
Medullary carcinoma of the duodenum treated with pembrolizumab: a case report
Highlight box
Key findings
• This is the first report of medullary carcinoma of the duodenum as well as the first medullary carcinoma to be treated with pembrolizumab in the first line setting.
What is known and what is new?
• Medullary carcinoma of the small intestine is exceedingly rare. All nine previously reported cases were treated with surgical resection. Our case highlights a potential alternative treatment option in the setting of medullary carcinoma of the small intestine with unresectable disease.
What is the implication, and what should change now?
• Further case data will be needed to determine whether the success from immune checkpoint inhibitors in MSI-H colorectal carcinomas can be translated to the treatment of MC of the colon or small intestine.
Introduction
Medullary carcinoma (MC) is a unique histopathologic subtype of colorectal cancer characterized by a solid growth pattern with poor glandular differentiation and prominent intraepithelial lymphocytic infiltrate (1). A population-based analysis using the Surveillance Epidemiology and End Results (SEER) database found that MCs accounted for 5–8 cases for every 10,000 colon cancers diagnosed (2). However, even more uncommon are cases of MC of the small intestine, with only nine cases described in the literature (3-7). In all of these reported cases, the tumors were located either in the ileum, jejunum, or not specified. Given the rarity of the tumor and limited data available, the best treatment for this patient group remains unclear. For localized tumors, surgical resection appears to be the mainstay of treatment based on previous reports. Here, we report the case of a patient with unresectable microsatellite instability-high (MSI-H) MC of the duodenum treated with pembrolizumab. We present the following case in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-755/rc).
Case presentation
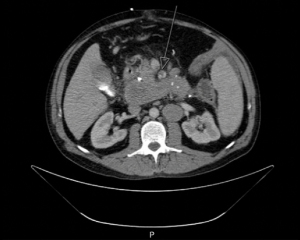
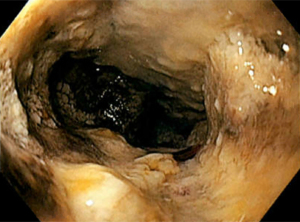
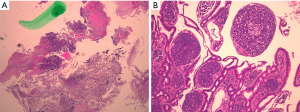
A 50-year-old man with history of adenocarcinoma of the proximal descending colon status post hemicolectomy and adjuvant treatment with chemotherapy with capecitabine and oxaliplatin in 2019 presented with abdominal pain and distension for two weeks. He also reported nausea, vomiting, decreased appetite, and constipation with black stools. He had a 10 pack-year smoking history, but no history of alcohol or drug use. Family history was notable for Lynch syndrome in two of his maternal uncles. Physical exam was significant for diffuse abdominal tenderness without any distension, rebound, or guarding. Laboratory evaluation including a complete blood count and comprehensive metabolic panel was remarkable for microcytic anemia with a hemoglobin of 7.3 (13.5–17.5 g/dL). Computed tomography (CT) of the abdomen and pelvis revealed a 10.7 cm by 4.3 cm mass in the mid-portion of the duodenum abutting the pancreatic head (Figure 1). Esophagogastroduodenoscopy (EGD) demonstrated circumferential, partially obstructing, intrinsic stenosis of the duodenum with ampullary involvement and likely invasion into the pancreatic head and common bile duct (Figure 2). Endoscopic biopsy of the primary tumor demonstrated sheets and nests of malignant cells with extensive tissue necrosis (Figure 3A,3B), suggestive of poorly differentiated carcinoma. Immunohistochemical staining was diffusely and strongly positive for pancytokeratin (AE1/AE3) and SATB2. Proliferation index assessed by Ki-67 was 80% by nuclear staining. The malignant cells were negative for CK20, CK7, CDX2, synaptophysin, CD56, calretinin, p63, p40, NKX3.1, TTF-1, Arginase-1, and S100. Of note, there was loss of MLH1 and PMS2 expression. Next-generation sequencing via Omniseq Advance assay determined the tumor as MSI-H with tumor mutation burden of 37.4/Mb and PD-L1 expression of 60% as well as identified a BRCA2 c.6361_6362 deletion mutation. Staging with CT of the chest with contrast showed no evidence of intrathoracic disease. Positron emission tomography (PET) scan redemonstrated circumferential duodenal wall thickening and hypermetabolic activity with an SUVmax of 26.4, as well as PET-avid epigastric, retroperitoneal, and periaortic lymphadenopathy suggestive of metastases (Figure 4A,4B). BRAFV600E mutation molecular testing was negative. MLH1 promoter methylation testing was positive. Genetic testing was also performed and showed the heterozygous MLH germline c.350C>T mutation. He was started on immunotherapy with pembrolizumab 200 mg every 3 weeks. At cycle 3 of his treatment, his pain had significantly improved, and he continued to tolerate diet and gain weight. He had no immunotherapy-related toxicities. On repeat PET/CT, he was found to have stable disease without worsening of obstruction. Unfortunately, the patient was then lost to follow-up.

