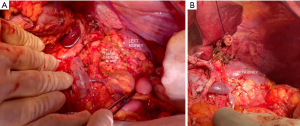
Here, lymphadenectomy is more straightforward. Retrieval of splenic hilum and splenic artery nodes (Nos. 10, 11p, and 11d) as well as nodes of the inferior pancreatic border (No. 18) are the accepted rule. Resection of common hepatic, CA and SMA nodes (Nos. 8, 9, and 14) should be considered, especially for more proximal tumors such as those located in the body. Although we favor routine extended lymphadenectomy, evidence remains debatable (56,57).
Porto-mesenteric axis resection is not exclusive to pancreatic head tumors and might be needed in distal pancreatectomies. Reconstruction follows fairly the same principles (80). In contrast, tumors in the body and tail are prone to an unfortunate condition: early local invasion of the celiac axis.
Thus the Appleby procedure, first described for gastric cancer, was readdressed as a novel treatment for pancreatic cancer, consisting in distal pancreatectomy with celiac axis resection (DP-CAR), which maintains hepatic perfusion through the pancreas head arcade flow into the gastroduodenal artery (GDA) (81). As expected, complications include high morbidity and mortality, with special consideration of hepatic or gastric ischemia (82). Several propositions to prevent this issue include preoperative embolization of either the hepatic or left gastric arteries, left gastric artery preservation when possible and even reconstruction of the HA, defined as a modified Appleby (81).
Truty et al. brought clarification to this subject with a tumor extent classification. The authors state that most reports of DP-CAR include only a pure celiac axis invasion, or a class 1 tumor. Further subdivided as 1A, when collaterals are sufficient, 1B, when reconstruction is needed, or even 1C when partial gastrectomy should be obligatory. More locally advanced tumors fit into the class 2 category with further lateral extension and invasion of the common hepatic artery (CHA) of proper hepatic artery (PHA)/GDA bifurcation. When the SMA is invaded, the tumor is then classified as a class 3. This classification is useful not only for scientifical uniformity but also for therapy guidance, as some class 2 or class 3 tumors should be considered for total pancreatectomy (TP) (40).
TP
There are several reasons for the TP procedure to have fallen out of use. Among them are the intrinsic perioperative morbidity, difficult to control diabetes and exocrine insufficiency (83). However, recent progress in both surgical expertise and clinical management of these metabolic consequences contributed to reconsideration of this type of resection for treatment of pancreatic cancer (84). Early postoperative outcomes are now reported as satisfactory and even comparable to partial pancreatectomy, with higher R0 margin resection but no clear oncological benefit or impact in overall survival (84,85).
Recent data emphasize that the long-term survival of patients undergoing TP is more dependent on the biology of the tumor than on the physiological changes caused by the apancreatic state. This procedure should no longer be avoided in stringently selected patients with appropriate indications (86).
Strong indications are still lacking, but the most common oncological drivers for this procedure are multifocal PDAC, combination with other parenchymal diseases or tumors at the neck of the pancreas (87,88). Although of relatively low incidence in high-volume centers, when present, POPF can lead to sepsis and via the erosion of vessels, to life-threatening hemorrhage. Thus, patients with high risk of pancreatic fistula specially in the context of arterial resection should benefit from TP (71,86,89). We believe that other indications that might justify TP are patient related features such as insulin dependent diabetes or a predominantly atrophic pancreas.
Mode of dissection follows what was later described for other pancreatic surgeries. Triangle dissection, combined with complex vascular resections are often performed as depicted in this case after neoadjuvant therapy (90). A particular issue in this operation is pronounced devascularization of the stomach and risk of gastric complications. Gastric venous congestion (GVC) is a frequent albeit not well-known finding after TP, especially when splenectomy and resection of the coronary vein are performed.
