
Short-term clinical effects of robot-assisted esophagectomy with thoracic duct resection
Highlight box
Key findings
• RAE-TDR may help to improve total and metastatic LN harvest. However, RAE-TDR did not lead to a decrease in the local recurrence rate within the short-term follow-up period.
What is known and what is new?
• The question of whether TDR and surrounding LN dissection are necessary steps in esophageal cancer surgery has been controversial.
• No previous related study has investigated the short- and long-term outcomes of RAE-TDR for esophageal cancer. RAE-TDR did not lead to a decrease in the local recurrence rate within the short-term follow-up period.
What is the implication, and what should change now?
• Nonselective RAE-TDR is not routinely recommended for patients with esophageal cancer. To explore whether thoracic duct resection can improve the prognosis of patients is necessary in a prospective multicenter study.
Introduction
Despite multimodality treatment plans playing an increasingly important role in the treatment of patients with esophageal squamous cell carcinoma (ESCC), esophagectomy with lymph node (LN) dissection remains one of the most effective methods (1). However, a standard procedure to enhance the control of local recurrence and distal metastasis still needs to be defined. Additionally, the 5-year survival of patients with ESCC remains unsatisfactory due to the occurrence of LN metastasis at the early stage (2).
ESCC LN metastasis occurs both vertically and horizontally (3). Mediastinal thoracic duct lymph nodes (TDLNs) lie within the adipose and connective tissue between the descending aorta and azygos vein (4). A complete lymphadenectomy should include the resection of the thoracic duct (TD), TDLNs, and surrounding tissues. The incidence of TDLN metastasis is reported to be approximately 2–11% (5). Previous studies have revealed that thoracic duct resection (TDR) is associated with both reduced hematogenous and local recurrence in patients with more advanced ESCC; thus, TDR leads to improved clinical outcomes (6,7). However, this procedure has raised debates due to concerns about its perioperative complications, including higher intraoperative bleeding, more frequent Clavien–Dindo (CD) classifications for grade ≥ II complications, and pulmonary morbidities (8,9). To date, there has been limited research on whether TDR definitively improves patients’ short- and long-term outcomes.
Robot-assisted minimally invasive esophagectomy (RAMIE) facilitates complex, minimally invasive surgical procedures and has multiple advantages, including an enlarged 3-dimensional (3D) view, 540° wrist articulation, and greater magnification (10,11). Furthermore, RAMIE offers precise dissection in the narrow working space in the posterior mediastinum, and is oncologically acceptable in terms of its shorter operating time, lower blood loss, and reduced pulmonary complications (12).
To our knowledge, no previous study has investigated the short- and long-term outcomes of robot-assisted esophagectomy with thoracic duct resection (RAE-TDR) for esophageal cancer. We prospectively analyzed a cohort of ESCC patients who underwent RAE and explored the effects of TDR on intra- and post-operative complications. We also examined recurrence-free survival (RFS) and overall survival (OS) at 1-year after surgery to evaluate the efficacy of RAE-TDR. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-985/rc).
Patients and methods
This single-center cohort study comprised 200 consecutive patients diagnosed with ESCC who received McKeown RAE at the Shanghai Chest Hospital between January 2019 and July 2020. Between 2019 and 2020, 127 consecutive ESCC patients received standard McKeown RAE surgery without TDR. From 2020 onward, esophagectomy with extended LN and TDR was executed in 73 patients. All participants were diagnosed preoperatively by endoscopy, enhanced contrast computed tomography (CT), and biopsy. Primarily resectable ESCC was defined as cT1–4a, N0–2, and M0 according to the staging system of the American Joint Committee for Cancer (seventh edition) (13). In our study, surgeons performing ≥40 RAE cases annually were considered sufficiently skilled, and RAE was completed with the help of the da Vinci surgical system. This study was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1734) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all patients.
