
An unusual paraesophageal and diaphragmatic SDHA-deficient gastrointestinal stromal tumor (GIST) metastases case report
Highlight box
Key findings
• SDHA-deficient GISTs have unique tumor biology and can metastasize to unusual locations such as the mediastinum.
What is known and what is new?
• Surgical management remains the gold standard in the treatment of wild-type GIST.
• Minimally invasive surgical techniques may be a useful approach in certain cases.
What is the implication, and what should change now?
• Conventional chemotherapies have limited effectiveness in the treatment of wild-type GISTs, however, novel agents such as temozolomide and rogaratinib are currently being evaluated in clinical trials.
• A multidisciplinary team is essential in the management of these rare tumors.
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common non-epithelial, mesenchymal neoplasm of the gastrointestinal tract, constituting a fifth of all soft tissue sarcomas, thereby making them the most common single type of sarcoma overall (1,2) .These cases tend to be sporadic, accounting for 5,000 to 6,000 cases per year in the United States and a clinical incidence of 10–13 per million population per year, with the vast majority arising in the stomach (50–60%) or small intestine (30–40%). These tumors share a common stem cell origin with the interstitial cells of Cajal (ICC) which reside in the myenteric plexus and serve as a regulator of peristalsis via autonomic smooth muscle regulation (2,3). Approximately 10–20% of patients with GISTs present with overt metastases at the time of diagnosis, as GISTs tend to spread to the liver hematogenously or peritoneum and omentum via intra-peritoneal dissemination (1,3,4). Extra-abdominal spread to bones, lungs, and lymph nodes is rare, with lymphatic spread accounting for 1–2% of GIST metastases (3).
Activating mutations in the genes encoding tyrosine kinase receptors, KIT and platelet-derived growth factor receptor alpha (PDGFRA), are the primary known mechanisms involved in tumorigenesis by way of smooth muscle and neural differentiation in conjunction with uninhibited proliferation. Approximately 75% of GISTs exhibit KIT overexpression, whereas 10–15% alternatively overexpress PDGFRA (2,3). The minority of GISTs that do not harbor KIT/PDGFRA activating mutations, commonly referred to as wild-type GISTs (WT GISTs), can be linked to other genetic alterations including the mutational or epigenetic silencing of succinate dehydrogenase (SDH) genes (SDHA, SDHB, SDHC, SDHD), or mutations in neurofibromatosis type 1 (NF1). Epigenetic or biallelic loss of the SDH complex allows for accumulation of succinate and defective intracellular energy metabolism, ultimately yielding activation of downstream oncogenic pathways (4). SDH-deficient GISTs encompass the majority of WT GISTs, with loss-of-function mutations in the catalytic SDHA subunit being the most common variant, accounting for up to 30% of SDH-deficient cases (5). Germline or somatic mutations in SDHx have also been associated with the presence of other tumor types, as in the setting of Carney-Stratakis syndrome (hereditary SDH-deficient GIST and paragangliomas) or Carney’s triad [paragangliomas, pulmonary chondroma, and GISTs (+/− SDH-deficient)] (5). Nonetheless, similar to KIT/PDGFRA mutant GIST, extra-abdominal metastasis is exceedingly rare, with metastases predominantly localizing to the liver, omentum, peritoneum, and lymph nodes (5,6).
As such, this case report highlights a novel presentation of SDHA-deficient GIST metastasizing to paraesophageal and intra-diaphragmatic sites. Given its complexity, a detailed discussion of the pre-operative work up, intra-operative surgical approach and a summary of current care guidelines will be reviewed. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-714/rc).
Case presentation
This is a case of an otherwise healthy 44-year-old female who presented for the management of metastatic SDHA-deficient GIST. She was initially diagnosed 12 years prior after an evaluation for severe abdominal pain which identified an 11 cm perigastric mass and multiple liver metastases consistent with wild-type GIST (negative KIT or PDGFR mutations) on percutaneous biopsy. She had a history of confirmed germline SDHA-deletion in multiple family members including her mother, aunt and children. Neither the patient or her family members had a known history of associated of paragangliomas or pulmonary chondromas, suggestive of a syndromic process. She was a former smoker and had no other pertinent history. Thus, upon diagnosis she underwent a total gastrectomy, small bowel resection and partial hepatectomy, followed by a short course of imatinib which was transitioned to everolimus for therapy intolerance. She was maintained on everolimus for close to 12 months until she developed evidence of disease progression mainly localized to her liver. She additionally underwent two abdominal metastasectomies for recurrent disease, both laden with post-operative complications. Furthermore, she received courses of stereotactic body radiation (SBRT) for management of her spinal lesions.