GVC might explain the high morbidity and mortality rates of TP. Loos et al. in a recent study with 585 patients investigated postoperative complications caused by impaired venous drainage of the stomach after TP. GVC was observed in 27.9% [163] of the patients requiring partial or total gastrectomy. Factors such as body mass index, ASA class IV, neoadjuvant therapy, completion pancreatectomy, high blood loss, splenectomy, coronary vein resection, and combined vascular resection were independently associated with intraoperative GVC. Adequate decision making for partial gastrectomy during TP is crucial. Therefore, reconstruction or at least preservation of gastric outflow is paramount in diminishing postoperative morbidity and mortality (Figure 8) (91).
Video 1 demonstrates a complex pancreatic head cancer resected with a type IV TP, with both arterial and venous resection. Several techniques described in this paper are illustrated.
Multivisceral resection
A significant proportion of patients (50–55%) present with distant metastatic disease at diagnosis, most commonly to the liver. As expected, the worst prognosis of an already severe disease is usually managed with supportive and palliative care (20). Even with the advances in neoadjuvant chemotherapy, surgical treatment of oligometastatic disease is perhaps the most debatable of all extended pancreatic resection discussions. Evidence of oncological benefit is limited although it has been increasingly performed in high volume centers (92). A recent single center report already explores this setting and demonstrates that some patients with liver only metastases but good preoperative prognostic index and optimal neoadjuvant chemotherapy response might have acceptable outcomes after resection (93). Furthermore, a cohort of 173 patients, including those with liver, peritoneal and distant lymph node metastatic disease reported encouraging survival outcomes, with overall survival of 25.5 months of those with complete pathological response (ypM0) confirmed after resection (94).
Minimally invasive pancreatic resection
In parallel to advances in open procedures’ rising complexity, laparoscopic and robotic pancreatic surgery’s current debate moved from feasibility towards reproducibility and questioning of the benefits that deem them worth performing. Less blood loss accompanied by short hospital stay and early recovery are promises that these methods keep delivering and should be accounted for, however, true oncological radicality and long-term survival are still under evaluation (95).
Minimally invasive distal pancreatomy (MIDP) contemplates both laparoscopic and robotic methods that have evolved greatly, in particular due to the nature of less complexity resection and no anastomosis needed. The LEOPARD prospective, randomized patient-blinded trial showed reduced time to functional recovery, less delayed gastric emptying and maintained overall rate of complications favoring MIDP, but with few resections regarding malignant tumors (95,96). In the setting of PDAC exclusively, oncological outcomes remain unclear. Although suggested the same as open surgery by some studies, retrospective design, small size and surgeon decision allocation are common problems with probably more aggressive tumors being submitted to open surgery, leading to bias (97). The DIPLOMA multicenter propensity score matched study demonstrates higher R0 resections but less lymph node retrieval with MIDP, reinforcing the challenges in interpreting these results (98). The group is currently performing a RCT with the same name aiming to better answer this question (99).
Minimally invasive pancreatoduodenectomy (MIPD) composes a different scenario. It remains a good operation in few highly experienced hands, and with fewer evidence supporting this approach (95). Lessons from the LEOPARD-2 trial and its early cessation due to unexpected high mortality rate with laparoscopic PD suggests that this type of operation might be a better fit for the robotic approach, with its advantageous learning curve and ergonomy (100,101). Interestingly enough, a recent meta analysis showed that both laparoscopic and robotic methods have similar oncological outcomes with lower postoperative complications than open surgery, with the remark that the robotic PD is associated with a lower conversion rate than the laparoscopic alternative (102).
Nonetheless, we believe that open surgery should be the standard of care for pancreatic cancer both for younger surgeons and for more complex resections, such as type 2, 3, or 4 PD or TP. A more mature and experienced surgeon can contemplate a minimally invasive learning curve and should start by less demanding DP or even type 1 PD. Although we expect more evidence in the early future.
Conclusions
In this paper we aim to discuss the various possibilities regarding the surgical management of pancreatic cancer. It is a complex subject with multiple and intricate variables, but it is clear that both systemic and surgical therapies have a fundamental and complementary role.