The standard esophagectomy conducted in our study was defined as a 3-incision McKeown surgery (left neck, left chest, and abdomen) with at least 2 fields of (thoracoabdominal) lymphadenectomy according to the Japanese Classification of Esophageal Cancer (14-16). The surgical procedures are described in our previous publication (17). Complete mesoesophageal resection involving TDR was performed as follows: after the azygos vein dissection, TD and the surrounding soft tissue, including the LNs, were dissected from the left mediastinum pleural surface to the esophageal side starting from the superior mediastinum. Continuing down to the descending aorta, the TD and surrounding soft tissue were completely removed, including the LNs, between the azygos vein and the aorta. The excision boundary ranged from the intersection of the TD with the left recurrent laryngeal nerve (RLN) to the level of the 10th thoracic vertebra. A schematic diagram of the robot-assisted mesoesophagectomy with TDR is shown in Figure 1. All patients were followed up in the outpatient clinic every 1 to 3 months. The follow-up visits were terminated following death or at 1-year after surgery.

Statistical analysis
The statistical analysis in our study was performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA). The continuous variables were expressed as the mean ± standard deviation (M ± SD), and differences between the groups were analyzed using the Mann–Whitney U test or Student’s t-test according to the homoscedasticity of variance. The categorical variables in different groups were assessed using the chi-squared test or Fisher’s exact test. The survival curves were estimated using the Kaplan–Meier method, and the survival distribution was compared using the log-rank test. Statistical significance was considered when P≤0.05.
Results
Patient characteristics
A total of 200 ESCC patients underwent McKeown RAE with at least 2-field LN dissection at Shanghai Chest Hospital between January 2019 and July 2020. The TD-resected group comprised 73 patients and the TD-preserved group comprised 127 patients. The patient characteristics are presented in Table 1. The outcomes of the 2 groups were comparable in terms of the baseline characteristics, cTNM stage, pTNM stage, and preoperative therapy. The mean age of patients in the TD-resected group was 63.5±10.3 years, and that of the TD-preserved group was 65.9±7.5 years (P=0.056). The TD-resected group comprised 63 males and 10 females, whereas the TD-preserved group comprised 104 males and 23 females (P=0.418).
Table 1
| Characteristics | TD-resected (n=73) | TD-preserved (n=127) | P value |
|---|---|---|---|
| Age, years, M ± SD | 63.5±10.3 | 65.9±7.5 | 0.056 |
| Gender, n (%) | 0.418 | ||
| Male | 63 (86.3) | 104 (81.9) | |
| Female | 10 (13.7) | 23 (18.1) | |
| BMI, n (%) | 0.234 | ||
| <18.5 kg/m2 | 3 (4.1) | 9 (7.1) | |
| 18.5–24 kg/m2 | 40 (54.8) | 80 (63.0) | |
| >24 kg/m2 | 30 (41.1) | 38 (29.9) | |
| ASA score, n (%) | 0.923 | ||
| 2 | 47 (64.4) | 79 (62.2) | |
| 3 | 26 (35.6) | 47 (37.0) | |
| 4 | 0 | 1 (0.8) | |
| Tumor location, n (%) | 0.15 | ||
| Upper | 7 (9.6) | 13 (10.2) | |
| Middle | 40 (54.8) | 52 (40.9) | |
| Lower | 26 (35.6) | 62 (48.8) | |
| cT stage, n (%) | 0.287 | ||
| T1 | 5 (6.8) | 9 (7.1) | |
| T2 | 16 (21.9) | 35 (27.6) | |
| T3 | 50 (68.5) | 83 (65.4) | |
| T4 | 2 (2.7) | 0 | |
| cN stage, n (%) | 0.135 | ||
| N0 | 12 (16.4) | 34 (26.8) | |
| N1 | 41 (56.2) | 69 (54.3) | |
| N2 | 20 (27.4) | 22 (17.3) | |
| N3 | 0 | 2 (1.6) | |
| cTNM stage, n (%) | 0.103 | ||
| I | 4 (5.5) | 9 (7.1) | |
| II | 17 (23.3) | 49 (38.6) | |
| III | 50 (68.5) | 67 (52.8) | |
| IVa | 2 (2.7) | 2 (1.6) | |
| pT stage, n (%) | 0.852 | ||
| T0 | 12 (16.4) | 16 (12.6) | |
| T1 | 13 (17.8) | 27 (21.3) | |
| T2 | 12 (16.4) | 20 (15.7) | |
| T3 | 36 (49.3) | 64 (50.4) | |
| pN stage, n (%) | 0.304 | ||
| N0 | 31 (42.5) | 65 (51.2) | |
| N1 | 25 (34.2) | 33 (26.0) | |
| N2 | 9 (12.3) | 21 (16.5) | |
| N3 | 8 (11.0) | 8 (6.3) | |
| pTNM stage, n (%) | 0.348 | ||
| I | 19 (26.0) | 32 (25.2) | |
| II | 15 (20.5) | 39 (30.7) | |
| III | 31 (42.5) | 48 (37.8) | |
| IVa | 8 (11.0) | 8 (6.3) |
TD, thoracic duct; M ± SD, mean ± standard deviation; BMI, body mass index; ASA, American Society of Anesthesiologists.
