
Time-trends in liver cancer incidence and mortality rates in the U.S. from 1975 to 2017: a study based on the Surveillance, Epidemiology, and End Results database
Highlight box
Key findings
• The incidence of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) increased from 2.641/100,000 person-years in 1975 to 8.657/100,000 person-years in 2017. The mortality rate increased from 2.808/100,000 person-years in 1975 to 6.648/100,000 person-years. The incidence and mortality rates of males, non-Hispanic Blacks, and older individuals exhibited a more rapid increase.
What is known and what is new?
• Previous studies have examined the overall cancer statistics.
• The incidence and mortality trends of liver and intrahepatic bile duct cancer from 1975 to 2017 have been estimated in the U.S., including in different age, gender, race and geographical location.
What is the implication, and what should change now?
• In states with high mortality, establishing a screening program may assist in early diagnosis and intervention before the clinical manifestation becomes fatal.
Introduction
Liver cancer was the third most common cause of cancer mortality globally in 2020, accounting for 8.3% of all cancer deaths (1). The principal histologic type of liver cancer is hepatocellular carcinoma (HCC), accounting for up to 90% of primary liver tumors (2). Intrahepatic cholangiocarcinoma (ICC), which involves malignant cells arising from the epithelial cells of the intrahepatic bile duct, is the second most common type of liver cancer (3). In the United States (U.S.), the projected incidence of liver and intrahepatic bile duct cancer in 2021 was 42,230 cases, with an estimated 30,230 deaths (4). Although the highest incidence was observed in Asia, the incidence trend in the U.S. is increasing (5).
Several risk factors of HCC and ICC are modifiable, such as exposure to aflatoxin, blue-green algal toxin, ethanol abuse (alcohol), metabolic syndrome, obesity, diabetes, non-alcoholic fatty liver disease (NAFLD), and dietary factors (5,6). The hepatitis B and C viruses are also strongly correlated with HCC, which can be prevented by vaccination (7). Clinical diagnosis involves biomarker evaluation of liver function, ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and liver biopsy (2,8). The common treatments for early cancer stages include antiviral treatment of hepatitis, surgical resection, radiation, transarterial chemoembolization (TACE), local radiofrequency ablation (RFA), and organ transplantation (6,8,9). At the advanced stage when the aforementioned treatments are no longer appropriate, sorafenib, a multi-kinase inhibitor, is regarded as the first-line chemotherapeutic agent (10).
A previous study has examined the overall cancer statistics (4). However, more detailed statistics regarding liver cancer have not been provided. In this study, we evaluated the incidence and mortality trends of liver and intrahepatic bile duct cancer in the U.S. from 1975 to 2017 based on the data in the Surveillance, Epidemiology, and End Results (SEER) database. Furthermore, the 5-year survival was used to evaluate the mortality rate trends of different cancer metastasis. Understanding the changing trend of incidence and mortality of lung cancer can not only improve people’s awareness of its risk, but also be associated with health policies, which is conducive to relevant departments to timely adjust relevant medical security policies, implement health education and clinical intervention. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-25/rc).
Methods
Study design and data source
This was a retrospective cohort study. SEER is a program conducted by the National Cancer Institute (NCI), which aims to provide cancer statistics to reduce cancer burdens in the U.S. The information included in the cancer registries is strictly handled to protect the confidentiality of the participants (11). The International Classification of Diseases for Oncology (ICD-O) 3rd edition codes C22.0 and C22.1 were used to identify HCC and ICC patients (12). The 1969–2018 SEER was accessed to retrieve data for the analysis; however, only the 1975–2017 SEER database was utilized due to missing essential variables in the other SEER years. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Data collection
Age, gender, race, metastasis, tumor site, and tumor grade of patients were extracted from the SEER database. Four cancer grades were determined using the variable “Grade”, including grade I, grade II, grade III, and grade IV. Metastasis, including localized, regionalized, and distant, was based on the NCI’s definition (13). Localized indicated cancer that had not spread; regional referred cancer that had spread to nearby lymph nodes, tissues, or organs; while distant denoted cancer that had spread to a distant part of the body.
Gender-, race-, metastasis-, site-, and grade-specific analyses were performed to investigate the incidence. As for the mortality rate, only gender- and race-specific analyses were performed, as the other specific characteristics were not collected when examining the mortality rate.
