
Diagnosis and treatment of postoperative bleeding in patients after gastrectomy: a retrospective case series study
Highlight box
Key findings
• Postoperative hemorrhage for gastric cancer is an infrequent complication. The clinical situation is more complicated and the reoperation rate is higher in extra-intestinal hemorrhage. TAE is a safe and effective method if the hemodynamics are stable in delayed exsanguination.
What is known and what is new?
• Re-operation is currently recognized as a method for early-onset extra-intestinal bleeding. As for delayed extra-intestinal bleeding, the choice of re-operation or TAE is still controversial.
• Extra-intestinal hemorrhage is more likely to occur in delayed bleeding, and it is usually complex bleeding. Most cases are associated with an anastomotic fistula. TAE is a safe and effective means of hemostasis if the hemodynamics is stable.
What is the implication, and what should change now?
• The clinical situation is more complicated and the re-operation rate is higher in patients with extra-intestinal hemorrhage. TAE is a safe and effective method if the hemodynamics is stable.
Introduction
Owing to the advances in surgical techniques and perioperative patient care in recent decades, the incidence of postoperative bleeding following gastrectomy has been decreasing. The incidence of post-gastrectomy bleeding is reported to be 0.6–4% and has a high mortality (1-7). Hemorrhagic complications after gastric cancer (GC) surgery are infrequent and can be managed with conservative treatment, endoscopic intervention, radiologic intervention and re-laparotomy; however, they can be life-threatening in some patients. Post-gastrectomy bleeding can be classified as either an early or delayed type based on its timing. Early postoperative hemorrhage (EPH) is defined as hemorrhage occurring within 6 days postoperatively, while delayed postoperative hemorrhage (DPH) often occurs after 7 days. Moreover, post-gastrectomy bleeding can also be divided into intra-intestinal and extra-intestinal bleeding according to the location of the bleeding. There are significant differences in terms of the pathophysiology and clinical manifestations between intra- and extra-luminal bleeding, and it is also difficult to determine the reasonable management of the bleeding. However, there have been few studies that deal with postgastrectomy bleeding.
This study aims to identify proper therapeutic management for PH by analyzing the clinical presentation, diagnostic measures and means of treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1203/rc).
Methods
Patients selection
This study was a retrospective analysis performed in a single high-volume GC center. The patients’ characteristics and medical therapy data between May 2014 and September 2019 were abstracted from medical records of the Department of Surgery of Fujian Cancer Hospital, Fuzhou, China. Patients who were diagnosed with postoperative bleeding at the discharge diagnosis and met the definition of postoperative bleeding were included. A total gastrectomy or a distal or proximal subtotal gastrectomy with regional lymph node dissection (D1+ or D2) was performed according to the 4th edition Japanese Stomach Cancer Treatment Guidelines. Gastrointestinal surgeons with sufficient experience in gastrectomy and D2 lymphadenectomy performed or supervised all of the operations. The tumor stages of GC were classified according to the 8th edition Tumor Node Metastasis (TNM) classification of the Union for International Cancer Control (UICC). Mortality was calculated based on the patient’s survival status at the time of discharge. Patients who underwent exploratory laparotomy, bypass surgery, invasion of the adjacent organs, distant metastasis, or gastric stump carcinoma, and those with incomplete or missing medical records were not included in this study. All participants gave informed consent before taking part. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Fujian Cancer Hospital (No. K2022-209-01).
Variables and definitions
Postoperative hemorrhage was defined as bleeding with a hemoglobin level decrease of more than 20 g/L within 24 hours. The diagnostic procedures of PH were considered as follows: clinical manifestation, blood routine, blood pressure measurement, bedside ultrasonography, abdominocentesis, or angiography and so on. Based on its origin, the bleeding was classified as either intra-intestinal bleeding and extra-intestinal bleeding. intra-intestinal bleeding was diagnosed according to its symptoms, such as melena, hematemesis, or hematochezia, with a drop in hemoglobin levels (≥2 mg/dL in 24 h), or based on endoscopic findings. extra-intestinal bleeding was diagnosed by a bloody abdominal drain or abdominal distension, with radiological findings and a drop in hemoglobin. Also, based on the occurrence of bleeding on the day after the operation, it can be divided into early [postoperative days (POD) ≤6 d] and delayed (POD ≥7 d) bleeding.
