
A comparison of two methods of lymph node dissection along the left recurrent laryngeal nerve in McKeown minimally invasive esophagectomy
Highlight box
Key findings
• The novel method of lymph node dissection along the left RLN in the semi-prone position during McKeown MIE is safe and reliable.
What is known and what is new?
• Many efforts have been directed to dissect more LNs around the RLN and to lower the incidence of RLN palsy.
• To dissect LNs along the left RLN more thoroughly with a lower risk of nerve palsy, we modified the previously reported methods and applied a novel method, which involves the en bloc dissection of LNs along the left RLN during McKeown MIE in the semi-prone position.
What are the implications, and what should change now?
• Although the LNs also be removed using the conventional method, the left RLN is not fully visible. Meanwhile, our novel lymphadenectomy along the left RLN not only increased the number of LNs but also decreased the incidence of hoarseness.
Introduction
Esophageal cancer is one of the most common malignant tumors around the world, ranking seventh in terms of incidence and sixth in terms of mortality worldwide (1,2). Unfortunately, more than 50% of esophageal cancer cases occur in China, making it the fifth most common malignant tumor and the fourth leading cause of cancer-related death in the country (3,4). In Western countries, the main pathological type of esophageal cancer is adenocarcinoma. In contrast, in China, more than 90% of esophageal cancers are squamous cell carcinomas that are most commonly located in the thoracic esophagus (4). Radical esophagectomy combined with neoadjuvant therapy has been considered the mainstream and effective treatment strategy for esophageal cancer (5). However, due to the high complication and mortality rates of open esophagectomy (OE), minimally invasive esophagectomy (MIE) has been widely accepted since the 1990s owing to its advantages, including less surgical trauma, fewer complications, and lower mortality rate (5-9).
Although neoadjuvant therapy has been commonly applied and demonstrated to increase the survival rate of esophageal cancer patients, the overall survival rate is still fairly low (6,7,10). Esophageal cancer presents with bidirectional, skipping lymph node (LN) metastasis including to the neck, mediastinum, and abdomen areas. Therefore, LN metastasis is one of the most important prognostic factors leading to the poor prognosis of esophageal cancer (10). It has been reported that the metastasis rate of bilateral laryngeal LNs was the highest in thoracic esophageal cancer, and it may even exceed 10% in clinical N0 superficial thoracic esophageal cancer (11). To improve survival and provide adequate staging, a radical approach of lymphadenectomy is necessary, which involves the dissection of abdominal and thoracic LNs, as well as the dissection of the LNs around the bilateral recurrent laryngeal nerve (RLN) (12). However, completely dissecting the LNs around the RLN is challenging due to the complex anatomy, and the narrow space of the upper mediastinum not only increases the difficulty of dissection and prolongs the surgical learning curve but it can also easily lead to RLN injury (13).
Numerous efforts have been directed to achieve the dissection of more LNs around the RLN and to lower the incidence of RLN palsy. Noshiro et al. adopted the prone position in thoracoscopic esophagectomy to provide enhanced exposure and surgical vision around the left RLN (14). Oshikiri et al. proposed a method of LN dissection along the left RLN during prone esophagectomy, known as the Bascule method, which focused on the anatomical concept of the esophageal mesenteriolum (15). Based on these methods, Zhang et al. introduced a modified Bascule method to completely remove the esophageal mesenteriolum to skeletonize the left RLN (16). The results showed that this modified Bascule method could obtain more lymph nodes along the left RLN. The above results showed that the improved method gradually improved the efficiency of lymph node dissection, while maintaining a relatively acceptable rate of recurrent laryngeal nerve injury. Lessons had been learned from the above methods and more efforts could be done. To dissect LNs along the left RLN more thoroughly with a lower risk of nerve palsy, we modified the previously reported methods and applied a novel method, which involves the en bloc dissection of LNs along the left RLN during McKeown MIE in the semi-prone position. Herein, we retrospectively reviewed and analyzed 244 cases of patients who underwent lymphadenectomy along the left RLN during McKeown MIE using the aforementioned novel method versus a conventional method. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1273/rc).