Anticancer treatment, including neoadjuvant chemotherapy, neoadjuvant chemoradiotherapy, neoadjuvant chemotherapy combined with immunotherapy, definitive chemoradiotherapy, and radiotherapy, was performed in 29 and 39 patients in the TD-resected and TD-preserved groups, respectively; however, there were no statistical differences between the 2 groups in terms of the preoperative treatment (P=0.195) or tumor regression status (P=0.096).
Intra- and post-operative parameters
The operative time for the thoracic phase (P=0.088) and total time (P=0.091) of RAE were calculated, and no significant differences were observed between the TD-resected group and TD-the preserved group. The bleeding volume of the TD-resected group was significantly lower than that of the TD-preserved group (P=0.008). In terms of complete resection, there were no differences in the distribution of the R0 resection status between the 2 groups (P=0.387). As Table 2 shows, 63 patients (86.3%) and 113 patients (89.0%) underwent R0 resection in the TD-resected and TD-preserved groups, respectively. However, 9 patients (12.3%) in the TD-resected group and 14 patients (11.0%) in the TD-preserved group had a positive circumferential resection margin (R1 resection). Further, a residual tumor was confirmed in the tracheal membrane of 1 patient in the TD-resected group (R2 resection).
Table 2
| Parameters | TD-resected | TD-preserved | P value |
|---|---|---|---|
| Intraoperative | |||
| Duration of operation (min), M ± SD | |||
| Total | 283.2±55.9 | 262.1±46.8 | 0.091 |
| Thoracic phase | 98.0±21.5 | 93.1±18.2 | 0.088 |
| Bleeding (mL), M ± SD | 139.0±48.8 | 160.6±57.9 | 0.008* |
| Resection status, n | 0.387 | ||
| R0 | 63 | 113 | |
| R1 | 9 | 12 | |
| R2 | 1 | 2 | |
| Postoperative | |||
| CD grade > II, n | 11 | 17 | 0.741 |
| CD grade > III, n | 8 | 8 | 0.242 |
| Pneumonia, n | 8 | 8 | 0.242 |
| Chylothorax, n | 6 | 6 | 0.361 |
| RLN palsy, n | 12 | 28 | 0.342 |
| Anastomotic leakage, n | 2 | 5 | 1.0 |
| Duration of drainage (day), M ± SD | 4.4±2.7 | 4.4±3.2 | 0.999 |
| Length of ICU stay (day), M ± SD | 1.5±1.4 | 1.4±1.7 | 0.643 |
| Length of hospital stay (day), M ± SD | 9.5±4.5 | 9.6±4.3 | 0.882 |
*P<0.05. TD, thoracic duct; M ± SD, mean ± standard deviation; CD, Clavien-Dindo; RLN, recurrent laryngeal nerve; ICU, intensive care unit.