Statistical analysis
The Statistical Package for Social Science (SPSS) version 26.0 for Windows (IBM, Chicago, IL, USA) was used for all statistical analyses. Most of the analyses are descriptive. The results were expressed as percentages or as the mean ± standard deviation (SD). Univariate analysis was performed using the chi-square test or Fisher’s exact test to determine the associations between the variables and bleeding complications. All tests were two sided, with a priori significance level set at P<0.05.
Results
Basic characteristics of patients
A total of 2,978 patients underwent radical gastrectomy, and 85 patients (2.85%) suffered postoperative bleeding. The clinical characteristics of these 85 patients are summarized in Table 1. There were 67 men and 18 women, and four patients died, with a bleeding-related mortality rate of 4.7%.
Table 1
| Characteristics | Total (n, %) | Intra-Intestinal, n | Extra-Intestinal, n | P value |
|---|---|---|---|---|
| Sex | ||||
| Male | 67 (78.8) | 35 | 32 | 0.052 |
| Female | 18 (21.2) | 11 | 7 | |
| Age (years), mean ± SD | 59.09±10.7 | 58.07±11.67 | 60.31±9.50 | 0.340 |
| Previous abdominal operation | 16 (18.8) | 10 | 6 | 0.327 |
| Neoadjuvant chemotherapy | 20 (23.5) | 10 | 10 | 0.673 |
| Surgical approach method | ||||
| Open | 39 (45.9) | 20 | 19 | 0.629 |
| Laparoscopic | 46 (54.1) | 26 | 20 | |
| Primary operation | ||||
| STG | 27 (31.8) | 17 | 10 | 0.264 |
| TG | 58 (68.2) | 29 | 29 | |
| Extent of lymphadenectomy | ||||
| D1 | 9 (10.6) | 1 | 8 | 0.019 |
| D2 | 38 (44.7) | 21 | 17 | |
| D2+ | 38 (44.7) | 24 | 14 | |
| Combined resection | 5 (5.9) | 2 | 3 | 0.516 |
| TNM stage | ||||
| I | 19 (22.4) | 10 | 9 | 0.205 |
| II | 23 (27.1) | 15 | 8 | |
| III | 37 (43.5) | 20 | 17 | |
| IV | 6 (7.1) | 1 | 5 |
SD, standard deviation; STG, subtotal gastrectomy; TG, total gastrectomy; TNM, tumor node metastasis.
Clinical characteristics of intra-intestinal and extra-intestinal hemorrhages
Table 2 summarizes the clinical manifestations and characteristics of intra-intestinal and extra-intestinal hemorrhages. There were 46 cases of intra-intestinal hemorrhage and 39 cases of extra-intestinal hemorrhage. All cases of intra-intestinal bleeding were anastomotic bleeding, and 32 cases (32/39) of extra-intestinal bleeding were arterial bleeding. The incidence of intraperitoneal infection and anastomotic leakage in the intra-intestinal bleeding group was higher than that in the extra-intestinal bleeding group (48.72% vs. 13.04%, P=0.001; 30.77% vs. 4.35%, P=0.002). Also, 78.26% (36/46) of intra-intestinal hemorrhage cases were diagnosed by clinical symptoms; 8.70% (4/46) were diagnosed by computerized tomography (CT), and 6.52% (3/46) were diagnosed by B-ultrasound. As for extra-intestinal hemorrhage, 35.90% (14/39) of cases were diagnosed by clinical manifestations, 38.46% (15/39) were diagnosed by CT, and 10.26% (4/39) were diagnosed by angiography, 15.38% (6/39) were diagnosed by B-ultrasound.
