
Comparison of efficacy and safety between endoscopic mucosal dissection and resection in the treatment of early gastrointestinal cancer and precancerous lesions: a systematic review and meta-analysis
Highlight box
Key findings
• This meta-analysis found that endoscopic mucosal dissection (EMD) for early gastrointestinal cancer and precancerous lesions can improve the complete tumor resection rate and reduce intraoperative bleeding, complications, operation time, and hospital stay and cost.
What is known and what is new?
• Improving the clinical diagnostic rate of early gastrointestinal cancer and precancerous disease is crucial for the treatment of this condition.
• This study systematically evaluated and analyzed the clinical effect of EMD of early gastrointestinal cancer and precancerous lesions by meta-analysis to evaluate the safety and effectiveness of EMD.
What is the implication, and what should change now?
• EMD has certain advantages in the treatment of early cancer of digestive tract, but due to insufficient included literatures and heterogeneous interference among literatures, whether it can be used as the first treatment mode needs to be further explored.
Introduction
Early gastrointestinal cancer and precancerous lesions are limited to the mucosa or submucosa and improving the clinical diagnosis rate of early gastrointestinal cancer and precancerous disease is very important for the treatment of this condition. In recent years, with the continuous changes in people’s lifestyles and dietary structures, the incidence of upper gastrointestinal cancer has increased every year, seriously affecting people’s lives, health, and safety (1-3). In the past, the first choice of treatment for early gastrointestinal cancer and precancerous lesions was radical surgery immediately after diagnosis, with a 5-year survival rate of 95%. However, this treatment involves many postoperative complications and high hospitalization costs, making it a controversial option (4,5).
With the increasing maturity of endoscopic diagnosis and treatment technology, gastrointestinal endoscopy has transformed from a diagnostic technology to one that integrates both diagnosis and treatment. EMD may be used as a curative treatment for gastrointestinal adenomas and early cancers confined to the mucosa and submucosa, with minimal risk of lymph node and distant metastases (6,7). It can not only safely and efficiently remove tumor tissue but also reduces the trauma to patients. Compared with surgical resection, EMD for early gastrointestinal cancer has less trauma, faster recovery and fewer complications. However, relative tumor clearance is not as good as surgical resection, and there may be a high rate of recurrence.
Although EMD has begun to be used in the treatment of gastrointestinal tumors abroad, it has been introduced in China for a relatively short period of time and is often used as a complementary treatment for surgical treatment (8). At present, there is still controversy on the clinical efficacy and safety of EMD compared with surgical resection and whether it can be used as the first choice for the treatment of early gastrointestinal cancer. It has been suggested that EMD for the early stage of the digestive tract has comparable clinical efficacy and prognosis to conventional radical surgery, but has less impact on the digestive tract (4). It has also been reported that EMD is superior to surgical resection in terms of early clinical efficacy and safety (9,10). But there are objections to this claim. One of these studies found that patients who underwent EMD for early gastric cancer showed higher complications than those who underwent surgical resection (11). Although these studies have reported endoscopic mucosal dissection for early gastrointestinal cancer, there is still a lack of reliable evidence given the small sample size of the studies. The purpose of this study was to evaluate the safety and efficacy of EMD in the treatment of early gastrointestinal cancer by means of Meta-analysis, to provide evidence-based medical research for the clinical treatment of early gastrointestinal cancer and precancerous lesions. We present the following article in accordance with the PRISMA reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-32/rc).
Methods
Search strategy
Literature on EMD for early gastrointestinal tract cancer and precancerous lesions was retrieved from the PubMed, Web of Science, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), Wanfang, cqvip.com (VIP) databases, websites and citation searching, and the retrieval was updated to November 29, 2022. Chinese and English search terms included endoscopic therapy, mucosal dissection, digestive tract early cancer, precancerous lesions, clinical effect, application value, clinical analysis, and so on.
Inclusion criteria
Types of research
The published articles were randomized controlled studies. The main content of the research was the clinical effect and application value of EMD for early digestive tract cancer and precancerous lesions.
Research object
(I) Studies involving patients with early digestive tract cancer and precancerous lesions aged ≥18 years old; (II) studies without gender or nationality restrictions; (III) articles with patients who were not combined with other major diseases.