The postoperative comorbidities were also compared, but no significant differences were detected between the 2 groups (P=0.432). Notably, 23 (31.5%) and 47 (37.0%) patients in the TD-resected and TD-preserved groups, respectively, experienced at least 1 complication after surgery. The rates of CD stage II–III complications, including chylothorax (P=0.36), left RLN palsy (P=0.34), and anastomotic leakage (P=1.0), were comparable between the 2 groups. Pulmonary complications occurred in 8 TD-resected and 8 TD-preserved patients (P=0.242). The thoracic drainage times were 4.4±2.7 days in the TD-resected group and 4.4±3.2 days in the TD-preserved group, and there was no difference between the 2 groups (P=0.999). The lengths of postoperative intensive care unit (ICU) and hospital stays in the TD-resected patients were 1.5±1.4 days and 9.5±4.5 days, respectively, and did not differ significantly to those of the TD-preserved patients whose ICU and hospital stays were 1.4±1.7 days (P=0.643) and 9.6±4.3 days (P=0.882), respectively.
The number of total retrieved nodes and mediastinal nodes were significantly higher in the TD-resected group than in the TD-preserved group (P=0.006 and P=0.002, respectively). As for the number of tumor-infiltrated LNs, there were no differences between the 2 groups irrespective of the operation phase (see Table 3). However, 8 patients (11%) in the TD-resected group had metastatic TD LNs. We also compared the number of total and metastatic mediastinal LNs according to cT stage and found that more mediastinal LNs were retrieved (P<0.001) in the TD-resected group than in the TD-preserved group (see Table 4), especially No. 109R (P=0.006), No. 110 (P=0.049), and No. 111 (P<0.001) LNs; however, there were no significant differences in the metastatic mediastinal LNs between the 2 groups. We compared the metastatic and total mediastinal LNs in cT stage 3/4 tumors, and found that more mediastinal LNs were harvested in the TD-resected group than in the TD-preserved group (P<0.001; see Table 5). In the TD-resected group, 2 (9.50%) and 6 (11.53%) patients had metastatic TDLNs in cT stage 1–2 and cT stage 3–4, respectively (P>0.05).
Table 3
| Location of lymph nodes | TD-resected | TD-preserved | P value |
|---|---|---|---|
| Total LNs | 29.0±11.1 | 25.1±8.5 | 0.006* |
| Cervical | 2.9±4.4 | 1.9±3.8 | 0.119 |
| Thoracic | 19.5±8.0 | 16.1±5.5 | 0.002* |
| Abdominal | 6.6±4.1 | 7.0±3.7 | 0.394 |
| Total positive LNs | 2.3±3.7 | 1.7±2.8 | 0.210 |
| Cervical | 0.2±0.6 | 0.1±0.6 | 0.659 |
| Thoracic | 1.3±2.3 | 1.0±1.9 | 0.350 |
| Abdominal | 0.9±1.9 | 0.6±1.3 | 0.164 |
*P<0.05. Data are presented as mean ± standard deviation. LN, lymph node; TD, thoracic duct.
Table 4
| Location of lymph nodes | Metastatic | Total | |||||
|---|---|---|---|---|---|---|---|
| TD-resected | TD-preserved | P value | TD-resected | TD-preserved | P value | ||
| 105 | 0.05±0.23 | 0.06±0.30 | 0.84 | 1.16±1.43 | 1.23±1.57 | 0.78 | |
| 106R | 0.44±0.96 | 0.38±0.98 | 0.67 | 2.59±2.23 | 2.79±1.91 | 0.51 | |
| 106L | 0.12±0.55 | 0.14±0.45 | 0.80 | 2.37±1.94 | 2.08±1.78 | 0.28 | |
| 107 | 0.11±0.42 | 0.06±0.26 | 0.26 | 2.59±1.90 | 3.12±2.13 | 0.08 | |
| 108 | 0.07±0.30 | 0.09±0.41 | 0.64 | 2.03±1.80 | 1.61±1.62 | 0.10 | |
| 109R | 0.05±0.23 | 0.02±0.12 | 0.12 | 1.86±1.78 | 1.20±1.54 | 0.006* | |
| 109L | 0.03±0.16 | 0.08±0.35 | 0.24 | 1.73±1.94 | 1.54±1.86 | 0.50 | |
| 110 | 0.19±0.61 | 0.12±0.37 | 0.29 | 1.71±1.61 | 1.26±1.52 | 0.049* | |
| 111 | 0.05±0.23 | 0.05±0.25 | 0.83 | 0.96±0.97 | 0.42±0.74 | <0.001* | |
| TDLN | 0.15±0.49 | NA | 1.59±1.83 | NA | |||
| Total | 1.27±2.30 | 0.99±1.85 | 0.35 | 18.59±7.52 | 15.24±5.18 | <0.001* | |
*P<0.05. Data are presented as mean ± standard deviation. TD, thoracic duct; TDLN, thoracic duct lymph node; NA, not available.