Statistical analysis
Age-specified incidence, age-standardized incidence and mortality, 5-year relative survival, race-specific accumulative incidence and mortality, and geographic-specific accumulative mortality were calculated in different groups. Changes in trends of liver cancer incidence and mortality were assessed using Joinpoint regression. The annual percent change (APC) was used to characterize the trends of cancer rates over time (14). The APC, as well as the average APC (AAPC), a summary measure of a trend over a certain period, were calculated using the NCI’s Joinpoint Regression Program (version 4.8.0.1, Statistical Methodology and Applications Branch, Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, Information Management Services, Inc.) (15). The age-standardized incidence and mortality rates, calculated by the NCI’s SEER*Stat software (version 8.3.9) (16), were standardized to the U.S. standard population in 2000 and expressed as per 100,000 persons. The APC in rates was quantified using the NCI’s Joinpoint Regression Program (version 4.8.0.1). Other statistical tests were performed using the SAS software 9.4 (SAS Institute, Inc., Cary, NC, USA). The trend chart was plotted using GraphPad Prism software (version 8.0.1, GraphPad Prism software, San Diego, CA, USA). Mortality maps were drawn using the Apache ECharts (Apache Software Foundation, Wilmington, DE, USA). All statistical significance tests were two-sided, and P<0.05 was considered statistically significant.
Results
Age-specified incidence
The liver cancer incidence trends of different age groups from 1975 to 2017 are presented in Figure 1. From birth to death, the incidence rate of liver cancer increased from 2.641/100,000 person-years to 8.658/100,000 person-years (AAPC =4.48, 95% confidence interval (CI): 4.32–4.62, P<0.001). The steepest incidence rate increase was observed in the 60–69-year-old age group (AAPC =4.40, 95% CI: 4.10–4.70, P<0.001). The incidence was the most stable from birth to 49 years old (AAPC =2.76, 95% CI: 1.96–3.46, P=0.009), and increased slowly at 70 years and older (AAPC =2.73, 95% CI: 2.53–2.93, P=0.010). A rapid increase in liver cancer incidence was also observed in the 50–59-year-old age group (AAPC =4.66, 95% CI: 3.96–5.26, P<0.001).
The APC of each joinpoint was displayed in Table 1. The most substantial incidence increase from birth to death was observed in 2001–2011, corresponding to an APC of 6.09. From birth to 49 years, the incidence increased from 1975–2005 (APC =5.22) but decreased from 2005–2017 (APC =−4.01). At 50–59 years, a steep increase in cancer incidence occurred in 1998–2007 (APC =10.28), while a sharp decrease appeared in 2012–2017 (APC =−7.36). A rapid increase was also observed in 1982–1996 (APC =5.01) and 2004–2017 (APC =6.85) in the 60–69-year-old age group. For participants aged 70 years or older, the most significant APC was 2.30 in 1991–2017.
Table 1
| Incidence | Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | AAPC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | Years | APC | Years | APC | Years | APC | Years | APC | 1975–2017 | ||||||
| Age (years) | ||||||||||||||||
| Birth to death | 1975–1984 | 2.21* | 1984–1998 | 5.15* | 1998–2001 | 1.11 | 2001–2011 | 6.09* | 2011–2017 | 1.92* | 4.48* | |||||
| Birth to 49 | 1975–2005 | 5.22* | 2005–2017 | −4.01* | 2.76* | |||||||||||
| 50 to 59 | 1975–1998 | 3.53* | 1998–2007 | 10.28* | 2007–2012 | 2.11 | 2012–2017 | −7.36* | 4.66* | |||||||
| 60 to 69 | 1975–1982 | 0.60 | 1982–1996 | 5.01* | 1996–2004 | 0.89 | 2004–2017 | 6.85* | 4.40* | |||||||
| 70+ | 1975–1988 | 1.31* | 1988–1991 | 10.71 | 1991–2017 | 2.30* | 2.73* | |||||||||
The trends were analyzed using the Joinpoint Regression Program (version 4.8), allowing up to 5 joinpoints. *, the APC or AAPC was significantly different from zero (P<0.05). AAPC, average annual percent change; APC, annual percent change.
Age-standardized incidence
Figure 2 and Table 2 demonstrate the age-standardized liver cancer incidence trends. The overall incidence increased significantly from 2.641/100,000 person-years in 1975 to 8.657/100,000 person-years in 2017 (AAPC =3.42, 95% CI: 3.28–3.62, P<0.001). The APC of each joinpoint was as follows: 1975–1984 (APC =1.27), 1984–1998 (APC =4.76), 2001–2009 (APC =4.67), and 2009–2015 (APC =1.94).