Interventions
Patients in the observation group were treated with gastrointestinal endoscopy or endoscopic submucosal dissection, and patients in the control group were treated with conventional surgical resection or endoscopic resection.
Outcome indicators
Main outcome indicators: (I) complete tumor resection rate after treatment (%); (II) cumulative intraoperative blood loss (mL); (III) operation time (minutes); (IV) incidence of complications (%); (V) treatment cost (10,000 yuan).
Exclusion criteria
(I) Repeatedly published literature (we selected studies with complete data from similar articles published by the same author); (II) reviews, news, reviews, meta-analyses, and other types of research; (III) studies in which we were unable to obtain the full text and those with incomplete or unextractable outcome indicators.
Literature screening and data extraction
According to the above inclusion and exclusion criteria, two researchers first independently screened the literature, extracted the data, and evaluated the literature quality. Next, the two researchers conducted cross-checks; if there were any disagreements, a third researcher was brought in to discuss and make a decision. The extracted data related to the included literature included the following: (I) general information of included literature: first author, year of publication, sample size, average age, intervention measures, etc.; (II) outcome data: raw data of outcome indicators; (III) methodological information: randomization, allocation blinding, measurement blinding, and loss-to-follow-up data.
Literature quality evaluation
The Cochrane recommended bias evaluation tool was used to evaluate the literature quality of the included research, and the evaluation index included 6 items: (I) random method; (II) allocation blinding; (III) researcher and researcher blinding; (IV) data integrity; (V) optional report; (VI) others. Each item was rated as high risk, low risk, or unclear risk. If all quality criteria were met, the study had a low risk of bias, if any one or more of them were not met, the study had an unclear risk of bias, and if all were not met, there was a high risk of bias.
Statistical analysis
R (Version 4.2.1) software (Lucent Technologies, USA) was used for meta-analysis. Heterogeneity analysis was judged by the Q test and I2 value. If there was no statistical heterogeneity among the included studies (I2≤50%), the fixed effect model was used; if there was heterogeneity among the included studies (I2>50%), after further analyzing the sources of heterogeneity, a random effects model was used for analysis. For dichotomous and continuous variables, risk ratio (RR) and mean difference (MD) were used as effect size indicators, and the 95% confidence interval (CI) was calculated to analyze publication bias with the Egger test. If P>0.05, there is no publication bias. All the statistical indexes were bilateral, with P<0.05 as a significant difference.
Results
Literature search and screening results
After searching the databases, websites and citation searching, 1,925 articles were obtained, and 1,687 were removed after reading the titles and abstracts. After obtaining the full texts of 230 articles, full-text browsing was performed. Six repeated publications were excluded. Twenty-eight articles had no outcome indicators, and 120 articles had no comparison. Among the remaining articles, 43 studies that did not meet the requirements of the intervention measures of the observation group, and 25 reviews or news were excluded. Finally, 10 studies were included. The literature screening process and the general information of the included literature are shown in Figure 1 and Table 1, respectively.
Table 1
| First author | Year | Sample sizes (observation group/control group) | Average age (years) | Sex (male/female) | Inventions | Outcome indicators | Risk of bias | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Observation group | Control group | Observation group | Control group | |||||||
| Fan (5) | 2019 | 147/147 | 49.12/46.03 | 83/64 | 82/65 | Gastrointestinal endoscopy | Conventional treatment | ②③⑤ | Unclear | |
| Nan (12) | 2018 | 27/26 | 56.23/56.20 | 13/14 | 12/14 | Endoscopic submucosal dissection | Conventional treatment | ①②③④ | Unclear | |
| Wu (13) | 2019 | 45/45 | 42.3/42.4 | 28/17 | 29/16 | Endoscopic submucosal dissection | Conventional treatment | ①②④⑤⑥ | Unclear | |
| Yan (3) | 2019 | 34/34 | 54.39/55.47 | 20/14 | 18/16 | Gastrointestinal endoscopy | Conventional treatment | ①③⑤⑥ | Low | |
| Li (14) | 2020 | 60/60 | 56.25/55.73 | 35/25 | 37/23 | Endoscopic submucosal dissection | Conventional treatment | ①②③④⑤ | Low | |
| Song (1) | 2022 | 94/94 | 46.17/46.56 | 45/49 | 48/46 | Gastrointestinal endoscopy | Conventional treatment | ②③④⑤⑥ | Low | |
| Song (10) | 2021 | 30/30 | 40.54/41.54 | 16/14 | 15/15 | Gastrointestinal endoscopy | Conventional treatment | ①②③④⑤ | Low | |
| Gu (9) | 2020 | 95/95 | 45.67/47.63 | 50/45 | 48/47 | Gastrointestinal endoscopy | Conventional treatment | ②④⑤ | Unclear | |
| Zhang (15) | 2020 | 20/20 | 60.5/62.8 | 11/9 | 10/10 | Endoscopic submucosal dissection | Conventional treatment | ①④ | Unclear | |
| Li (4) | 2019 | 60/60 | 53.3/52.6 | 29/31 | 33/27 | Gastrointestinal endoscopy | Conventional treatment | ③ | Low | |
Outcome indicators included: ① tumor resection rate after treatment (%); ② cumulative intraoperative blood loss (mL); ③ operation time (minutes); ④ incidence of complications (%); ⑤ hospitalization time (days); ⑥ treatment cost (10,000 CNY).