Table 2
| Variables | Intra-intestinal (n=46) | Extra-intestinal (n=39) | P value |
|---|---|---|---|
| Onset time (days), n (%) | |||
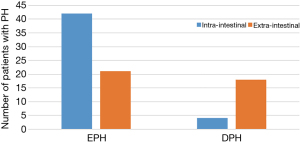
| EPH (POD ≤6) | 42 (91.30) | 21 (53.85) | <0.001 |
| DPH (POD ≥7) | 4 (8.70) | 18 (46.15) | |
| Hb drop (mg/dL), mean ± SD | 46.5±18.8 | 46.0±19.4 | 0.831 |
| Bleeding site, n (%) | |||
| Anastomosis | 46 (100.00) | 3 (7.69) | |
| Artery | 0 | 32 (82.05) | |
| Unknown | 0 | 4 (10.26) | |
| Abdominal infection, n (%) | 0.001 | ||
| Yes | 6 (13.04) | 19 (48.72) | |
| No | 40 (86.96) | 20 (51.28) | |
| Anastomotic fistula, n (%) | |||
| Yes | 2 (4.35) | 12 (30.77) | 0.002 |
| No | 44 (95.65) | 27 (69.23) | |
| Confirmative diagnostic tools, n (%) | |||
| Clinical symptoms | 36 (78.26) | 14 (35.90) | |
| Endoscopy | 3 (6.52) | 0 | |
| Angiography | 0 | 4 (10.26) | |
| CT | 4 (8.70) | 15 (38.46) | |
| Ultrasonography | 3 (6.52) | 6 (15.38) | |
| Therapeutic approach, n (%) | |||
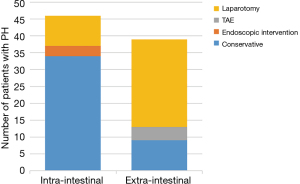
| Conservative | 34 (73.91) | 9 (23.08) | |
| Endoscopic intervention | 3 (6.52) | 0 | |
| Radiologic intervention | 0 | 4 (10.26) | |
| Laparotomy | 9 (19.57) | 26 (66.67) | <0.001 |
| Bleeding-related mortality | 1 (2.17) | 3 (7.69) | 0.329 |
EPH, early postoperative hemorrhage; DPH, delayed postoperative hemorrhage; POD, postoperative day; Hb, haemoglobin; SD, standard deviation; CT, computerized tomography.
Occurrence time of intra-intestinal and extra-intestinal bleeding
Figure 1 summarizes the differences in the timing of intra-intestinal and extra-intestinal bleeding. The incidence of delayed bleeding in the external bleeding group was higher than that in the internal bleeding group (46.15% vs. 8.70%, P<0.001). The bleeding time of intra-intestinal hemorrhage was 3 days, while that of external bleeding was 6 days.
Comparison of the delayed bleeding reoperation and transcatheter arterial embolization (TAE) groups
Table 3 summarizes the clinical characteristics of the delayed bleeding reoperation and TAE groups. A total of 11 patients underwent reoperation to stop the bleeding. In the reoperation group, eight patients had hemodynamic instability, eight had an abdominal infection, and three patients died of bleeding-related complications. Moreover, a total of four patients underwent TAE hemostasis, all of whom were hemodynamically stable, and two of them had an abdominal infection, both of which were successfully hemostatic.
Table 3
| Variables | Surgery (n=11) | TAE (n=4) |
|---|---|---|
| Hemodynamic instability, n (%) | 8 (72.73) | 1 (25.00) |
| Abdominal infection, n (%) | 7 (63.64) | 2 (50.00) |
| Anastomotic fistula, n (%) | 7 (63.64) | 2 (50.00) |
| Bleeding-related mortality, n (%) | 3 (27.27) | 0 |
TAE, transcatheter arterial embolization.
Differences in the treatment methods between intra-intestinal and extra-intestinal hemorrhages
Figure 2 summarizes the treatment of intra-intestinal and extra-intestinal bleeding. Patients with external bleeding were more likely to undergo reoperation for hemostasis than those with internal bleeding (66.67% vs. 19.57%, P<0.001). Thirty-four patients (34/46) with intra-intestinal hemorrhage were treated conservatively, and one patient died of multiple organ failure. Furthermore, eight patients (8/46) with intra-intestinal hemorrhage underwent endoscopy first, of which three patients were successfully hemostatic under endoscopy, and five patients were successfully hemostatic due to the inability of endoscopy to explore the bleeding site. Additionally, four patients with intra-intestinal hemorrhage were directly treated with exploratory laparotomy for hemostasis, and the hemostasis was successful. Among the patients with extra-intestinal bleeding, 26 (26/39) patients chose re-laparotomy as the first choice for hemostasis, and three patients died due to bleeding-related complications. Nine patients (9/39) were treated conservatively, and four patients (4/39) underwent interventional embolization and achieved successful hemostasis.