Table 2
| Incidence | Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | Trend 6 | AAPC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | Years | APC | Years | APC | Years | APC | Years | APC | Years | APC | 1975–2017 | |||||||
| Overall | 1975–1984 | 1.27* | 1984–1998 | 4.76* | 1998–2001 | 0.22 | 2001–2009 | 4.67* | 2009–2015 | 1.94* | 2015–2017 | −2.48 | 3.42* | ||||||
| Gender | |||||||||||||||||||
| Male | 1975–1984 | 1.64 | 1984–1998 | 4.60* | 1998–2001 | 0.89 | 2001–2011 | 4.42* | 2011–2017 | −0.03 | 3.41* | ||||||||
| Female | 1975–1983 | 0.54 | 1983–1999 | 4.26* | 1999–2002 | −1.18 | 2002–2011 | 3.96* | 2011–2017 | 1.37 | 3.03* | ||||||||
| Race | |||||||||||||||||||
| White | 1975–1987 | 1.59* | 1987–1998 | 5.36* | 1998–2001 | −0.62 | 2001–2012 | 4.70* | 2012–2017 | 0.51 | 3.58* | ||||||||
| Black | 1975–2002 | 2.91* | 2002–2009 | 6.24* | 2009–2017 | 0.00 | 3.24* | ||||||||||||
| Site | |||||||||||||||||||
| Primary | 1975–1984 | 1.21 | 1984–1998 | 4.32* | 1998–2001 | 0.45 | 2001–2009 | 4.27* | 2009–2015 | 1.62* | 2015–2017 | −3.31 | 3.12* | ||||||
| Secondary | 1975–1981 | 11.20* | 1981–1984 | −8.80 | 1984–1997 | 9.82* | 1997–2002 | 1.01 | 2002–2011 | 7.00* | 2011–2017 | 2.29* | 5.59* | ||||||
| Metastasis | |||||||||||||||||||
| Localized | 1975–1990 | 1.38* | 1990–1996 | 12.54* | 1996–1999 | 4.52 | 1999–2007 | 8.34* | 2007–2015 | 3.82* | 2015–2017 | −4.86 | 5.91* | ||||||
| Regional | 1975–1993 | 3.02* | 1993–1997 | 11.40* | 1997–2000 | 0.47 | 2000–2005 | 6.17* | 2005–2011 | 4.06* | 2011–2017 | −0.42 | 4.11* | ||||||
| Distant | 1975–1985 | −0.42 | 1985–1999 | 3.43* | 1999–2002 | −2.25 | 2002–2017 | 2.53* | 2.04* | ||||||||||
| Grade | |||||||||||||||||||
| I | 1975–1990 | 5.58* | 1990–1998 | 9.53* | 1998–2008 | 3.03* | 2008–2017 | −2.50* | 3.71* | ||||||||||
| II | 1975–1992 | 7.75* | 1992–2005 | 9.83* | 2005–2012 | 4.10* | 2012–2017 | −1.87 | 6.48* | ||||||||||
| III | 1975–1995 | 5.49* | 1992–2010 | 3.31* | 2010–2017 | −0.52 | 3.45* | ||||||||||||
| IV | 1975–1986 | −7.23* | 1986–1999 | 4.11 | 1999–2017 | −2.26* | −0.65 | ||||||||||||
The trends were analyzed using the Joinpoint Regression Program (version 4.8), allowing up to 5 joinpoints. *, the APC or AAPC is significantly different from zero (P<0.05). AAPC, average annual percent change; APC, annual percent change based on incidence rates age adjusted to the standard population.
The sex-specific and age-standardized incidence is shown in Figure 2A. Males exhibited a more rapid increase in cancer incidence, from 3.928/100,000 person-years to 13.128/100,000 person-years (AAPC =3.41, 95% CI: 3.21–3.61, P<0.001), than females [from 1.642/100,000 person-years to 4.783/100,000 person-years (AAPC =3.03, 95% CI: 2.91–3.21, P=0.001)]. The race-specific analysis (Figure 2B) revealed a steeper elevation of cancer incidence among non-Hispanic Blacks, from 4.740/100,000 person-years to 11.286/100,000 person-years (AAPC =3.24, 95% CI: 3.02–3.48, P<0.001) than non-Hispanic Whites [from 2.190/100,000 person-years to 7.608/100,000 person-years (AAPC =3.58, 95% CI: 3.42–3.83, P<0.001)].
As for metastasis (Figure 2C), the most substantial increase was detected in localized cancer, 0.598 to 3.875 (AAPC =5.91, 95% CI: 5.49–6.32, P<0.001), followed by regional cancer (AAPC =4.11, 95% CI: 3.82–4.43, P<0.001) and distant cancer (AAPC =2.04, 95% CI: 1.91–2.22, P=0.008). As shown in Figure 2D, the incidence of secondary cancer increased from 0.140/100,000 person-years to 1.556/100,000 person-years (AAPC =5.59, 95% CI: 5.29–5.94, P<0.001), while that of primary cancer increased from 2.502/100,000 person-years to 7.102/100,000 person-years (AAPC =3.12, 95% CI: 2.91–3.29, P=0.001).