Methods
Patients
This was a retrospective study. Patients who underwent McKeown MIE with lymphadenectomy along left RLN from January 2018 through August 2021 were selected. In total, 244 consecutive cases were enrolled in this study. All patients were diagnosed pathologically with esophageal cancer by electronic esophagogastroduodenoscopy and clinical tumor-node-metastasis (TNM) staging based on positron emission tomography-computed tomography (PET-CT), cervical and supraclavicular B-ultrasound, chest and abdominal contrast CT, or endoscopic ultrasonography. All patients were evaluated by a multidisciplinary expert team and given personalized treatment plans according to the treatment guidelines of the National Comprehensive Cancer Network (NCCN) and the Guidelines for the Diagnosis and Treatment of Esophageal Carcinoma of the Chinese Medical Association. All surgical operations were performed by one surgical team with expertise in OE and MIE. All operations were performed by one surgical team, which was proficient in OE and MIE.
Based on the two different techniques of lymphadenectomy along the left RLN, the patients were assigned into the following two groups: 77 patients received the conventional method (CM group) from January 2018 to June 2019, and 167 patients received the novel method (NM group) from 2019 July to August 2021. Hoarseness was subjectively assessed according to the auditory impression by the otolaryngologist. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from each participant. The research protocol of this clinical study was approved by the Ethics Committee of the Sir Run Run Shaw Hospital (No. 20220314-33).
Thoracic procedure and trocar locations
The patients were placed in a left semi-prone position with single-lumen endotracheal intubation, and artificial carbon dioxide (CO2) pneumothorax was established at a pressure of 6–8 mmHg. Four ports were distributed in the right thorax. A 12-mm port was located at the seventh intercostal space in the mid-axillary line for the camera, and another 12-mm port for the assistant was placed at the tenth intercostal space in the subscapular line. Two additional ports were used for the operation by the surgeon: a 5-mm port was located at the seventh intercostal space in the subscapular line and a 12-mm port was placed at the fifth intercostal space in the mid-axillary line.
Procedure of the novel method for lymphadenectomy along the left RLN
After disconnecting the azygos arch, the esophagus was mobilized from the upper mediastinum to the diaphragm hiatus while protecting the thoracic duct. A sling through the port was then used to suspend the esophagus forward to facilitate the dissection of LNs. The esophageal suspension method not only promotes the continuous traction of the esophagus in the direction of the incision but also moves the right lung forward (Figure 1A). Accordingly, the posterior mediastinum space was expanded to provide a larger operating space.
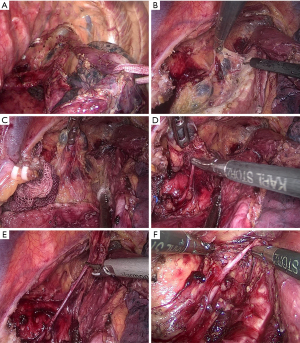
After dissecting the subcarinal LNs, the left main trachea and the lower part of the trachea were rotated forward with an attractor sucking a cotton swab to explore the tracheoesophageal groove. Under proper suspension and good anti-traction, the surface of the tracheoesophageal groove containing LNs, the left RLN, and fatty tissue was well-exposed. Starting from the area below the aortic arch, the anterior edge of the connective tissue was freed along the left main bronchus and then along the proximal direction of the trachea. The tracheal side of the connective tissue in the tracheal esophageal groove was then completely detached (Figure 1B). The surgeon lifted the connective tissue so that it detached from the trachea and expanded the three-dimensional connective tissue into a two-dimensional structure, which we called a two-dimensional membrane. In this two-dimensional membrane, the left RLN, LNs, and vessels were clearly displayed (Figure 1C).
Next, the surgeon separated the LNs in the two-dimensional membrane using forceps by a combination of blunt and sharp methods, starting at the root of the left RLN and moving from bottom to top along the left RLN (Figure 1D). Meanwhile, an ultrasonic knife or a regular knife was used to dissect the fibrous connective tissue and the vessels around the LNs with careful identification and protection of the nourishing vessels of the RLN (Figure 1E). If the vision was affected by bleeding, a small gauze strip was temporarily used to stop the bleeding and to preserve the more nourishing blood vessels around the left RLN. The left RLN was identified and the nerve sheath was protected. The procedure of en bloc lymphadenectomy along the left RLN was then completed, and the left inferior thyroid artery was occasionally visible (Figure 1F).