Discussion
In this study, we found that the incidence of postoperative bleeding in GC patients was 2.85%, intra-intestinal hemorrhage occurred mostly in the early postoperative period, and the success rate of conservative treatment was high. In total, 34 cases (34/42) of early intra-intestinal hemorrhage were treated conservatively, and one patient died. Intravenous bleeding was typically caused by the failure of blood vessels at the broken end of the digestive tract to close or reopen effectively, resulting in blood flowing into the digestive tract, which is clinically manifested as hematemesis or nasogastric tube drainage of fresh blood. Despite the obvious decrease in hemoglobin, the hemodynamics of patients are generally stable, and thus, the success rate of conservative treatment is high (8). In addition, with intravenous bleeding, the pressure in the intestinal cavity increases rapidly, which can also play a role in local compression and hemostasis. Conservative treatments, such as intravenous supplementation of clotting factors, hemostatic drugs, infusion of blood products, and close observation, are usually successful in stopping bleeding. If the amount of bleeding is large and the hemodynamic is unstable, it is necessary to actively stop the bleeding through endoscopy or surgery (9). Previous studies have reported the effectiveness of hemostasis under endoscopy using a hemostatic clip, heater coagulation hemostasis, epinephrine, and other means (4,10). However, endoscopy for early intra-intestinal bleeding usually involves the risk of anastomotic complications, and accurately determining the bleeding site under endoscopy may not be feasible. In this study, eight cases of early intra-intestinal hemorrhage underwent endoscopic examination, among which three cases were successfully hemostasis. Also, a large number of blood clots in the intestinal cavities were visible under endoscopy in five patients; however, observing the bleeding site was not possible in these cases, so they chose to perform abdominal exploration again for hemostasis, and all of them successfully achieved hemostasis.
Extra-intestinal bleeding after radical gastrectomy represents the most severe hemorrhage, manifesting as abdominal arterial bleeding or external anastomotic bleeding (or both). Compared with intra-intestinal bleeding, early extra-luminal bleeding is usually characterized by more significant bleeding, more unstable hemodynamics, a higher probability of reoperation, and higher mortality. Most of the extra-luminal bleeding is arterial, and thus, it is difficult to stop the bleeding by conservative treatment. Furthermore, the abdominal cavity space is larger, making it difficult to limit the hematoma. Persistent bleeding from the abdomen is ultimately managed with surgery or TAE.
In this study, 32 patients (32/39) with extra-intestinal blood were cases of arterial bleeding. In addition, for patients with delayed hemorrhage, the proportion of extra-intestinal blood was very high, with 18 cases (18/22) of delayed hemorrhage being extra-intestinal bleeding. The possible reasons for this are as follows: (I) during radical gastrectomy, to perform a thorough lymph node dissection, the surgeon usually performs "vascular skeletonization", which may damage the vascular outer membrane of the artery and cause postoperative arterial bleeding; (II) in cases where there is a postoperative digestive tract fistula, the leakage of intestinal fluid, pancreatic fluid, and/or bile corrodes the artery wall and causes bleeding; and (III) abdominal infection can also cause damage to the artery wall, and may also damage the anastomosis and form an anastomotic fistula, further damaging the artery membrane and causing bleeding. The most common incidence time of anastomotic fistula is about 1 week postoperatively, and it also takes some time for bleeding caused by digestive fluid or abdominal infection to corrode the artery, which may be a possible reason why bleeding in the delayed period is usually extra-intestinal bleeding. When extra-intestinal bleeding occurs, clinicians prefer to stop bleeding with re-operation or TAE, as compared to intravenous bleeding. In this study, 26 cases (26/39) of extra-intestinal bleeding were stopped by re-operation, and three patients died.