Different tumor grades also displayed distinct changing incidence patterns (Figure 2E). The incidence of grade II (AAPC =6.48, 95% CI: 5.72–7.18, P<0.001) tumors increased most substantially, followed by grade I (AAPC =3.71, 95% CI: 3.02–4.53, P<0.001) and grade III (AAPC =3.45, 95% CI: 2.98–4.01, P<0.001) tumors. Although the incidence of grade IV was numerically decreasing, the trend was not statistically significant.
Age-standardized mortality
The age-standardized mortality rate trend from 1975 to 2017 is illustrated in Figure 3 and Table 3. The overall mortality rate increased from 2.808/100,000 person-years in 1975 to 6.648/100,000 person-years (AAPC =2.41, 95% CI: 2.29–2.51, P<0.001). The sex-specific trend (Figure 3A) indicated a significant increase in the mortality rate of both sexes. The mortality rate of males increased from 3.846/100,000 person-years in 1975 to 9.700/100,000 person-years in 2017 (AAPC =2.51, 95% CI: 2.39–2.61, P<0.001). A less substantial increase was observed in the female population, from 2.047/100,000 to 4.057/100,000 person-years (AAPC =1.92, 95% CI: 1.82–2.02, P=0.006). As shown in Figure 3B, the mortality rate of the non-Hispanic Black population increased from 4.298/100,000 to 8.418/100,000 person-years (AAPC =1.84, 95% CI: 1.82–1.94, P=0.007). In the non-Hispanic White population, the mortality rate elevated from 2.621/100,000 to 6.287/100,000 person-years (AAPC =2.48, 95% CI: 2.38–2.58, P<0.001).
Table 3
| Mortality | Trend 1 | Trend 2 | Trend 3 | Trend 4 | Trend 5 | Trend 6 | AAPC | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Years | APC | Years | APC | Years | APC | Years | APC | Years | APC | Years | APC | 1975–2017 | |||||||
| Overall | 1975–1980 | 0.17 | 1980–1987 | 1.99* | 1987–1995 | 3.75* | 1995–2007 | 1.89* | 2007–2013 | 3.17* | 2013–2017 | 0.65 | 2.41* | ||||||
| Gender | |||||||||||||||||||
| Male | 1975–1985 | 1.50* | 1985–1996 | 3.78* | 1996–1999 | 0.31 | 1999–2013 | 2.66* | 2013–2017 | 0.58 | 2.51* | ||||||||
| Female | 1975–1984 | 0.23 | 1984–1995 | 3.13* | 1995–2008 | 1.17* | 2008–2013 | 3.25* | 2013–2017 | 1.32* | 1.92* | ||||||||
| Race | |||||||||||||||||||
| White | 1975–1978 | −1.23 | 1978–1987 | 1.65* | 1987–1996 | 3.90* | 1996–2000 | 0.54 | 2000–2013 | 2.78* | 2013–2017 | 1.40* | 2.48* | ||||||
| Black | 1975–1988 | 0.77* | 1988–1995 | 3.19* | 1995–2000 | 0.29 | 2000–2013 | 2.49* | 2013–2017 | 0.11 | 1.84* | ||||||||
The trends were analyzed using the Joinpoint Regression Program (version 4.8), allowing up to 5 joinpoints. *, the APC or AAPC is significantly different from zero (P<0.05). AAPC, average annual percent change; APC, annual percent change based on mortality rates age adjusted to the standard population.
5-year relative survival
Figure 4 displays the race-specific 5-year survival. The overall 5-year survival of the study population was 22.2%, with localized cancer (37.6%) showing higher survival rates than those of regional (14.2%) and distant (3.9%) cancers. The overall 5-year survival was 21.1% among non-Hispanic Black participants; for these participants, the survival rates of localized, regional, and distant cancers were 35.8%, 12.8%, and 1.2%, respectively. As for non-Hispanic White participants, the overall 5-year survival was 20.7%. Localized cancer also exhibited the highest survival (35.1%) in all three stages among non-Hispanic White participants. Similarly, distant cancer showed the lowest survival in the non-Hispanic White population (3.3%). Furthermore, the 5-year survival of regional cancer among these participants was 14.4%.