Complex vascular resections and reconstructions, recognizing patterns of dissection and defining proper lymphadenectomy compose an important set of tools concerning pancreatic surgery. Herewith a paramount and detailed knowledge of the patient’s anatomy with preoperative imaging contribute for a better and safer surgical outcome. We propose a vessel oriented technical approach with extended lymphadenectomy, following surgical oncologic principles, including specially the resection of the mesopancreas, aiming for the most favorable outcome and long-term survival. Based on current data regarding pancreatic cancer a more radical approach often brings more accomplishing results bringing as to believe that more is better.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francisco Schlottmann and Marco G. Patti) for the series “Current Management of Upper Gastrointestinal Malignancies” published in Journal of Gastrointestinal Oncology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-763/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-763/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-763/coif). The series “Current Management of Upper Gastrointestinal Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019;4:934-47. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol 2016;55:1158-60. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Lippi G, Mattiuzzi C. The global burden of pancreatic cancer. Arch Med Sci 2020;16:820-4. [Crossref] [PubMed]
- Bengtsson A, Andersson R, Ansari D. The actual 5-year survivors of pancreatic ductal adenocarcinoma based on real-world data. Sci Rep 2020;10:16425. [Crossref] [PubMed]
- Mizrahi JD, Surana R, Valle JW, et al. Pancreatic cancer. Lancet 2020;395:2008-20. [Crossref] [PubMed]
- Strobel O, Neoptolemos J, Jäger D, et al. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 2019;16:11-26. [Crossref] [PubMed]
- Traub B, Link KH, Kornmann M. Curing pancreatic cancer. Semin Cancer Biol 2021;76:232-46. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173-80. [Crossref] [PubMed]
- Fergus J, Nelson DW, Sung M, et al. Pancreatectomy in Stage I pancreas cancer: national underutilization of surgery persists. HPB (Oxford) 2020;22:1703-10. [Crossref] [PubMed]
- Gooiker GA, van Gijn W, Wouters MW, et al. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg 2011;98:485-94. [Crossref] [PubMed]
- Henry AC, Schouten TJ, Daamen LA, et al. Short- and Long-Term Outcomes of Pancreatic Cancer Resection in Elderly Patients: A Nationwide Analysis. Ann Surg Oncol 2022;29:6031-42. [Crossref] [PubMed]
- Shamali A, De'Ath HD, Jaber B, et al. Elderly patients have similar short term outcomes and five-year survival compared to younger patients after pancreaticoduodenectomy. Int J Surg 2017;45:138-43. [Crossref] [PubMed]
- Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7.
- Evans DB, George B, Tsai S. Non-metastatic Pancreatic Cancer: Resectable, Borderline Resectable, and Locally Advanced-Definitions of Increasing Importance for the Optimal Delivery of Multimodality Therapy. Ann Surg Oncol 2015;22:3409-13. [Crossref] [PubMed]
- Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology 2018;18:2-11. [Crossref] [PubMed]
- Schneider M, Hackert T, Strobel O, et al. Technical advances in surgery for pancreatic cancer. Br J Surg 2021;108:777-85. [Crossref] [PubMed]
- Anger F, Döring A, van Dam J, et al. Impact of Borderline Resectability in Pancreatic Head Cancer on Patient Survival: Biology Matters According to the New International Consensus Criteria. Ann Surg Oncol 2021;28:2325-36. [Crossref] [PubMed]
- Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA 2021;326:851-62. [Crossref] [PubMed]
- Khakoo S, Petrillo A, Salati M, et al. Neoadjuvant Treatment for Pancreatic Adenocarcinoma: A False Promise or an Opportunity to Improve Outcome? Cancers (Basel) 2021;13:4396. [Crossref] [PubMed]
- Versteijne E, Suker M, Groothuis K, et al. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol 2020;38:1763-73. [Crossref] [PubMed]
- Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol 2022;40:1220-30. [Crossref] [PubMed]
- Strobel O, Lorenz P, Hinz U, et al. Actual Five-year Survival After Upfront Resection for Pancreatic Ductal Adenocarcinoma: Who Beats the Odds? Ann Surg 2022;275:962-71. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Hackert T, Sachsenmaier M, Hinz U, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg 2016;264:457-63. [Crossref] [PubMed]
- Truty MJ, Kendrick ML, Nagorney DM, et al. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann Surg 2021;273:341-9. [Crossref] [PubMed]
- Ishikawa Y, Ban D, Matsumura S, et al. Surgical pitfalls of jejunal vein anatomy in pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2017;24:394-400. [Crossref] [PubMed]
- Vasiliadis KD. "Mesopancreas-first" radical resection of pancreatic head cancer following the Cattell-Braasch-Valdoni maneuver: Appreciating the legacy of pioneers in visceral surgery. Ann Hepatobiliary Pancreat Surg 2021;25:376-85. [Crossref] [PubMed]
- Miyazawa M, Kawai M, Hirono S, et al. Preoperative evaluation of the confluent drainage veins to the gastrocolic trunk of Henle: understanding the surgical vascular anatomy during pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci 2015;22:386-91. [Crossref] [PubMed]
- Aldouri A. Navigating the Root of the Mesentery: A Guided Approach to an Artery-First Pancreatoduodenectomy. J Pancreat Cancer 2017;3:78-83. [Crossref] [PubMed]
- Schmidt T, Strobel O, Schneider M, et al. Cavernous transformation of the portal vein in pancreatic cancer surgery-venous bypass graft first. Langenbecks Arch Surg 2020;405:1045-50. [Crossref] [PubMed]
- Nakata K, Higuchi R, Ikenaga N, et al. Precision anatomy for safe approach to pancreatoduodenectomy for both open and minimally invasive procedure: A systematic review. J Hepatobiliary Pancreat Sci 2022;29:99-113. [Crossref] [PubMed]
- Al-Saeedi M, Sauer HB, Ramouz A, et al. Celiac Axis Stenosis is an Underestimated Risk Factor for Increased Morbidity after Pancreatoduodenectomy. Ann Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- Martin DF. Computed tomography of the normal pancreatic uncinate process. Clin Radiol 1988;39:195-6. [Crossref] [PubMed]
- Zhu C, Ma L, Jia Z, et al. Novel morphological classification of the normal pancreatic uncinate process based on computed tomography. J Int Med Res 2020;48:300060520957453. [Crossref] [PubMed]
- Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery 2003;133:521-7. [Crossref] [PubMed]
- Nishino H, Zimmitti G, Ohtsuka T, et al. Precision vascular anatomy for minimally invasive distal pancreatectomy: A systematic review. J Hepatobiliary Pancreat Sci 2022;29:136-50. [Crossref] [PubMed]
- Truty MJ, Colglazier JJ, Mendes BC, et al. En Bloc Celiac Axis Resection for Pancreatic Cancer: Classification of Anatomical Variants Based on Tumor Extent. J Am Coll Surg 2020;231:8-29. [Crossref] [PubMed]
- Chandrasegaram MD, Goldstein D, Simes J, et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg 2015;102:1459-72. [Crossref] [PubMed]
- Strobel O, Hank T, Hinz U, et al. Pancreatic Cancer Surgery: The New R-status Counts. Ann Surg 2017;265:565-73. [Crossref] [PubMed]