Of note, the original pathology of the descending colon revealed poorly differentiated carcinoma with equivocal expression of MLH-1 and partial loss of PMS-2 protein expression. The tumor cells were negative for CK20, CK7, synaptophysin, CD56 but positive for cytokeratin (AE1/AE3) and CDX2.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
MC is a relatively new and distinct histologic subtype of colorectal carcinoma designated by the World Health Organization. It was formerly known as large cell adenocarcinoma with minimal differentiation because of its characteristic sheets of malignant cells with vesicular nuclei, prominent nucleoli, and abundant cytoplasm. However, it is difficult to differentiate MC and poorly differentiated adenocarcinoma based on histology alone since they are morphologically similar. Therefore, immunohistochemical analysis is essential for confirming the diagnosis of MC. The medullary phenotype of colon cancer has been demonstrated to have a strong association with microsatellite instability in at least 60% of cases (8,9). Several studies have sought to characterize the immunohistochemical profile of MC, determining that a significantly higher proportion of MC, as opposed to poorly differentiated colorectal carcinomas, showed loss of staining for mutl homolog1 (MLH-1) and for the intestinal transcription factor CDX2 (9-11). In addition, calretinin staining has been found to be strongly positive in 73% of MCs compared to only 12% of poorly differentiated colonic carcinomas (11). It should be noted that calretinin was negative in our patient, which appears to argue against MC. However, the overall morphology and the aberrant immunohistochemical pattern with loss of CDX2 and CK20 as well as the absent expression of the mismatch repair proteins MLH1 and PMS2 were compatible with a medullary subtype (11).
MC of the small intestine is an exceedingly rare diagnosis. Only nine other cases have been reported in the medical literature (3-7). The tumors were located in the jejunum (4,5), ileum (6,7), or in an unspecified part of the small intestine (3). All but two of these cases were MSI-H, consistent with our patient. Interestingly, the first reported case of MC of the small intestine by Peycru et al. was found to be microsatellite stable (7). The MSI status of the other patient (6) was not specified. Compared to MC of the colon, it is difficult to determine the prognosis of this disease as most of these cases lack information regarding follow-up. Of the cases with follow-up data reported, one patient remained disease-free 6 years after resection (5), one patient remained disease-free at 1 year after resection (6), and one patient remained disease-free at an unspecified follow-up date (4).
We contribute the first case of MC of the small intestine located in the duodenum as well as the first MC to be treated with immunotherapy. Considering that colorectal metastasis can morphologically mimic a primary small intestinal or upper gastrointestinal carcinoma, we sought to exclude the possibility of metastatic colon cancer. Original pathology from the descending colon was compared with the pathology from the duodenum, revealing the discordance of CDX2 expression between the two tumors and suggesting our patient’s lesion to more likely represent a primary duodenal carcinoma rather than metastatic recurrence of his previous colorectal carcinoma. BRAF molecular testing and MLH1 hypermethylation testing were pursued to distinguish between a germline mutation and somatic inactivation of MLH1. Given the patient’s family history of Lynch syndrome, genetic testing for a germline MLH1 mutation was also performed, which indeed revealed a heterozygous MLH germline c.350C>T mutation. Interestingly, the patient’s tumor tested negative for the BRAFV600E mutation however tested positive for MLH1 promoter hypermethylation, suggesting a sporadic mutation. Although Lynch Syndrome is characterized by abnormal IHC for MMR proteins and microsatellite instability, it is certainly possible that this patient with Lynch Syndrome had developed a sporadic duodenal tumor due to abnormal methylation of the MLH1 gene promoter.
MC is a rare histologic tumor with no standard treatment. However, consistent with other tumors of the gastrointestinal tract, surgical resection appears to be the mainstay of treatment for those with localized disease in which the role of adjuvant systemic therapy is unclear (3-7). Our patient’s case was discussed at our institution’s multidisciplinary meeting, and he was deemed not a candidate for surgical resection in the setting of PET-avid epigastric, retroperitoneal, and periaortic lymphadenopathy suggestive of metastases. In the metastatic disease setting, given the majority of the cases are MSI-H and extrapolating from KEYNOTE-177, single agent pembrolizumab might be an appropriate option as first line for these tumors (12). In addition, this tumor was found to be TMB-high, which further supported the choice of pembrolizumab as a viable treatment option with better tolerability compared to cytotoxic regimens (13). However, treatment duration was relatively short, and PET/CT only showed stable disease after three cycles of pembrolizumab. As KEYNOTE-177 demonstrated a median time to response of 2.2 months with pembrolizumab, it remains to be seen whether the magnitude of benefit from immune checkpoint inhibitors in MSI-H MC is similar to that of MSI-H colorectal cancers.