Literature quality evaluation
Among the 10 included literatures, if one or more non-conformities are found according to Cochrane literature quality evaluation, the risk of bias is unclear. All items of the 5 articles were met, with low risk of bias. as shown in Table 1.
Meta-analysis results
Complete tumor resection rate
Five articles (10,12-15) that measured the rate of complete tumor resection after treatment as an outcome indicator. The results showed that the heterogeneity among the studies was small (I2=32%), so the random effect model was used for analysis. The difference in the complete tumor resection rate between the observation and control groups was statistically significant. Also, EMD for early digestive tract cancer and precancerous lesions has a higher tumor resection rate than that of the control group (RR =1.25, 95% CI: 1.15–1.35, P<0.01, Figure 2).

Cumulative intraoperative blood loss
Six articles (1,3,5,12,14,16) measured cumulative intraoperative blood loss as an outcome indicator. The results showed high between-study heterogeneity (I2=94%), which may have been due to differences in the level of surgery in different medical units. Therefore, a random effects model was used for analysis. The results showed that the difference in the cumulative blood loss between the observation group and the control group was statistically significant, that is, the cumulative blood loss in the EMD for early digestive tract cancer and precancerous lesions was much lower than that of the control group (MD =−26.51, 95% CI: −30.08 to −22.93, P<0.01, Figure 3).

Operation time
Eight studies (1,3,5,9,10,12-14) measured operation time as an outcome indicator. The results showed high between-study heterogeneity (I2=100%), which may have been due to differences in the level of surgery performed by different medical institutions. In particular, the average operation time reported by Nan et al. (12) was much longer than that in the other studies. Therefore, a random effects model was used for analysis. The results showed that the difference in operation time between the observation and control groups was statistically significant, that is, the operation time of EMD for early digestive tract cancer and precancerous lesions was shorter than that of the control group (MD =−49.39, 95% CI: −67.99 to −30.80, P<0.01, Figure 4).

Postoperative complications
Eight studies (1,4,9,10,12-15) measured postoperative complications as an outcome indicator. The results showed no heterogeneity between the studies (I2=0%). Therefore, the fixed effect model was used for analysis, indicating that the difference in postoperative complications between the observation and control groups was statistically significant, that is, the incidence of postoperative complications of EMD for early digestive tract cancer and precancerous lesions was lower than that of the control group (RR =0.29, 95% CI: 0.20–0.42, P<0.01, Figure 5).
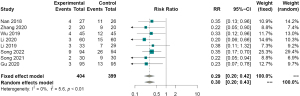
Hospital stay
Seven articles (1,3,5,9,10,13,14) measured hospital stay as an outcome indicator. The results showed high between-study heterogeneity (I2=99%), the sources of which were differences in treatment across medical units. Therefore, a random effects model was used for analysis. The results showed that the difference in hospitalization time between the observation and control groups was statistically significant. The hospitalization time for EMD for early digestive tract cancer and precancerous lesions was lower than that of the control group (MD =−6.50, 95% CI: −8.57 to −4.42, P<0.01, Figure 6).