Procedure of the conventional method for lymphadenectomy along the left RLN
After mobilizing and suspending the esophagus, the subcarinal LNs were dissected using the same procedure as that in the NM group described above. Tissues including the left RLN and LNs in the left tracheoesophageal groove were exposed, and the surgeon bluntly separated the tissues using forceps to separate the LNs from the left RLN. Subsequently, the LNs were dissected by the surgeon after separating them from the middle section of the left RLN using a blunt and sharp motion with his left hand while holding the LNs with his right hand.
Statistical analysis
The demographic parameters of the patients were expressed using descriptive statistics. The continuous variables were expressed as the mean ± standard deviation for normally distributed data or as the median (interquartile range) for non-normally distributed variables. An independent sample t-test was used for comparison between the two groups, while the Mann-Whitney U test and Pearson’s χ2 test were performed to examine the differences between the two groups. Statistical analysis was completed using SPSS® version 20 (SPSS, Inc., Chicago, IL, USA), with P<0.05 indicating a statistically significant difference.
Results
Demographic parameters
A total of 244 cases were included in this study. Among them, 77 cases of LN dissection around the left RLN were performed via the conventional method (CM group), and 167 cases of LN dissection around the left RLN were carried out using the novel method (NM group). The demographic characteristics of the patients, neoadjuvant therapy, and tumor characteristics of the entire cohort are presented in Table 1. There were no significant differences in age, body mass index (BMI), smoking and drinking history, and rate of comorbidities between the two groups (P>0.05). However, the percentages of neoadjuvant chemotherapy and neoadjuvant immunotherapy were significantly higher in the NM group compared to those in the CM group (chemotherapy: 28.7% vs. 15.6%, P=0.027; immunotherapy: 8.4% vs. 0, P=0.009, respectively). Histological analysis indicated that the majority of the cancers were squamous cell carcinomas.
Table 1
| Characteristic | CM group (n=77) | NM group (n=167) | P value |
|---|---|---|---|
| Age (years)* | 64.99±7.20 | 64.80±7.86 | 0.861 |
| Sex, n (%) | 0.276 | ||
| Male | 66 (85.7) | 151 (90.4) | |
| Female | 11 (14.3) | 16 (9.6) | |
| BMI (kg/m2)* | 22.28±3.28 | 21.87±2.94 | 0.320 |
| History of smoking | 36 (46.8) | 59 (35.3) | 0.089 |
| History of drinking | 32 (41.6) | 66 (39.5) | 0.729 |
| ASA, n (%) | 0.336 | ||
| I | 1 (1.3) | 0 (0.0) | |
| II | 75 (97.4) | 165 (98.8) | |
| III | 1 (1.3) | 2 (1.2) | |
| Comorbidity, n (%) | |||
| Hypertension | 25 (32.5) | 58 (34.7) | 0.729 |
| Diabetes mellitus | 3 (3.9) | 7 (4.2) | 0.914 |
| Cardiovascular disease | 6 (7.8) | 13 (7.8) | 0.998 |
| Obstructive lung disease | 4 (5.2) | 5 (3.0) | 0.397 |
| Cerebrovascular disease | 2 (2.6) | 10 (6.0) | 0.255 |
| Neoadjuvant chemotherapy, n (%) | 12 (15.6) | 48 (28.7) | 0.027# |
| Neoadjuvant radiotherapy, n (%) | 2 (2.6) | 1 (0.6) | 0.188 |
| Neoadjuvant immunotherapy, n (%) | 0 (0.0) | 14 (8.4) | 0.009# |
| Endoscopic submucosal dissection, n (%) | 2 (2.6) | 3 (1.8) | 0.681 |
| Histological type, n (%) | 0.031# | ||
| Squamous cell carcinoma | 73 (94.8) | 166 (99.4) | |
| Adenocarcinoma | 1 (1.3) | 1 (0.6) | |
| Others | 3 (3.9) | 0 (0.0) | |
| Tumor location, n (%) | 0.652 | ||
| Upper | 5 (6.5) | 7 (4.2) | |
| Middle | 30 (39.0) | 61 (36.5) | |
| Lower | 42 (54.5) | 99 (59.3) |
*, values were expressed as mean ± SD; #, statistically significant difference. ASA, American Society of Anesthesiologists; BMI, body mass index; CM, conventional method; NM, novel method; SD, standard deviation.