Race-specific accumulative incidence and mortality
Table 4 demonstrates the race-specific cumulative incidence and mortality. The overall accumulative incidence was 306.53/100,000 person-years, with the accumulative incidence of the non-Hispanic White participants (241.63/100,000 person-years) being lower than that of non-Hispanic Black participants (422.98/100,000 person-years). The accumulative incidence, as well as the mortality rate, was approximately two times higher in males than females across all races. Also, the accumulative incidence of secondary cancer (268.44/100,000 person-years) was substantially higher than that of primary cancer (38.09/100,000 person-years). Grade II tumors exhibited the highest accumulative incidence (38.17/100,000 person-years).
Table 4
| Variables | All races | Non-Hispanic Whites | Non-Hispanic Blacks | Others* |
|---|---|---|---|---|
| Incidence | ||||
| Overall | 306.53 | 241.63 | 422.98 | 1,078.19 |
| Male | 436.95 | 342.42 | 639.07 | 1,500.00 |
| Female | 181.35 | 144.63 | 224.52 | 660.46 |
| Primary cancer | ||||
| Primary | 38.09 | 33.36 | 48.54 | 91.51 |
| Secondary | 268.44 | 208.27 | 374.44 | 986.67 |
| Metastasis | ||||
| Localized | 98.64 | 76.8 | 134.46 | 364.58 |
| Regional | 68.52 | 51.21 | 104.69 | 266.90 |
| Distant | 53.97 | 42.41 | 81.15 | 181.38 |
| Grade | ||||
| I | 26.75 | 20.93 | 37.7 | 94.81 |
| II | 38.17 | 30.41 | 51.91 | 131.1 |
| III | 27.39 | 21.29 | 36.71 | 102.89 |
| IV | 3.56 | 2.96 | 4.15 | 11.29 |
| Mortality rates | ||||
| Overall | 255.72 | 235.4 | 299.85 | 963.26 |
| Male | 338.46 | 306.91 | 425.61 | 1,322.09 |
| Female | 177.31 | 167.24 | 186.4 | 613.31 |
Rates are expressed per 100,000 population and age-adjusted to the 2000 U.S. standard population. Others* include American Indian/Alaska Native, Asian/Pacific Islander.
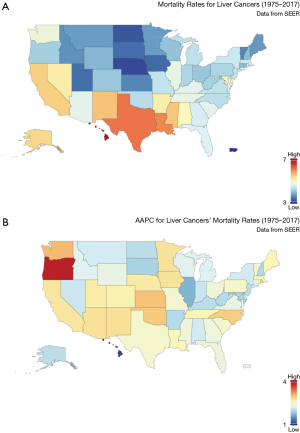
Geographic-specific accumulative mortality
The mortality rate varied geographically, as summarized in Figure 5. The highest mortality rate was observed in Hawaii (6.996/100,000 person-years) and was comparatively low in the middle-northern area of the U.S. (Figure 5A). The most rapid increase was detected in Oregon state (AAPC =3.82, 95% CI: 3.22–4.12, P=0.021), while the slowest increase was in Hawaii (Figure 5B).
Discussion
Global prevalence
In 2020, the incidence of liver cancer ranks sixth worldwide, with approximately 906,000 new cases (17). Globally, the risk of liver cancer is highest in Asian countries, particularly in China and the Republic of Korea, and sub-Saharan Africa (17,18). Although the current morbidity rate in the U.S. is not as prominent as that in Asian countries, a significant increase is projected due to aging and obesity (19,20). In 2030, the greatest increase in liver cancer incidence among women is anticipated in the non-Hispanic Black American population (4.0%). Non-Hispanic White American men are projected to have the second highest increase in morbidity (2.6%) (19). Our study revealed a substantial increase in liver cancer incidence rate, from 2.641/100,000 person-years in 1975 to 8.657/100,000 person-years in 2017, which is consistent with the previous projection. The increase in new liver cancer incidence in the U.S. is mainly attributed to the immigration of infected persons from highly endemic countries (21).
Pathophysiology
High heterogeneity is observed in the cellular morphology and genetic landscape of liver cancers; for instance, catenin Beta 1 (CTNNB1) mutated tumors are large, well-differentiated, cholestatic, exhibit a microtrabecular and pseudoglandular pattern, and do not have an inflammatory infiltrate (22). Hepatocytes and hepatic progenitor cells are the two main cell types that play essential roles in hepatocarcinogenesis (23). In a mouse model, hepatic progenitor cells, lineage-committed hepatoblasts, and differentiated adult hepatocytes lead to tumorigenesis through various cell-specific activation pathways (24). Isolated hepatic progenitor cells are also able to produce liver carcinomas similar to human HCC, highlighting the tumor-initiating role of these cells (25). Adult hepatocytes are another crucial cellular origin of HCC. Following sequential genomic insults, hepatocytes can dedifferentiate into precursor cells that transform into HCC cells and transdifferentiate into biliary-like cells, which transform into ICC (26).