Table 5
| Location of lymph nodes | Metastatic | Total | |||||
|---|---|---|---|---|---|---|---|
| TD-resected | TD-preserved | P value | TD-resected | TD-preserved | P value | ||
| 105 | 0.06±0.24 | 0.09±0.37 | 0.58 | 1.31±1.62 | 1.21±1.68 | 0.66 | |
| 106R | 0.51±1.05 | 0.51±1.19 | 0.61 | 2.45±1.74 | 2.84±1.79 | 0.52 | |
| 106L | 0.16±0.65 | 0.19±0.53 | 0.59 | 2.16±1.66 | 2.01±1.69 | 0.72 | |
| 107 | 0.14±0.49 | 0.07±0.30 | 0.62 | 2.51±1.96 | 3.28±2.29 | 0.40 | |
| 108 | 0.10±0.36 | 0.11±0.45 | 0.59 | 2.02±1.77 | 1.48±1.55 | 0.86 | |
| 109R | 0.06±0.24 | 0.01±0.11 | 0.61 | 1.49±1.42 | 1.33±1.70 | 0.69 | |
| 109L | 0.02±0.14 | 0.11±0.42 | 0.56 | 1.24±1.39 | 1.49±1.96 | 0.54 | |
| 110 | 0.24±0.69 | 0.15±0.42 | 0.63 | 1.67±1.67 | 1.24±1.52 | 0.81 | |
| 111 | 0.06±0.24 | 0.08±0.32 | 0.59 | 0.84±0.98 | 0.40±0.75 | 0.77 | |
| TDLN | 0.20±0.57 | NA | 1.69±1.86 | NA | |||
| Total | 1.57±2.58 | 1.31±2.12 | 0.74 | 17.39±6.01 | 15.29±5.44 | 0.022* | |
*P<0.05. Data are presented as mean ± standard deviation. TD, thoracic duct; TDLN, thoracic duct lymph node; NA, not available.
Relationship between TDR and short-term outcomes
Across all stages, the OS at 1 year after surgery of the TD-resected group (94.52%) was higher than that of the TD-preserved group (90.55%); however, the survival curves did not differ significantly (P=0.402, log-rank test; see Figure 2A). Additionally, the RFS at 1 year of the TD-resected patients (76.71%) was lower than that of the TD-preserved patients (85.04%), with the curves being similar (P=0.996; see Figure 2B).
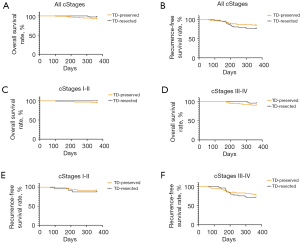
We further analyzed the 1-year OS and RFS according to the clinical stages. The 1-year OS of the stage I–II and stage III–IV patients were similar regardless of the TDR status (P=0.953 and P=0.317) (see Figure 2C,2D). Additionally, 1-year RFS was found to be irrelevant to TDR status in stage I–II (P=0.976) and stage III–IV (P=0.903) patients (see Figure 2E,2F).
The postoperative recurrence patterns are shown in Table 6. The incidence of hematogenous recurrence at 1 year after surgery was significantly lower in the TD-preserved group (7.9%) than in the TD-resected group (17.8%; P=0.034). However, the liver, bone, lung, brain, and other hematogenous metastases were similar in the 2 groups. The incidences of LN recurrence (P>0.99) and local recurrence (P>0.99) during the 1-year follow-up did not differ between the 2 groups.