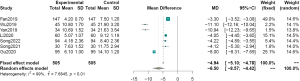
Hospital expenses
Three articles (1,3,13) measured the cost of hospitalization as an outcome indicator. The results showed high between-study heterogeneity (I2=89%), the sources of which were differences in treatment across medical units. Therefore, a random effects model was used for analysis. The results showed that there was a statistically significant difference in hospitalization expenses between the observation and control groups. The hospitalization expenses for EMD for early digestive tract cancer and precancerous lesions were lower than those of the control group (MD =−3.91, 95% CI: −4.16 to −3.67, P<0.01, Figure 7).

Publication bias analysis
The Egger test was used to analyze publication bias. The results showed that there was no significant publication bias for complete tumor resection rate (P=0.33), cumulative intraoperative blood loss (P=0.57), operation time (P=0.62), hospital stay (P=0.32), postoperative complications (P=0.29), and hospital costs (P=0.60).
Discussion
Gastrointestinal cancer is one of the most important cancers worldwide. The incidence of digestive tract cancer in China is rising rapidly, and its morbidity and mortality rates are closely behind those of lung cancer, which has the highest incidence in China (17,18). Early gastrointestinal cancers include rectal cancer, biliary tract cancer, gastric cancer, esophageal cancer, pancreatic cancer, colorectal cancer, and hepatocellular carcinoma (19). In general, early gastrointestinal cancers and precancerous lesions have no obvious clinical manifestations, and the external shape of lesions in the early stage of onset does not change significantly, so they are easily overlooked. Therefore, early screening and treatment of gastrointestinal cancer in the early stage or before the occurrence of cancer are extremely effective measures to reduce the incidence of upper gastrointestinal cancer and control its progress (1).
The main treatment of early gastrointestinal cancer is resection and endoscopic treatment, endoscopic treatment can be divided into EMD and endoscopic resection. It has been demonstrated that EMD has more advantages than surgical resection and endoscopic resection, such as less trauma, early healing, and low cost (20). However, whether this method can replace the resection as the first choice of treatment for early gastrointestinal cancer has not been agreed.
The results of this study found that the tumor resection rate of EMD in the treatment of early gastrointestinal cancer has exceeded that of surgical resection or endoscopic resection, which may be due to the fact that EMD can clearly demonstrate the pipeline wall layer and then accurately judge the extent of the lesion and its relationship with the surrounding tissue (21). Therefore, the complete resection rate of early gastrointestinal cancer and precancerous lesions can be improved. EMD for the treatment of early cancer of the digestive tract is relatively small, which can not only significantly reduce the amount of intraoperative blood loss but also shorten the operation time. Previous studies have shown that traditional treatment methods require en bloc resection to ensure the integrity of the obtained cancerous tissue in diagnosing the disease. However, endoscopic treatment avoids a large resection area, which can reduce the postoperative complications of patients while ensuring the therapeutic effect (22). Based on the above advantages, the hospitalization time and treatment cost for patients treated with endoscopy will be reduced accordingly, ensuring better clinical efficacy and safety.
There were some limitations of this study that should be noted. Firstly, the number of randomized controlled trials (RCTs) included in the study was relatively small (n=10), and the number of patients included was also relatively insufficient, which may have had a certain impact on the conclusion. Secondly, although EMD has been widely used, the research on early digestive tract cancer and precancerous lesions remains insufficient. Thirdly, there may be differences in the level of medical equipment and doctors in different medical units, resulting in a certain degree of heterogeneity among the included studies. Finally, many of the included articles did not specifically report on the allocation blinding method or the blinding of participants and personal. There may have also been a certain risk of bias in the included literature.
In summary, EMD for early digestive tract cancer and precancerous lesions can increase the complete resection rate of tumors, and reduce intraoperative blood loss, complications, operation time, as well as hospitalization time and cost. It is a safe and effective method of diagnosis and treatment and has clinical application value.
Conclusions
EMD has an advantage in the treatment of early gastrointestinal cancer. However, due to the lack of literature, the heterogeneity of literature interference, whether it can be used as a first-line treatment needs to be further explored.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-32/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Song Y, Qu Y, Li L, et al. Application Value of the Diagnosis during Early Carcinoma of Upper Digestive Tract Based on Optical Enhanced Endoscopic Technique. Comput Math Methods Med 2022;2022:9587070. [Crossref] [PubMed]
- Li J, Xu Q, Huang ZJ, et al. CircRNAs: a new target for the diagnosis and treatment of digestive system neoplasms. Cell Death Dis 2021;12:205. [Crossref] [PubMed]
- Yan Z, Zhang ZQ, Wang J. Clinical effect analysis of endoscopic treatment of early digestive tract cancer and precancerous lesions. Health for Everyone 2019;55.