Risk factors
Several risk factors have been established, including hepatitis, alcohol intake, aflatoxin exposure, metabolic diseases, and certain demographic characteristic (6,27,28). The risk factors of liver cancer vary from region to region. In the U.S., the main risk factors are listed below.
Hepatitis
Viral hepatitis infection, particularly the hepatitis B virus (HBV), is the dominant risk factor for liver cancer (29-31). The hepatitis virus is an enveloped, coated, double-stranded DNA virus that replicates via reverse transcription. Hepatitis viral infection may be acute, chronic, or long-lasting, causing liver inflammation and damage (32). The primary mode of infection of HBV, hepatitis C virus (HCV), and hepatitis D virus (HDV) is through vertical transmission (30,33,34), while the hepatitis A (HAV) and hepatitis E (HEV) viruses are transmitted through fecal-oral contact (35,36). HBV is the most common chronic infection worldwide. Approximately 30% of the world’s population is currently or previously infected (37). Since there is no cure for chronic HBV infection currently, universal vaccination is recommended by the World Health Organization (WHO) (38). The timely screening, diagnosis, assessment of disease severity, and antiviral treatment to reduce the viral load are crucial for disease control. There are 862,000 ongoing cases of HBV in the U.S. Immigration from endemic high-risk countries is the leading cause of new cases in the the U.S. (39). Since HBV is commonly non-symptomatic, the Center for Disease Control and Prevention (CDC) estimates that 68% of HBV carriers are not aware of the infection (40). Therefore, national routine tests and screening are strongly recommended, particularly for people with a high risk of infection (41).
Behavioral risk factors
The behavioral risk factors of liver cancer, such as alcohol abuse, smoking cigarettes, and environmental exposure to aflatoxin, are highly modifiable (42-44). Alcohol abuse is a key cause of liver disease worldwide (20). Alcohol consumption stimulates the activity of cytochrome P450s, which is an enzyme that mediates the production of reactive oxygen species (ROS) in the liver. Heavy alcohol consumption is associated with a 68–87% increased risk of HCC and ICC (45). Due to its highly reactive property, ROS induces oxidative stress and damages cellular molecules (46). Moreover, alcohol consumption acts synergistically with other factors, such as chronic liver disease, hepatitis, and other behavioral factors (47).
The carcinogens derived from tobacco use are mainly metabolized in the liver (48). The Liver Cancer Pooling Project, a consortium consisting of 14 prospective cohort studies, discovered a strong link between smoking and the risk of HCC and ICC (45). The risk of HCC and ICC increases 47–86% among smokers, as compared to non-smokers. Aflatoxin, which is produced by fungi Aspergillus flavus and A. parasiticus, is another carcinogenic toxin associated with the risk of liver cancer. Food crops, such as maize, peanuts, and corn, are common carriers of aflatoxin. Approximately 14–19% of HCC cases are associated with aflatoxin exposure (49).
Health conditions
Other health conditions may exacerbate or precede the development of liver cancer, such as metabolic syndrome, obesity, and diabetes (28). Obesity-induced steatosis is believed to play a role in the development of HCC (50). NAFLD often coexists with obesity and diabetes (51,52) and is independently associated with the incidence of liver disease (53). It is estimated that NAFLD accounts for 13–38.2% of HCC cases in the U.S. (54).
Demographics
Similar to many other cancers, liver cancer risk increases substantially among elderly individuals (5). In North America, the median age of onset is 60 years and older (55). In our study, the prevalence was higher, and the increasing trend was steeper in older age groups compared to younger age groups. Gender is another risk factor for liver cancer. The morbidity and mortality are 2 to 3 times higher in men than women. In 2020, the global incidence of liver cancer was 2.31 times higher in men than women, while the mortality rate was 2.28 times higher in men than women (17). In the U.S., the estimated number of new cases in 2021 was 29,890 among men and 12,340 among women, corresponding to a male-to-female ratio of 2.42:1. The global mortality ratio of men to women in 2021 is expected to be 2.04:1. The findings of our study are consistent with those of previous studies, demonstrating a higher and more rapid increase in the incidence of liver cancer.