Early-onset extra-intestinal bleeding is caused by technical failure (11). Re-operation is currently recognized as a method for exact hemostasis. In the present study, early and late extra-intestinal bleeding occurred in 25 cases and reoperation was performed in 15 cases. In these cases, the vital signs deteriorated rapidly and there was obvious intra-abdominal bleeding, which usually required emergency re-laparotomy to control the success of the bleeding. Only one patient was successfully treated by TAE because of a ruptured pseudoaneurysm on the 6th day postoperatively.
Arterial erosion, and potential arterial wall injury during lymph node dissection are the two main reasons for the delayed extra-intestinal bleeding (11,12). The erosion of arterial vessels resulting from intra-abdominal contamination of enteric, pancreatic, and/or bile juice from a leaking anastomosis can cause DPH and lead to pseudoaneurysm, which, in turn, can rupture and lead to delayed extra-intestinal bleeding (13). As for delayed extra-intestinal bleeding, the choice of re-operation or TAE is still controversial. Encouraging results have been reported after TAE, which is an effective, stand-alone treatment modality for achieving hemostasis in patients with pseudoaneurysms, with a reported success rate of between 79% and 83% (5,14). TAE has certain advantages for bleeding caused by pseudoaneurysms. The key to the success of TAE treatment is to locate the responsible vessel and embolize it quickly and effectively. Since there is a wide range of communicating branches in the celiac artery system, embolization should be carried out not only at the proximal end but also at the distal end if necessary. Even after effective embolization, some patients will bleed again and require interventional embolization. Yang et al. compared the safety and efficacy of TAE and re-laparotomy in the treatment of delayed bleeding. The re-bleeding rate in the TAE group was higher than that in the re-operation group (36.48% vs. 25.0%) (15). Not all delayed extra-intestinal bleeding is caused by a ruptured pseudoaneurysm. Park et al. showed that TAE has no obvious advantage over re-operation for extra-intestinal bleeding caused by non-aneurysms (3). TAE has certain risks for patients with splenic artery bleeding after subtotal gastrectomy. Perfusion of the remnant stomach through the short gastric artery is very important. Complete embolization of the spleen trunk will significantly affect the blood supply of the remnant stomach and spleen. In the study of Han et al., there were two cases of remnant stomach or spleen infarction caused by splenic artery embolization, resulting in death (5).
On the other hand, delayed extra-intestinal bleeding is usually complex bleeding, and most cases are associated with an anastomotic fistula. Pancreatic studies have also confirmed that 100% of patients with complex postoperative bleeding have a pancreatic fistula (11). According to previous reports, 42% of GC patients with delayed postoperative bleeding had a pre-abdominal infection, and 62% were confirmed to have anastomotic or pancreatic leakage (3). This is consistent with the results of our study, in which eight cases (8/13) of delayed extra-intestinal bleeding were confirmed to have an anastomotic fistula or abdominal infection before bleeding. Even if interventional embolization is successful, the abdominal infection cannot be resolved. Re-operation can stop the bleeding, remove the abdominal abscess, and close the fistula, thereby reducing the inflammatory stimulation and digestive juice corrosion of blood vessels, which prevents the occurrence of recurrent bleeding. The difficulty of re-operation lies in abdominal adhesion, which cannot correctly locate the bleeding point, and thus, care must be taken in the second operation to avoid damage to abdominal organs, and retention of the jejunostomy tube can be considered for postoperative nutritional support.
Finally, patients who suffer delayed extra-intestinal bleeding are usually sent to the intensive care unit (ICU) for further monitoring and treatment. In our hospital, the location of the radiology department is not in the same building as the surgical operating rooms; therefore, if interventional embolization is to be performed, it takes a long time to transport patients to the radiology department. Also, the radiology department does not possess complete resuscitation and intubation equipment. If the intervention fails, it may be too late to send the patient back to the operating room for a second operation. Therefore, surgeons are usually reluctant to send a patient with hemodynamic instability to the radiology department.
In this study, hemodynamic instability was observed in 72.73% (8/11) of patients in the re-operation group for delayed hemorrhage and 25% (1/4) of patients in the TAE group. Patients with delayed extra-intestinal hemorrhage are more often treated with re-operation, and therefore, patients with delayed extra-intestinal bleeding included in this study were more likely to undergo surgery. Ideally, if there is interventional radiation equipment in the surgical operating room as well as the full support of an anesthesiologist, interventional embolization can be used as a first-line treatment. In this situation, if this intervention fails, the next operation can be carried out immediately, and if the condition of interventional embolization is not sufficient, timely open hemostasis is an effective means to save the patient’s life.