- Leonhardt CS, Niesen W, Kalkum E, et al. Prognostic relevance of the revised R status definition in pancreatic cancer: meta-analysis. BJS Open 2022;6:zrac010. [Crossref] [PubMed]
- Campbell F, Cairns A, Duthie F, et al. G091 Dataset for histopathological reporting of carcinomas of the pancreas, ampulla of Vater and common bile duct. 2019. Available online: https://www.rcpath.org/static/34910231-c106-4629-a2de9e9ae6f87ac1/G091-Dataset-for-histopathological-reporting-of-carcinomas-of-the-pancreas-ampulla-of-Vater-and-common-bile-duct.pdf
- Fernandes ESM, Strobel O, Girão C, et al. What do surgeons need to know about the mesopancreas. Langenbecks Arch Surg 2021;406:2621-32. [Crossref] [PubMed]
- Inoue Y, Saiura A, Yoshioka R, et al. Pancreatoduodenectomy With Systematic Mesopancreas Dissection Using a Supracolic Anterior Artery-first Approach. Ann Surg 2015;262:1092-101. [Crossref] [PubMed]
- Inoue Y, Saiura A, Oba A, et al. Optimal Extent of Superior Mesenteric Artery Dissection during Pancreaticoduodenectomy for Pancreatic Cancer: Balancing Surgical and Oncological Safety. J Gastrointest Surg 2019;23:1373-83. [Crossref] [PubMed]
- Hackert T, Strobel O, Michalski CW, et al. The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford) 2017;19:1001-7. [Crossref] [PubMed]
- Klotz R, Hackert T, Heger P, et al. The TRIANGLE operation for pancreatic head and body cancers: early postoperative outcomes. HPB (Oxford) 2022;24:332-41. [Crossref] [PubMed]
- Sanjay P, Takaori K, Govil S, et al. 'Artery-first' approaches to pancreatoduodenectomy. Br J Surg 2012;99:1027-35. [Crossref] [PubMed]
- Ironside N, Barreto SG, Loveday B, et al. Meta-analysis of an artery-first approach versus standard pancreatoduodenectomy on perioperative outcomes and survival. Br J Surg 2018;105:628-36. [Crossref] [PubMed]
- Jiang X, Yu Z, Ma Z, et al. Superior mesenteric artery first approach can improve the clinical outcomes of pancreaticoduodenectomy: A meta-analysis. Int J Surg 2020;73:14-24. [Crossref] [PubMed]
- Nakao A. Isolated pancreatectomy using mesenteric approach. J Hepatobiliary Pancreat Sci 2022;29:293-300. [Crossref] [PubMed]
- Nakao A. The Mesenteric Approach in Pancreatoduodenectomy. Dig Surg 2016;33:308-13. [Crossref] [PubMed]
- Hirono S, Kawai M, Okada KI, et al. MAPLE-PD trial (Mesenteric Approach vs. Conventional Approach for Pancreatic Cancer during Pancreaticoduodenectomy): study protocol for a multicenter randomized controlled trial of 354 patients with pancreatic ductal adenocarcinoma. Trials 2018;19:613. [Crossref] [PubMed]
- Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2014;156:591-600. [Crossref] [PubMed]
- Japan Pancreas Society. Classification of Pancreatic Carcinoma. 4th ed. Tokyo: Kanehara Press, 2017.
- Schneider M, Strobel O, Hackert T, et al. Pancreatic resection for cancer-the Heidelberg technique. Langenbecks Arch Surg 2019;404:1017-22. [Crossref] [PubMed]
- Erdem S, Bolli M, Müller SA, et al. Role of lymphadenectomy in resectable pancreatic cancer. Langenbecks Arch Surg 2020;405:889-902. [Crossref] [PubMed]
- Safi SA, Rehders A, Haeberle L, et al. Para-aortic lymph nodes and ductal adenocarcinoma of the pancreas: Distant neighbors? Surgery 2021;170:1807-14. [Crossref] [PubMed]
- Strobel O, Hinz U, Gluth A, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg 2015;261:961-9. [Crossref] [PubMed]
- Hartwig W, Vollmer CM, Fingerhut A, et al. Extended pancreatectomy in pancreatic ductal adenocarcinoma: definition and consensus of the International Study Group for Pancreatic Surgery (ISGPS). Surgery 2014;156:1-14. [Crossref] [PubMed]