Racial disparities in liver cancer incidence also exist in the U.S. (56). The prevalence of liver cancer is the highest among American Indian/Alaska Native (15.7%), followed by Hispanics (13.5%), Asian/Pacific Islanders (12.6%), non-Hispanic Blacks (11.0%), and non-Hispanic Whites (7.1%) (4). Although the race and ethnicities in the SEER database were not as specific as in the previous study, we detected similar results regarding the incidence among non-Hispanic Blacks and non-Hispanic Whites.
Limitations of the study
Certain variables, such as race, were not specified during the data collection process. Therefore, a more specific subgroup analysis was not performed. However, the findings of this study allow for general interpretations of the incidence and mortality rates of liver cancer in the U.S. Future studies may examine the incidence and mortality rates from a more comprehensive perspective and provide more detailed information about a population with distinct characteristics.
Additionally, the morbidity and mortality statistics and trends revealed in the current study provide a fundamental guide for future research to examine the potential causes of shifts in the incidence rate, such as healthcare system infrastructure, environmental exposure, immigration, etc. Adjustments in policies and the development of prevention programs may be implemented to reduce the morbidity and mortality of liver cancer. As for increasing morbidity rates caused by immigration, a more comprehensive health examination and complete vaccination may reduce the morbidity of liver cancer. In states with high mortality, establishing a screening program may assist in early diagnosis and intervention before the clinical manifestation becomes fatal.
Conclusions
The incidence of HCC and ICC increased from 2.641/100,000 person-years in 1975 to 8.657/100,000 person-years in 2017. The mortality rate increased from 2.808/100,000 person-years in 1975 to 6.648/100,000 person-years in 2017. The incidence and mortality rates of males, non-Hispanic Blacks, and older individuals exhibited a more rapid increase. Future studies may focus on exploring the underlying reasons for incidence and mortality shifts from 1975–2017, which will assist in the adjustment of policy and the advancement of early detection.
Acknowledgments
Funding: This research was supported by the Science and Technology Planning Project of Guangzhou City (No. 202102010171), the Chinese foundation for hepatitis prevention and control-Tian Qing liver disease research fund subject (No. TQGB20200048), the CSCO-Roche solid tumor research project (No. Y-Roche2019/2-0041), and the Medical Scientific Research Foundation of Guangdong Province (No. A2021110).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-25/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-25/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. The Global Cancer Observatory-Cancer today. Published December 2020. Accessed April 22, 2021. Available online: http://gco.iarc.fr/today/home
- Asafo-Agyei KO, Samant H. Hepatocellular Carcinoma. StatPearls Publishing; 2021. Accessed April 22, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559177/
- El-Diwany R, Pawlik TM, Ejaz A. Intrahepatic Cholangiocarcinoma. Surg Oncol Clin N Am 2019;28:587-99. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Sayiner M, Golabi P, Younossi ZM. Disease Burden of Hepatocellular Carcinoma: A Global Perspective. Dig Dis Sci 2019;64:910-7. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Lotfollahzadeh S, Recio-Boiles A, Babiker HM. Liver Cancer. StatPearls Publishing; 2020. Accessed April 22, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448337/
- Lo EC. N Rucker A, Federle MP. Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: Imaging for Diagnosis, Tumor Response to Treatment and Liver Response to Radiation. Semin Radiat Oncol 2018;28:267-76. [Crossref] [PubMed]
- Gomaa AI, Waked I. Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol 2015;7:673-87. [Crossref] [PubMed]
- Niu L, Liu L, Yang S, et al. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim Biophys Acta Rev Cancer 2017;1868:564-70. [Crossref] [PubMed]
- National Cancer Institute. About the SEER Program. Surveillance, Epidemiology, and End Results Program. Accessed November 19, 2020. Available online: https://seer.cancer.gov/about/index.html
- Percy C, Fritz A, Jack A, et al. International Classification of Diseases for Oncology. 3rd edition. World Health Organization, 2000.
- National Cancer Institute. Cancer Staging - National Cancer Institute. Diagnosis and staging. Published September 3, 2015. Accessed April 28, 2021. Available online: https://www.cancer.gov/about-cancer/diagnosis-staging/staging
- Fay MP, Tiwari RC, Feuer EJ, et al. Estimating average annual percent change for disease rates without assuming constant change. Biometrics 2006;62:847-54. [Crossref] [PubMed]
- National Cancer Institute. Joinpoint Regression Program. Joinpoint Trend Analysis Software. Published March 15, 2021. Accessed April 28, 2021. Available online: https://surveillance.cancer.gov/joinpoint/
- National Cancer Institute. SEER*Stat Software. Statistical software. Published March 15, 2021. Accessed April 28, 2021. Available online: https://seer.cancer.gov/seerstat/index.html
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Petrick JL, Florio AA, Znaor A, et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer 2020;147:317-30. [Crossref] [PubMed]
- Valery PC, Laversanne M, Clark PJ, et al. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology 2018;67:600-11. [Crossref] [PubMed]
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019;70:151-71. [Crossref] [PubMed]
- Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin Liver Dis 2016;20:607-28. [Crossref] [PubMed]
- Calderaro J, Couchy G, Imbeaud S, et al. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol 2017;67:727-38. [Crossref] [PubMed]
- Tirnitz-Parker JEESchneller D, Angel P. Cellular Origin of Hepatocellular Carcinoma. 2019.