Surgical outcomes and pathological characteristics
The perioperative outcomes and pathological findings are shown in Table 2. The median operation time was 225 minutes in the CM group and 240 minutes in the NM group. The median intraoperative blood loss was 100 mL in both groups. The percentage of patients who underwent ligation of the thoracic duct was significantly higher in the CM group (41.6%, 32/77) than that in the NM group (23.4%, 39/167, P=0.004). The median number of harvested LNs in total, from the abdomen, or from the left RLN was markedly higher in the NM group than that in the CM group (P=0.006 in total, P=0.038 from the abdomen, P=0.044 from the left RLN, Table 2). However, the median number of harvested LNs from the chest or right RLN was not significantly different between the two groups (P>0.05, Table 2). In addition, there were no significant differences between the two groups in terms of postoperative hospital stay, TNM stage, perineural invasion, or vascular invasion.
Table 2
| Outcomes | CM group (n=77) | NM group (n=167) | P value |
|---|---|---|---|
| Operative time (min)* | 225 (207.5, 280) | 240 (215, 265) | 0.638 |
| Intraoperative blood loss (mL)* | 100 (50, 100) | 100 (50, 100) | 0.773 |
| Ligation of thoracic duct, n (%) | 32 (41.6) | 39 (23.4) | 0.004# |
| Restore fluid diet time (d)* | 9 (8, 10.5) | 9 (9, 11) | 0.005# |
| Postoperative hospital stay (d)* | 11 (10, 14) | 11 (10, 13) | 0.252 |
| Total lymph node dissection (n)* | 27 (21.5, 36) | 32 (25, 41) | 0.006# |
| Chest lymph node dissection (n)* | 18 (13, 23.5) | 20 (14, 25) | 0.119 |
| Abdomen lymph node dissection (n)* | 9 (6.5, 14) | 11 (7, 16) | 0.038# |
| Lymph node dissection around right RLN (n)* | 3 (1.5, 5) | 3 (1, 5) | 0.988 |
| Lymph node dissection around left RLN (n)* | 2 (1, 4) | 3 (1, 6) | 0.044# |
| Pathological tumor category, n (%) | 0.119 | ||
| T1 | 23 (29.9) | 43 (25.7) | |
| T2 | 10 (13.0) | 41 (24.6) | |
| T3 | 44 (57.1) | 83 (49.7) | |
| Pathological node category, n (%) | 0.546 | ||
| N0 | 38 (49.4) | 75 (44.9) | |
| N1 | 19 (24.7) | 50 (29.9) | |
| N2 | 17 (22.1) | 30 (18.0) | |
| N3 | 3 (3.9) | 12 (7.2) | |
| TNM stage, n (%) | 0.548 | ||
| Stage I | 25 (32.5) | 45 (26.9) | |
| Stage II | 18 (23.4) | 35 (21.0) | |
| Stage III | 31 (40.3) | 74 (44.3) | |
| Stage IV | 3 (3.9) | 13 (7.8) | |
| Vascular invasion, n (%) | 11 (14.3) | 31 (18.6) | 0.411 |
| Perineural invasion, n (%) | 12 (15.6) | 27 (16.2) | 0.908 |
*, values were expressed as the median (interquartile range); #, statistically significant difference. RLN, recurrent laryngeal nerve; TNM, Tumor Node Metastasis; CM, conventional method; NM, novel method.
Postoperative complications
The postoperative complications of the two groups are presented in Table 3. There were no significant differences in postoperative complications between the groups except for the incidence of wound infection, which was substantially higher (9.1%, 7/77) in the CM group compared to the NM group (0.6%, 1/167, P=0.001, Table 3). The incidence of anastomotic leakage was 2.6% in the entire cohort, and it was slightly but not significantly higher in the CM group (5.2%, 4/77) compared to the NM group (1.2%, 2/167, P=0.061). All cases of anastomotic leakage were healed using non-surgical management. The incidence of hoarseness was not significantly different between the groups (2.6% vs. 1.8%, P=0.681), and all cases recovered within 6 months after surgery with conservative treatment.