- Li T, Xiang PLB. Therapeutic effects of endoscopic mucosal resection on the recovery and prognosis of early gastric cancer. J BUON 2019;24:1087-91.
- Fan X. Value analysis of digestive endoscopy in the diagnosis and treatment of early digestive tract cancer. Electronic Journal of Clinical Medicine Literature 2019;6:139-40.
- Ang TL, Seewald S. Endoluminal resection and tissue acquisition. Curr Treat Options Gastroenterol 2014;12:140-53. [Crossref] [PubMed]
- Kim HH, Park MI, Park SJ, et al. Myocardial infarction thought to be provoked by local epinephrine injection during endoscopic submucosal dissection. J Clin Med Res 2011;3:143-6. [Crossref] [PubMed]
- Nan R, Lei YF, Liu BY. Analysis of curative effect of endoscopic mucosal dissection in the treatment of early upper gastrointestinal cancer and precancerous lesions. Chinese Folk Remedies 2018;26:84.
- Gu J. Clinical value analysis of digestive endoscopy in the diagnosis and treatment of early gastrointestinal cancer. Chinese Community Doctors 2020;36:104-5.
- Song XH, Jiang CY. Observation on the curative effect of endoscopic mucosal dissection in the treatment of early upper gastrointestinal cancer and precancerous lesions. Chinese Community Doctors 2021;37:53-4.
- Li H, Han X, Su L, et al. Laparoscopic radical gastrectomy versus traditional open surgery in elderly patients with gastric cancer: Benefits and complications. Mol Clin Oncol 2014;2:530-4. [Crossref] [PubMed]
- Nan R, Lei YF, Liu BY. Endoscopic mucosal dissection in the treatment of upper gastrointestinal cancer and precancerous lesions. China's Naturopathy 2018;26:84-5.
- Wu HH, Dong J, Zhang MQ, et al. Clinical efficacy of ESD in the treatment of early digestive tract cancer and precancerous lesions and its impact on the quality of life of patients. Modern Digestive and Interventional Diagnosis and Treatment 2019;24:996-8.
- Li M. Clinical analysis of endoscopic treatment for early cancer and precancerous lesions of digestive tract. Oriental Medicated Diet 2020;12:255.
- Zhang P. Clinical effect analysis of endoscopic treatment of early digestive tract cancer and precancerous lesions. Medical Aesthetics 2020;29:75-6.
- Song SY. Endoscopic treatment of upper gastrointestinal tumors. Yonsei Med J 1999;40:559-68. [Crossref] [PubMed]
- Ding R, Hong W, Huang L, et al. Examination of the effects of microRNA-145-5p and phosphoserine aminotransferase 1 in colon cancer. Bioengineered 2022;13:12794-806. [Crossref] [PubMed]
- Xia R, Zeng H, Liu W, et al. Estimated Cost-effectiveness of Endoscopic Screening for Upper Gastrointestinal Tract Cancer in High-Risk Areas in China. JAMA Netw Open 2021;4:e2121403. [Crossref] [PubMed]
- Lv X, Xu G. Regulatory role of the transforming growth factor-β signaling pathway in the drug resistance of gastrointestinal cancers. World J Gastrointest Oncol 2021;13:1648-67. [Crossref] [PubMed]
- Draganov PV, Aihara H, Karasik MS, et al. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology 2021;160:2317-2327.e2. [Crossref] [PubMed]
- Su Q, Peng J, Chen X, et al. Role of endoscopic ultrasonography for differential diagnosis of upper gastrointestinal submucosal lesions. BMC Gastroenterol 2021;21:365. [Crossref] [PubMed]
- Zhang N, Lu B. Expression of macrophage inhibitory cytokine-1 in early gastric cancer cases treated using endoscopic mucosal resection and the correlation with prognosis. Oncol Lett 2017;14:1967-70. [Crossref] [PubMed]
(English Language Editor: A. Kassem)