On follow up imaging after completion of her SBRT therapy, she was found to have new FDG-avid masses to the right diaphragm and paraesophageal region (Figure 1). Given her history and the unusual location of these newly discovered positron emission tomography (PET) avid lesions, an extensive pre-operative workup was conducted to determine its etiology. Inflammatory process, GIST recurrence, or syndromic versus non-syndromic-related primaries were included on the differential.
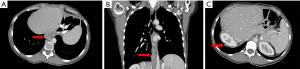
Multi-modality imaging confirmed a 2.6 cm × 2 cm posterior mediastinal mass and a 1.2 cm diaphragmatic mass adjacent to the liver dome. Biochemical and laboratory analysis were not consistent with a systemic infectious process or the presence of a catecholamine-secreting paraganglioma. Thus, an esophagogastroduodenoscopy (EGD) and an endoscopic ultrasound (EUS)-guided biopsy was performed to obtain a tissue diagnosis. Histopathologic analysis confirmed an epithelioid GIST recurrence. Due to her history of multiple abdominal metastasectomies and the unique location of the tumors, a minimally invasive, robotic-assisted thoracoscopic approach was employed.
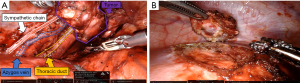
To accomplish this, ports were placed in the standard fashion for posterior mediastinal robotic surgery (Figure S1). The tumor was identified as a conglomeration of multiple nodules that coalesced into one large mass extending from the diaphragm to nearly the level of the subcarinal space. The parietal pleura was opened, and the tumor was dissected free by skeletonizing underlying structures while preserving key structures (azygous vein, thoracic duct, esophagus, aorta) (Figure 2A). Frozen section of a portion of the mass was confirmed to be consistent with GIST histology. The diaphragmatic metastasis was identified, and a full-thickness section of diaphragm was resected en-bloc with tumor utilizing bipolar cautery (Figure 2B), followed by primary diaphragm repair. She recovered appropriately in the post-anesthesia care unit without event and was subsequently discharged on post-operative day 8 after an unremarkable hospital course. Final histologic and molecular analysis of the posterior mediastinal and diaphragmatic masses demonstrated recurrent SDHA-deficient GISTs (c667delG mutation at chromosome 5) (Figure 3).
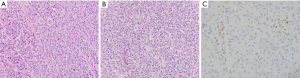
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Herein, we describe the first known presentation of recurrent foci of SDHA-deficient GIST to the mediastinum and diaphragm, including its complex clinical and operative management. Inactivating mutations in the SDHA catalytic subunit are the most common cause of SDH-deficient GIST (5). They generally localize to the stomach, have a male predilection and a median age of onset at 34 years, versus other subunit types at 21 years (6).
Overall, SDH-deficient tumors tend to have a low mitotic activity, indolent behavior, and favorable long-term survival. However, these tumors are highly metastatic, with 60–80% of variant carriers developing distant disease in their lifetimes (7). SDH-deficient GISTs lack the tyrosine kinase binding domains seen in KIT/PDGFR-related GISTs, which causes WT GISTs to be essentially non-responsive to the conventional tyrosine kinase inhibitor (TKI) therapy, imatinib. Some patients with SDH-deficient GIST may derive clinical benefit from later-generation TKIs, namely sunitinib and regorafenib, given these agents’ vascular endothelial growth factor inhibition as opposed to their TKI activity (8). Other therapies such as DNA-damage inducing temozolomide (TMZ), have shown promising results in a retrospective single institutional study in patients with TKI-refractory SDH-deficient GIST. Albeit a limited cohort, all five patients (100%) had evidence of partial response or stabilization of disease progression despite their metastatic disease burden on presentation (9). Thus, active accrual and evaluation of TMZ’s efficacy in patients with advanced SDH-deficient GISTs is currently ongoing in Phase II trials [NCT 03556384]. Furthermore, the use of a novel small molecule fibroblast growth factor receptor (FGFR) inhibitor, rogaratinib, in SDH-deficient GISTs is currently in phase II clinical trials [NCT 04595747], although its clinical benefit is yet to be fully determined. Radiotherapy has shown some efficacy in locally advanced and metastatic GISTs (10), however, given the central location of the lesions in our patient’s case, surgical resection was recommended over SBRT due to the risk of esophageal toxicity.