- Mihaljevic AL, Hackert T, Loos M, et al. Not all Whipple procedures are equal: Proposal for a classification of pancreatoduodenectomies. Surgery 2021;169:1456-62. [Crossref] [PubMed]
- Filho JELP, Tustumi F, Coelho FF, et al. The impact of venous resection in pancreatoduodectomy: A systematic review and meta-analysis. Medicine (Baltimore) 2021;100:e27438. [Crossref] [PubMed]
- Peng C, Zhou D, Meng L, et al. The value of combined vein resection in pancreaticoduodenectomy for pancreatic head carcinoma: a meta-analysis. BMC Surg 2019;19:84. [Crossref] [PubMed]
- Delpero JR, Sauvanet A. Vascular Resection for Pancreatic Cancer: 2019 French Recommendations Based on a Literature Review From 2008 to 6-2019. Front Oncol 2020;10:40. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Oba A, Ito H, Ono Y, et al. Regional pancreatoduodenectomy versus standard pancreatoduodenectomy with portal vein resection for pancreatic ductal adenocarcinoma with portal vein invasion. BJS Open 2020;4:438-48. [Crossref] [PubMed]
- Fortner JG, Kim DK, Cubilla A, et al. Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg 1977;186:42-50. [Crossref] [PubMed]
- Younan G, Tsai S, Evans DB, et al. Techniques of Vascular Resection and Reconstruction in Pancreatic Cancer. Surg Clin North Am 2016;96:1351-70. [Crossref] [PubMed]
- Tee MC, Krajewski AC, Groeschl RT, et al. Indications and Perioperative Outcomes for Pancreatectomy with Arterial Resection. J Am Coll Surg 2018;227:255-69. [Crossref] [PubMed]
- Bachellier P, Addeo P, Faitot F, et al. Pancreatectomy With Arterial Resection for Pancreatic Adenocarcinoma: How Can It Be Done Safely and With Which Outcomes?: A Single Institution's Experience With 118 Patients. Ann Surg 2020;271:932-40. [Crossref] [PubMed]
- Del Chiaro M, Rangelova E, Halimi A, et al. Pancreatectomy with arterial resection is superior to palliation in patients with borderline resectable or locally advanced pancreatic cancer. HPB (Oxford) 2019;21:219-25. [Crossref] [PubMed]
- Inoue Y, Oba A, Ono Y, et al. Radical Resection for Locally Advanced Pancreatic Cancers in the Era of New Neoadjuvant Therapy-Arterial Resection, Arterial Divestment and Total Pancreatectomy. Cancers (Basel) 2021;13:1818. [Crossref] [PubMed]
- Hackert T, Weitz J, Büchler MW. Splenic artery use for arterial reconstruction in pancreatic surgery. Langenbecks Arch Surg 2014;399:667-71. [Crossref] [PubMed]
- Diener MK, Mihaljevic AL, Strobel O, et al. Periarterial divestment in pancreatic cancer surgery. Surgery 2021;169:1019-25. [Crossref] [PubMed]
- Loos M, Kester T, Klaiber U, et al. Arterial Resection in Pancreatic Cancer Surgery: Effective After a Learning Curve. Ann Surg 2022;275:759-68. [Crossref] [PubMed]
- Truty MJ. Commentary on: Periarterial divestment in pancreatic cancer surgery. Surgery 2021;169:1041-3. [Crossref] [PubMed]
- Zhou Q. Assessement of postoperative long-term survival quality and complications associated with radical antegrade modular pancreatosplenectomy and distal pancreatectomy: a meta-analysis and systematic review. BMC Surg 2019;19:12. [Crossref] [PubMed]
- Hackert T, Schneider L, Büchler MW. Current State of Vascular Resections in Pancreatic Cancer Surgery. Gastroenterol Res Pract 2015;2015:120207. [Crossref] [PubMed]
- Klompmaker S, Boggi U, Hackert T, et al. Distal Pancreatectomy with Celiac Axis Resection (DP-CAR) for Pancreatic Cancer. How I do It. J Gastrointest Surg 2018;22:1804-10. [Crossref] [PubMed]