An important limitation of this case report is the lack of data available following the patient’s treatment with pembrolizumab. Although he demonstrated improvement in his symptoms and stable disease on PET/CT, we acknowledge that it is difficult to evaluate continued clinical or imaging response to immunotherapy as he was lost to follow up. It should also be noted that the gold standard for the classification of MC is via resection of the tumor. However, in our case, histological diagnosis was confirmed using core biopsy alone based on distinct microscopic and immunohistochemical characteristics.
Conclusions
To our knowledge, this is the first case of duodenal MC as well as the first report of MC to be treated with pembrolizumab in the first line setting. Given the rarity of the condition, specific management guidelines have yet to be established. In the last decade, immune checkpoint inhibitors have been demonstrated to provide durable clinical response, excellent tolerability, and in some cases, even cure in a wide range of tumor types. It remains to be seen whether the benefits of immunotherapy can be translated to the treatment of MC of the colon or small intestine. Further investigations are certainly warranted in this unique population.
Acknowledgments
This case was presented at the 2021 Precision Oncology Summit: Personalizing Treatments to Improve Patient Outcomes conference on October 3, 2021. The conference abstract was published by the International Journal of Cancer Care and Delivery in October 2021.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-755/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-755/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-755/coif). FD has disclosed receiving research grants from AstraZeneca, Bristol-Myers Squibb, Merck, Genentech/Roche, Taiho, Exelixis, Trishula, Leap Therapeutics; receiving consultancy honorarium from Natera, QED, Eisai, Exelixis, Genentech/Roche; and receiving speaker honorarium from Amgen, Eisai, Ipsen, Exelixis, Sirtex, Deciphera, Ipsen, and Natera. May Cho has disclosed receiving honorarium from Amgen, Incyte, Eisai, Ipsen, Astellas, Taiho, Exelixis, QED, I-Mab, Tempus, Seagen, HelioDx, Bayer, AstraZeneca, Genentech/Roche, Pfizer, Natera, Taiho, BMS, Basilea, DSI, and Helsinn. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pyo JS, Sohn JH, Kang G. Medullary carcinoma in the colorectum: a systematic review and meta-analysis. Hum Pathol 2016;53:91-6. [Crossref] [PubMed]
- Thirunavukarasu P, Sathaiah M, Singla S, et al. Medullary carcinoma of the large intestine: a population based analysis. Int J Oncol 2010;37:901-7. [PubMed]
- Shamekh R, Ghayouri M, Centeno B, et al. Microsatellite Instability Associated Medullary Carcinoma of the Small Intestine: Diagnostic Pitfall and Impact on Patient Care. Am J Clin Pathol 2014;142:A277. [Crossref]
- Slack D, Sandeep Sachidananda S, Zdankiewicz P, et al. Synchronous Medullary Carcinomas of the Small Bowel Presenting as Recurrent Small Bowel Obstruction. Surgery: Open Access 2019;1:003.
- Brcic I, Cathomas G, Vanoli A, et al. Medullary carcinoma of the small bowel. Histopathology 2016;69:136-40. [Crossref] [PubMed]
- Gonzalez HH, Sidhu S, Eisner T. A Rare Case of Medullary Carcinoma of the Ileum. Cureus 2018;10:e3721. [PubMed]
- Peycru T, Jarry J, Soubeyran I. Sporadic medullary carcinoma of the ileum. Clin Gastroenterol Hepatol 2011;9:A24. [Crossref] [PubMed]
- Gómez-Álvarez MA, Lino-Silva LS, Salcedo-Hernández RA, et al. Medullary colonic carcinoma with microsatellite instability has lower survival compared with conventional colonic adenocarcinoma with microsatellite instability. Prz Gastroenterol 2017;12:208-14. [Crossref] [PubMed]
- Hinoi T, Tani M, Lucas PC, et al. Loss of CDX2 expression and microsatellite instability are prominent features of large cell minimally differentiated carcinomas of the colon. Am J Pathol 2001;159:2239-48. [Crossref] [PubMed]
- Arai T, Esaki Y, Sawabe M, et al. Hypermethylation of the hMLH1 promoter with absent hMLH1 expression in medullary-type poorly differentiated colorectal adenocarcinoma in the elderly. Mod Pathol 2004;17:172-9. [Crossref] [PubMed]
- Winn B, Tavares R, Fanion J, et al. Differentiating the undifferentiated: immunohistochemical profile of medullary carcinoma of the colon with an emphasis on intestinal differentiation. Hum Pathol 2009;40:398-404. [Crossref] [PubMed]
- Shiu KK, Andre T, Kim TW, et al. KEYNOTE-177: Phase III randomized study of pembrolizumab versus chemotherapy for microsatellite instability-high advanced colorectal cancer. J Clin Oncol 2021;6. [Crossref]
- Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [Crossref] [PubMed]