With limited availability of effective treatment options, resection of the primary tumor and, when appropriate, locoregional and distant metastasectomies remains the mainstay in treatment of all WT GISTs. Multiple studies have shown the efficacy of metastasectomy in combination with first or second generation TKI’s for KIT/PDGFR-related GISTs, however, these outcomes are yet to be extrapolated to WT GISTs (11-13). Although no survival benefit has been associated with achieving an R0 compared to an R1 resection, R0 resection should be the goal. Additionally, surgical resection is most efficacious in the setting of symptomatology, obstruction, hemorrhage, or functional compromise for management of recurrent disease (14). Utility of an open versus minimally invasive surgical approach should be predicated by tumor size and location, the patient’s medical and surgical history, as well as surgeon experience. Classification tools have emerged that provide a decision framework for a minimally invasive approach to GISTs located in the stomach, however, there is a paucity of data regarding minimally invasive resections of metastatic lesions at other sites as described in this case (15).
Conclusions
Nonetheless, this report is strengthened by the uniqueness of its presentation and management, however, common to all case reports, its impact on general practice is often limited as patient outcomes may vary from case to case. Overall, these cases underscore the need for a comprehensive knowledge of tumor biology, a skilled surgical team, and multi-disciplinary involvement in order to optimize care and ensure favorable outcomes in this patient population.
Acknowledgments
We would like to acknowledge expert pathology review and representative photographs of resected lesions provided by Markku Miettinen, MD.
Funding: This research was conducted at the NIH using intramural research funds.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-714/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-714/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-714/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang DY, Wang X, Yuan WJ, et al. Metastatic pattern and prognosis of gastrointestinal stromal tumor (GIST): a SEER-based analysis. Clin Transl Oncol 2019;21:1654-62. [Crossref] [PubMed]
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. [Crossref] [PubMed]
- Keung EZ, Fairweather M, Raut CP. The Role of Surgery in Metastatic Gastrointestinal Stromal Tumors. Curr Treat Options Oncol 2016;17:8. [Crossref] [PubMed]
- Miettinen M, Killian JK, Wang ZF, et al. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol 2013;37:234-40. [Crossref] [PubMed]
- Miettinen M, Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs) - a review. Int J Biochem Cell Biol 2014;53:514-9. [Crossref] [PubMed]
- Neppala P, Banerjee S, Fanta PT, et al. Current management of succinate dehydrogenase-deficient gastrointestinal stromal tumors. Cancer Metastasis Rev 2019;38:525-35. [Crossref] [PubMed]
- Ibrahim A, Chopra S. Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumors. Arch Pathol Lab Med 2020;144:655-60. [Crossref] [PubMed]
- Ben-Ami E, Barysauskas CM, von Mehren M, et al. Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol 2016;27:1794-9. [Crossref] [PubMed]
- Yebra M, Bhargava S, Kumar A, et al. Establishment of Patient-Derived Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumor Models for Predicting Therapeutic Response. Clin Cancer Res 2022;28:187-200. [Crossref] [PubMed]
- Cuaron JJ, Goodman KA, Lee N, et al. External beam radiation therapy for locally advanced and metastatic gastrointestinal stromal tumors. Radiat Oncol 2013;8:274. [Crossref] [PubMed]
- Fairweather M, Balachandran VP, Li GZ, et al. Cytoreductive Surgery for Metastatic Gastrointestinal Stromal Tumors Treated With Tyrosine Kinase Inhibitors: A 2-institutional Analysis. Ann Surg 2018;268:296-302. [Crossref] [PubMed]
- Zhang X, Zhou Y, Wu X, et al. Cytoreductive surgery for metastatic gastrointestinal stromal tumors followed by sunitinib compared to followed by imatinib-a multi-center cohort study. Eur J Surg Oncol 2019;45:318-23. [Crossref] [PubMed]
- Yeh CN, Hu CH, Wang SY, et al. Cytoreductive Surgery may be beneficial for highly selected patients with Metastatic Gastrointestinal Stromal Tumors receiving Regorafenib facing Local Progression: A Case Controlled Study. J Cancer 2021;12:3335-43. [Crossref] [PubMed]
- Weldon CB, Madenci AL, Boikos SA, et al. Surgical Management of Wild-Type Gastrointestinal Stromal Tumors: A Report From the National Institutes of Health Pediatric and Wildtype GIST Clinic. J Clin Oncol 2017;35:523-8. [Crossref] [PubMed]
- Hagerty BL, Torres MB, Drake J, et al. Trends and Predictors of Failure of Minimally Invasive Surgery for Gastric GIST. J Gastrointest Surg 2021;25:1319-22. [Crossref] [PubMed]