- Heckler M, Hackert T. Surgery for locally advanced pancreatic ductal adenocarcinoma-is it only about the vessels? J Gastrointest Oncol 2021;12:2503-11. [Crossref] [PubMed]
- Del Chiaro M, Rangelova E, Segersvärd R, et al. Are there still indications for total pancreatectomy? Updates Surg 2016;68:257-63. [Crossref] [PubMed]
- Stoop TF, Ateeb Z, Ghorbani P, et al. Surgical Outcomes After Total Pancreatectomy: A High-Volume Center Experience. Ann Surg Oncol 2021;28:1543-51. [Crossref] [PubMed]
- Passeri MJ, Baker EH, Siddiqui IA, et al. Total compared with partial pancreatectomy for pancreatic adenocarcinoma: assessment of resection margin, readmission rate, and survival from the U.S. National Cancer Database. Curr Oncol 2019;26:e346-56. [Crossref] [PubMed]
- Kulu Y, Schmied BM, Werner J, et al. Total pancreatectomy for pancreatic cancer: indications and operative technique. HPB (Oxford) 2009;11:469-75. [Crossref] [PubMed]
- Reddy S, Wolfgang CL, Cameron JL, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg 2009;250:282-7. [Crossref] [PubMed]
- Crippa S, Belfiori G, Tamburrino D, et al. Indications to total pancreatectomy for positive neck margin after partial pancreatectomy: a review of a slippery ground. Updates Surg 2021;73:1219-29. [Crossref] [PubMed]
- Loos M, Al-Saeedi M, Hinz U, et al. Categorization of Differing Types of Total Pancreatectomy. JAMA Surg 2022;157:120-8. [Crossref] [PubMed]
- Fernandes ESM, Moraes-Junior JMA, Vasques RR, et al. Combined venous and arterial reconstruction in the triangle area after total pancreatoduodenectomy. Arq Bras Cir Dig 2022;35:e1643. [Crossref] [PubMed]
- Loos M, Mehrabi A, Ramouz A, et al. Gastric Venous Congestion After Total Pancreatectomy is Frequent and Dangerous. Ann Surg 2022;276:e896-904. [Crossref] [PubMed]
- Wei K, Hackert T. Surgical Treatment of Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 2021;13:1971. [Crossref] [PubMed]
- Takeda T, Sasaki T, Okamoto T, et al. Outcomes of pancreatic cancer with liver oligometastasis. J Hepatobiliary Pancreat Sci 2023;30:229-39. [Crossref] [PubMed]
- Hank T, Klaiber U, Hinz U, et al. Oncological Outcome of Conversion Surgery After Preoperative Chemotherapy for Metastatic Pancreatic Cancer. Ann Surg 2022; Epub ahead of print. [Crossref] [PubMed]
- van Hilst J, de Graaf N, Abu Hilal M, et al. The Landmark Series: Minimally Invasive Pancreatic Resection. Ann Surg Oncol 2021;28:1447-56. [Crossref] [PubMed]
- de Rooij T, van Hilst J, van Santvoort H, et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg 2019;269:2-9. [Crossref] [PubMed]
- Yang DJ, Xiong JJ, Lu HM, et al. The oncological safety in minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Sci Rep 2019;9:1159. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Klompmaker S, et al. Minimally Invasive versus Open Distal Pancreatectomy for Ductal Adenocarcinoma (DIPLOMA): A Pan-European Propensity Score Matched Study. Ann Surg 2019;269:10-7. [Crossref] [PubMed]
- van Hilst J, Korrel M, Lof S, et al. Minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma (DIPLOMA): study protocol for a randomized controlled trial. Trials 2021;22:608. [Crossref] [PubMed]
- van Hilst J, de Rooij T, Bosscha K, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol 2019;4:199-207. [Crossref] [PubMed]
- Strobel O, Büchler MW. Laparoscopic pancreatoduodenectomy: safety concerns and no benefits. Lancet Gastroenterol Hepatol 2019;4:186-7. [Crossref] [PubMed]
- Aiolfi A, Lombardo F, Bonitta G, et al. Systematic review and updated network meta-analysis comparing open, laparoscopic, and robotic pancreaticoduodenectomy. Updates Surg 2021;73:909-22. [Crossref] [PubMed]