Table 6
| Location | TD-resected (n=73), n (%) | TD-preserved (n=127), n (%) | P value |
|---|---|---|---|
| Hematogenous metastasis | 13 (17.8) | 10 (7.9) | 0.034* |
| Liver | 2 (2.7) | 4 (3.1) | >0.99 |
| Bone | 4 (5.4) | 3 (2.4) | 0.261 |
| Lung | 4 (5.4) | 4 (3.1) | 0.467 |
| Brain | 2 (2.7) | 1 (0.8) | 0.555 |
| Other | 6 (8.1) | 3 (2.4) | 0.076 |
| LN recurrence | 5 (6.8) | 9 (7.1) | >0.99 |
| Local | 3 (4.1) | 3 (2.4) | 0.670 |
| Distant | 5 (6.8) | 6 (4.8) | 0.534 |
| Local recurrence | 1 (1.4) | 3 (2.4) | >0.99 |
*P<0.05. TD, thoracic duct; LN, lymph node.
Discussion
The question of whether TDR and surrounding LN dissection are necessary steps in esophageal cancer surgery has been controversial. Concerns arise as to whether this approach increases surgical complications, whether it can control local recurrence, and whether it will lead to long-term harm due to its effects on humoral immune circulation. Thus, we sought to examine the use of RAE-TDR in homogenous, high-precision robotic surgery in which the learning curve had been completed.
Chylothorax and RLN palsy are the 2 most common complications after esophagectomy and are regarded as collateral damage to lymphadenectomy. RAE for esophageal cancer has been increasingly performed at several large international reference centers. The minimally invasive effect and higher efficacy of regional LN dissection have been demonstrated (10,17). Under the robot-assisted system, better visualization and a flexible but steady robot arm allows the surgeon to precisely and completely dissect the TD, TDLNs, LNs, and RLN without any other accidental injuries.
In our study, the number of dissected LNs in the thorax was significantly increased by TDR, which is consistent with previous reports (12,17). We also discovered that the number of dissected LNs in stations 109R, 110, and 111 was significantly higher in the TD-resected group than in the TD-preserved group. Despite of the insignificant statistical power, more metastatic mediastinal LNs, including positive TDLNs, were harvested in the TD-resected patients, which may have a favorable effect in preventing tumor metastasis.
There is still considerable debate about the long-term survival effects of TDR. The only possible positive effects of TDR are to achieve greater LN dissection and avoid the presence of occult metastatic LNs. The spatial relationship of the TD and its surrounding LNs is very difficult to define, especially if the TD is in close proximity to the middle and upper paraoesophageal LNs. In relation to LN metastasis in esophageal cancer, if the invasion is above T2, the metastatic LNs will significantly metastasize to the tumor side wall and have the potential to drain directly into the TD. Complete TDR is thus beneficial to obtaining the effect of a radical en bloc tumor resection. The complete resection of TD should include the TD, TDLNs, and adipose tissues surrounding the TD, as the incidence of TDLN metastasis has been reported to be approximately 2–11%, and some patients with ESCC have been reported to have TDLN recurrence after radical esophagectomy; thus, it has been proposed that joint resection with TDR may improve the long-term outcomes of patients (5).
Tanaka et al. conducted a retrospective, multi-institutional analysis in which they compared the outcomes of 2,269 patients receiving esophagectomy with and without TDR and concluded that TDR is associated with both reduced hematogenous and local recurrence in local advanced ESCC patients and thus leads to improved short- and long-term outcomes (6). However, some studies have found that TDR produces survival benefits even in T1 patients (7,18). This appears to contradict the previous rule of LN metastasis that states that LNs will metastasize up and down the submucosa and form lesions beside the recurrent laryngeal nerve and around the cardia in T1 patients.
In addition to greater tumor resection, TDR and its surrounding LN dissection may also have negative effects. In Matsuda et al.’s study, the TD-preserved group had a more favorable RFS curve than did the TD-resected group among patients who underwent esophagectomy for c-stage II–IV ESCC (18). Additionally, Hou et al. a conducted a multivariate Cox regression analysis and reported that TD ligation during esophagectomy unfavorably affected the OS of patients with esophageal cancer (19). Recently, a large-scale multicenter cohort study Japan suggested that TD resection can obtain more positive LNs, but it may lead to worse long-term survival and more distant organ metastasis. Our findings were consistent with this (20).