- Holczbauer Á, Factor VM, Andersen JB, et al. Modeling pathogenesis of primary liver cancer in lineage-specific mouse cell types. Gastroenterology 2013;145:221-31. [Crossref] [PubMed]
- Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 2006;125:1253-67. [Crossref] [PubMed]
- Sia D, Villanueva A, Friedman SL, et al. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017;152:745-61. [Crossref] [PubMed]
- Thun M, Linet MS, Cerhan JR, et al. editors. Cancer epidemiology and prevention. 4th edition. Oxford University Press, 2017.
- McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021;73:4-13. [Crossref] [PubMed]
- Ringelhan M, McKeating JA, Protzer U. Viral hepatitis and liver cancer. Philos Trans R Soc Lond B Biol Sci 2017;372:20160274. [Crossref] [PubMed]
- Holmes KKBertozzi SBloom BR, et al. Viral Hepatitis. 2017.
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76. [Crossref] [PubMed]
- National Institute of Diabetes and Digestive and Kidney Diseases. What Is Viral Hepatitis? | NIDDK. National Institute of Diabetes and Digestive and Kidney Diseases. Published May 2017. Accessed April 29, 2021. Available online: https://www.niddk.nih.gov/health-information/liver-disease/viral-hepatitis/what-is-viral-hepatitis
- Mysore KR, Leung DH. Hepatitis B and C. Clin Liver Dis 2018;22:703-22. [Crossref] [PubMed]
- Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat 2014;21:34-59. [Crossref] [PubMed]
- Linder KA, Malani PN, Hepatitis A. JAMA 2017;318:2393. [Crossref] [PubMed]
- Waqar S, Sharma B, Koirala J. Hepatitis E. StatPearls Publishing; 2020. Accessed April 29, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532278/
- Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053-63. [Crossref] [PubMed]
- Yuen MF, Chen DS, Dusheiko GM, et al. Hepatitis B virus infection. Nat Rev Dis Primers 2018;4:18035. [Crossref] [PubMed]
- Patel EU, Thio CL, Boon D, et al. Prevalence of Hepatitis B and Hepatitis D Virus Infections in the United States, 2011-2016. Clin Infect Dis 2019;69:709-12. [Crossref] [PubMed]
- Policy (OIDP) O of ID and H. Hepatitis B Basics. HHS.gov. Published April 20, 2016. Accessed April 30, 2021. Available online: https://www.hhs.gov/hepatitis/learn-about-viral-hepatitis/hepatitis-b-basics/index.html
- Krist AH, Davidson KW, et al. Screening for Hepatitis B Virus Infection in Adolescents and Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2020;324:2415-22. [Crossref] [PubMed]
- Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer 2015;112:580-93. [Crossref] [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264-1273.e1. [Crossref] [PubMed]
- Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer 2016;122:1757-65. [Crossref] [PubMed]
- Petrick JL, Campbell PT, Koshiol J, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer 2018;118:1005-12. [Crossref] [PubMed]
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health 2003;27:277-84.
- Grewal P, Viswanathen VA. Liver cancer and alcohol. Clin Liver Dis 2012;16:839-50. [Crossref] [PubMed]
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009;29-60. [Crossref] [PubMed]
- Kirch W, ed. Population Attributable Risk (PAR) Population attributable risk (PAR). In: Encyclopedia of Public Health. Netherlands: Springer, 2008:1117-8.
- Avgerinos KI, Spyrou N, Mantzoros CS, et al. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019;92:121-35. [Crossref] [PubMed]
- Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol 2018;14:99-114. [Crossref] [PubMed]
- Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism 2019;92:82-97. [Crossref] [PubMed]
- Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med 2016;67:103-17. [Crossref] [PubMed]
- Marrero JA, Fontana RJ, Su GL, et al. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology 2002;36:1349-54. [Crossref] [PubMed]
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- Islami F, Miller KD, Siegel RL, et al. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin 2017;67:273-89. [Crossref] [PubMed]
(English Language Editor: A. Kassem)