Table 3
| Outcomes | CM group (n=77) | NM group (n=167) | P value |
|---|---|---|---|
| Anastomotic leakage | 4 (5.2) | 2 (1.2) | 0.061 |
| Hoarseness | 2 (2.6) | 3 (1.8) | 0.681 |
| Wound infection | 7 (9.1) | 1 (0.6) | 0.001# |
| Pleural effusion | 6 (7.8) | 16 (9.6) | 0.650 |
| Pneumonia | 3 (3.9) | 12 (7.2) | 0.320 |
| Chylothorax | 1 (1.3) | 1 (0.6) | 0.573 |
| Arrhythmia | 0 (0.0) | 4 (2.4) | 0.171 |
| Pneumothorax | 2 (2.6) | 2 (1.2) | 0.424 |
| ICU stay | 2 (2.6) | 10 (6.0) | 0.255 |
| Secondary surgery | 1 (1.3) | 2 (1.2) | 0.947 |
| Liver dysfunction | 2 (2.6) | 1 (0.6) | 0.188 |
| Acute renal dysfunction | 0 (0.0) | 2 (1.2) | 0.335 |
| Pulmonary embolism | 0 (0.0) | 1 (0.6) | 0.496 |
| Death | 0 (0.0) | 1 (0.6) | 0.496 |
Data are presented as n (%). #, statistically significant difference. ICU, intensive care unit; CM, conventional method; NM, novel method.
Perioperative death occurred in one of the NM group patients who suffered from severe pneumonia and died of respiratory failure 30 days after surgery (Table 3). Although the incidences of pleural effusion (9.6%, 16/167) and intensive care unit (ICU) stay (6.0%, 10/167) were slightly (but not significantly) higher in the NM group compared to that of the CM group (pleural effusion: 7.8%, 6/77, P=0.650; ICU stay: 2.6%, 2/77, P=0.255), the incidence of other comorbidities was very low (Table 3).
LN metastasis status
The incidence of LN metastasis in the two groups is shown in Table 4. In this study, it was found that 131 out of 244 (53.7%) patients with esophageal cancer experienced LN metastasis. Specifically, the incidence of total LN metastasis was 50.7% (39/77) in the CM group and 55.1% (92/167) in the NM group, which was not significantly different. Similarly, there was no marked difference between the two groups in terms of the incidence of left RLN LN metastasis [CM: 15.6% (12/77) vs. 13.2% (22/167)].
Table 4
| Group | Incidence of lymph node metastasis | |
|---|---|---|
| CM group | NM group | |
| Total | 50.7% (39/77) | 55.1% (92/167) |
| Left RLN | 15.6% (12/77) | 13.2% (22/167) |
RLN, recurrent laryngeal nerve; CM, conventional method; NM, novel method.
Discussion
Currently, neoadjuvant therapy combined with esophagectomy and the dissection of potentially metastatic LNs remains the essential treatment for esophageal cancer (7). Previous studies have demonstrated that compared to OE, MIE has the advantages of less surgical trauma, fewer complications, lower mortality, as well as similar oncological outcomes (5-9,13). Consequently, various MIEs have been widely applied and performed in most academic centers. The submucosa of the esophagus contains a rich network of longitudinal lymphatic vessels, and therefore, once esophageal cancer has involved the submucosal layer, “skipping metastasis” may occur to the upper mediastinal area far away from the original lesion, including along the bilateral RLN (17). Previous studies have reported metastasis rates of between 20% and 40% in the RLN LN, and even up to 20% in patients with lower esophageal squamous cell cancer (ESCC) (12,18). Therefore, LN dissection in esophagectomy is an essential procedure for radical resection, which can not only provide more accurate staging but may also improve survival (12).
However, complete dissection of LNs around RLN is technically challenging and may lead to RLN paralysis resulting from thermal injury, squeezing, stretching, and nourishing vessel injury. RLN paralysis increases the incidence of postoperative complications, which include (but are not limited to) hoarseness and coughing, aspiration pneumonia, and even acute respiratory distress syndrome, as well as the increased risk of anastomotic leakage and prolonged postoperative hospital stay (13-20). Despite the continuous technological development and equipment updates, the incidence of RLN paralysis is still high, ranging from 2.9% to 69%. In particular, RLN paralysis on the left is more likely to occur compared to the right (12-18). Therefore, we used a novel method that involved en bloc dissection of the LNs in this area with sufficient exposure and precise surgery.