As an independent human organ, the TD should be protected. The TD is indispensable in immune function and nutrition circulation, as it is the largest lymphatic vessel and drains about 75% of the lymph, with rich ingested fats, proteins, T lymphocytes, and various immune components; thus, the ligation or resection of the TD may result in hemodynamic disturbances and malnutrition (21,22). The TD also lets intestinal chyle flow into the venous system, and the continued interruption of chyle flow can lead to immunosuppression and impaired B-lymphocyte–mediated immune function, which may worsen the outcomes of TD-resected patients (23). T follicular helper (Tfh) cells in germinal centers of secondary lymphoid organs are pivotal for humoral immunity. Circulating Tfh cells can inhibit micrometastasis of tumors. The TD is the main route of Tfh cell traffic from lymphatic system to blood system (24). Therefore, after thoracic duct resection, humoral immunity is impaired which may lead to more distant organ metastasis.
In our study, the intra- and post-operative parameters were almost comparable between the 2 groups except that the bleeding volume was lower in the TD-resected group. The 1-year OS, RFS, and recurrence patterns across all stages were similar among the TD-resected and TD-preserved patients. This is mainly due to the short follow-up time. However, the distant metastasis rate of the TD-resected group was much higher than that of TD-preserved group. This may have been due to immune system damage after TDR. Thus, it appears that TDR and peripheral LN dissection should be performed in patients with a high risk of LN metastasis to minimize the risk of surgery.
Our study had some limitations, including its small volume of samples and its recruitment of participants from only 1 center. Additionally, propensity score matching was not performed to minimize selection bias before the analysis, as the baseline characteristics were considered comparable. Finally, the follow-up period was too short to reach a significant end point.
In conclusion, the technique of RAE-TDR is safe, does not increase postoperative complications, and only slightly prolongs the operation time. RAE-TDR may help to improve the total and metastatic harvest of LNs, especially in patients with advanced ESCC, without increasing the intra- and postoperative incidence of adverse events. However, RAE-TDR does not lead to a decrease in the local recurrence rate in the short-term. Whether the increase in distant metastasis rate in the TD-resected group was associated with relevant immune system damage is unclear. Thus, nonselective RAE-TDR is not routinely recommended.
Conclusions
RAE-TDR may help to improve total and metastatic LN harvest, especially in patients with advanced ESCC without increasing the intra- and post-operative adverse events. However, RAE-TDR did not lead to a decrease in the local recurrence rate within the short-term follow-up period. Thus, nonselective RAE-TDR is not routinely recommended.
Acknowledgments
Thanks to AME English Editors (L. Huleatt and J.Gray) for polishing the manuscript language. The abstract has been presented as oral presentation at the ISDE 2022 Virtual Congress. We have obtained permission for reuse of the abstract.
Funding: This work was supported by the Shanghai Esophageal Cancer Cohort Database of Shanghai Hospital Development Center (No. SHDC2020CR6002) to ZGL and 2020 Shanghai “Rising Stars of Medical Talents” Youth Development Program to CGL.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-985/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-985/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-985/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-985/coif). All authors report that the study was supported by 2020 Shanghai “Rising Stars of Medical Talents” Youth Development Program to CGL, and the Shanghai Esophageal Cancer Cohort Database of Shanghai Hospital Development Center (No. SHDC2020CR6002) to ZGL. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1734) and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was taken from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Tachimori Y. Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: efficacy of lymph node dissection according to tumor location. J Thorac Dis 2017;9:S724-30. [Crossref] [PubMed]
- Schurink B, Defize IL, Mazza E, et al. Two-Field Lymphadenectomy During Esophagectomy: The Presence of Thoracic Duct Lymph Nodes. Ann Thorac Surg 2018;106:435-9. [Crossref] [PubMed]
- Udagawa H, Ueno M, Shinohara H, et al. Should lymph nodes along the thoracic duct be dissected routinely in radical esophagectomy? Esophagus 2014;11:204-10.