In this study, TAE intervention was performed in fewer cases, and statistical analysis could not be performed between the re-operation and TAE groups. The choice of intervention approach for postoperative bleeding in GC requires more large-scale studies. In this study, no statistical analysis was performed on the coagulation function of patients, because a large number of patients would have been transfused with plasma after surgery, which would affect the coagulation function indicators. Therefore, further studies are needed to confirm whether abnormal coagulation function is a risk factor for postoperative bleeding.
Conclusions
Bleeding after GC surgery is an infrequent complication that requires effective management. Compared with intra-intestinal hemorrhage, the clinical situation is more complicated and the re-operation rate is higher in patients with extra-intestinal hemorrhage. TAE is a safe and effective method if the hemodynamics is stable.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1203/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1203/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1203/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All participants gave informed consent before taking part. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Fujian Cancer Hospital (No. K2022-209-01).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20. [Crossref] [PubMed]
- Yu J, Huang C, Sun Y, et al. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA 2019;321:1983-92. [Crossref] [PubMed]
- Park JY, Kim YW, Eom BW, et al. Unique patterns and proper management of postgastrectomy bleeding in patients with gastric cancer. Surgery 2014;155:1023-9. [Crossref] [PubMed]
- Tanizawa Y, Bando E, Kawamura T, et al. Early postoperative anastomotic hemorrhage after gastrectomy for gastric cancer. Gastric Cancer 2010;13:50-7. [Crossref] [PubMed]
- Han K, Ahmed BM, Kim MD, et al. Clinical outcome of transarterial embolization for postgastrectomy arterial bleeding. Gastric Cancer 2017;20:887-94. [Crossref] [PubMed]
- Zhao D, Deng J, Cao B, et al. Short-term outcomes of D2 lymphadenectomy plus complete mesogastric excision for gastric cancer: a propensity score matching analysis. Surg Endosc 2022;36:5921-9. [Crossref] [PubMed]
- Li Z, Qian F, Zhao Y, et al. A comparative study on perioperative outcomes between robotic versus laparoscopic D2 total gastrectomy. Int J Surg 2022;102:106636. [Crossref] [PubMed]
- Yuan Y, Wang C, Hunt RH. Endoscopic clipping for acute nonvariceal upper-GI bleeding: a meta-analysis and critical appraisal of randomized controlled trials. Gastrointest Endosc 2008;68:339-51. [Crossref] [PubMed]
- Chak A, Cooper GS, Lloyd LE, et al. Effectiveness of endoscopy in patients admitted to the intensive care unit with upper GI hemorrhage. Gastrointest Endosc 2001;53:6-13. [Crossref] [PubMed]
- Kim KH, Kim MC, Jung GJ, et al. Endoscopic treatment and risk factors of postoperative anastomotic bleeding after gastrectomy for gastric cancer. Int J Surg 2012;10:593-7. [Crossref] [PubMed]
- Yekebas EF, Wolfram L, Cataldegirmen G, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg 2007;246:269-80. [Crossref] [PubMed]
- Correa-Gallego C, Brennan MF, D'Angelica MI, et al. Contemporary experience with postpancreatectomy hemorrhage: results of 1,122 patients resected between 2006 and 2011. J Am Coll Surg 2012;215:616-21. [Crossref] [PubMed]
- de Castro SM, Kuhlmann KF, Busch OR, et al. Delayed massive hemorrhage after pancreatic and biliary surgery: embolization or surgery? Ann Surg 2005;241:85-91.
- Song W, Yuan Y, Peng J, et al. The delayed massive hemorrhage after gastrectomy in patients with gastric cancer: characteristics, management opinions and risk factors. Eur J Surg Oncol 2014;40:1299-306. [Crossref] [PubMed]
- Yang J, Zhang XH, Huang YH, et al. Diagnosis and Treatment of Abdominal Arterial Bleeding After Radical Gastrectomy: a Retrospective Analysis of 1875 Consecutive Resections for Gastric Cancer. J Gastrointest Surg 2016;20:510-20. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