- Tanaka K, Yamasaki M, Sugimura K, et al. Thoracic Duct Resection Has a Favorable Impact on Prognosis by Preventing Hematogenous Spread of Esophageal Cancer Cells: A Multi-institutional Analysis of 2269 Patients. Ann Surg Oncol 2021;28:4402-10. [Crossref] [PubMed]
- Yoshida N, Nagai Y, Baba Y, et al. Effect of Resection of the Thoracic Duct and Surrounding Lymph Nodes on Short- and Long-Term and Nutritional Outcomes After Esophagectomy for Esophageal Cancer. Ann Surg Oncol 2019;26:1893-900. [Crossref] [PubMed]
- Oshikiri T, Takiguchi G, Miura S, et al. Thoracic Duct Resection During Esophagectomy Does Not Contribute to Improved Prognosis in Esophageal Squamous Cell Carcinoma: A Propensity Score Matched-Cohort Study. Ann Surg Oncol 2019;26:4053-61. [Crossref] [PubMed]
- Bao T, Wang YJ, Li KK, et al. Short- and long-term outcomes of prophylactic thoracic duct ligation during thoracoscopic-laparoscopic McKeown esophagectomy for cancer: a propensity score matching analysis. Surg Endosc 2020;34:5023-9. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Yang Y, Zhang X, Li B, et al. Short- and mid-term outcomes of robotic versus thoraco-laparoscopic McKeown esophagectomy for squamous cell esophageal cancer: a propensity score-matched study. Dis Esophagus 2020;33:doz080. [Crossref] [PubMed]
- Wang J, Wu N, Zheng QF, et al. Evaluation of the 7th edition of the TNM classification in patients with resected esophageal squamous cell carcinoma. World J Gastroenterol 2014;20:18397-403.
- McKeown KC. Trends in oesophageal resection for carcinoma with special reference to total oesophagectomy. Ann R Coll Surg Engl 1972;51:213-39.
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus 2017;14:37-65.
- Yang Y, Li B, Yi J, et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma: Early Results of a Multicenter Randomized Controlled Trial: the RAMIE Trial. Ann Surg 2022;275:646-53. [Crossref] [PubMed]
- Matsuda S, Takeuchi H, Kawakubo H, et al. Clinical outcome of transthoracic esophagectomy with thoracic duct resection: Number of dissected lymph node and distribution of lymph node metastasis around the thoracic duct. Medicine (Baltimore) 2016;95:e3839. [Crossref] [PubMed]
- Hou X, Fu JH, Wang X, et al. Prophylactic thoracic duct ligation has unfavorable impact on overall survival in patients with resectable oesophageal cancer. Eur J Surg Oncol 2014;40:1756-62. [Crossref] [PubMed]
- Oshikiri T, Numasaki H, Oguma J, et al. Prognosis of Patients with Esophageal Carcinoma following Routine Thoracic Duct Resection: A Propensity-matched Analysis of 12,237 Patients based on the Comprehensive Registry of Esophageal Cancer in Japan. Ann Surg 2021; Epub ahead of print. [Crossref]
- Zilversmit DB. The composition and structure of lymph chylomicrons in dog, rat, and man. J Clin Invest 1965;44:1610-22. [Crossref] [PubMed]
- Wasmuth-Pietzuch A, Hansmann M, Bartmann P, et al. Congenital chylothorax: lymphopenia and high risk of neonatal infections. Acta Paediatr 2004;93:220-4. [Crossref] [PubMed]
- Cavriani G, Domingos HV, Oliveira-Filho RM, et al. Lymphatic thoracic duct ligation modulates the serum levels of IL-1beta and IL-10 after intestinal ischemia/reperfusion in rats with the involvement of tumor necrosis factor alpha and nitric oxide. Shock 2007;27:209-13. [Crossref] [PubMed]
- Vella LA, Buggert M, Manne S, et al. T follicular helper cells in human efferent lymph retain lymphoid characteristics. J Clin Invest 2019;129:3185-200. [Crossref] [PubMed]