The full exposure of the surgical area is the first priority in LN dissection to reduce complications (21). Thoracoscopy provides a larger surgical field of view, which is more vivid than the field of open surgery. The semi-prone position provides good exposure: not only can the lungs fall forward due to gravity, but also mediastinal tissues can be naturally exposed by the artificial pneumothorax. Furthermore, with the help of single-lumen endotracheal intubation and artificial pneumothorax, the tissue around the esophagus and the gap between the esophagus and trachea are easy to expose and dissect. More importantly, the left semi-prone position can be quickly converted to the left lateral position when emergency open conversion is necessary (12). In this study, by combining the left semi-prone position and esophageal suspension, the trachea was pushed forward by an aspirator with a cotton swab to expose the tracheoesophageal groove, and the LNs around the left RLN were dissected by a blunt dissection method, which reduced damage to the tissue.
There are three important anatomical structures in the narrow space of the upper mediastinum: the left RLN that should be completely protected; the LNs that should be completely removed; and the nourishing vessels of the RLN that should be retained. The above structures are mixed together in a narrow space, complicating LN resection along the left RLN. Compared with the right RLN, the left RLN has a longer course from the aortic arch to the neck, and it is more likely to be damaged during lymphadenectomy. Thus, it is necessary to understand the interrelationship of these three anatomical structures and their anatomical logic. Oshikiri et al. (15) proposed the Bascule method as an effective procedure to dissect the LNs along the RLN and introduced the concept of the “esophageal mesenteriolum”, which integrated the left RLN, the LNs, and the trachea-esophageal artery in the left upper mediastinum into a two-dimensional membrane. In our procedures, the visual exposure of the field was greatly improved with the suspension of the esophagus and the forward movement of the trachea, and complete lymphadenectomy in this space was performed. This novel approach provided better exposure and greater operating space, and thus, the median number of dissected LNs along the left RLN using this novel method was significantly improved compared to that dissected via the conventional method.
Previous studies have reported the high incidence of LN metastases around bilateral RLNs in thoracic esophageal squamous cell cancer (11,12,14,16,17). Niwa et al. (22) found that the rate of LN metastasis to bilateral RLN was 15.8% among 342 patients, Zhang et al. (16) demonstrated that the incidence of metastasis to the left RLN LN was 18.6% in the entire cohort of 194 patients, and Tan et al. (23) reported that the frequency of LN metastasis along the left RLN was 8.7%. In our study, the incidence of left RLN LN metastasis was 13.9%, which was similar to the results reported in the aforementioned studies. Moreover, the number of harvested LNs along the left RLN via the novel method in this study was markedly greater than that by the conventional method. Since not all patients with hoarseness underwent laryngoscopy, for the purpose of comparative research in this study, hoarseness was considered to be the main criterion for evaluating RLN palsy. The incidence of hoarseness in the NM group was 1.8%, which was lower than that in the CM group (2.6%); however, the difference was not statistically significant. In addition, all patients with hoarseness recovered within 6 months after surgery with conservative treatment. These results indicated that our novel method was superior to the conventional method in terms of increasing the number of LN dissections along the left RLN without increasing the risk of left RLN injury.
Although LNs could also be removed using the conventional method, the left RLN was not fully visible, and the nourishing vessels were easily damaged. In contrast, the novel method applied in this study has several advantages compared to the conventional method. Firstly, by suspending the esophagus and turning the trachea forward with an attractor sucking a cotton swab, the left tracheal esophagus sulcus was clearly exposed, allowing the surgeon to easily identify and isolate the left RLN so that the LNs were dissected completely and safely. Secondly, without increasing the risk of injury to the left RLN and other complications, including anastomotic leakage and pneumonia, our novel method increased the number of LN dissections along the left RLN. Finally, this method reduced the use of energy devices while stripping the LNs, alleviating the damage to nutrient vessels and the impact on the blood supply of the left RLN, avoiding excessive tension on the nerves, thereby limiting the damage to the left RLN.
This study inevitably had some limitations that should be noted. Firstly, this was a single-institutional and retrospective cohort study with significant differences in the number of patients between the two groups and the lack of matching. Secondly, laryngoscopy was not performed to evaluate the vocal cord mobility of all patients, and hoarseness was considered to be the main criterion for evaluating RLN palsy. Thirdly, hoarseness was assessed by auditory impression, which was prone to subjective bias that could be overcome by applying voice analysis in a future study.
Conclusions
In conclusion, the results of this study showed that the novel method applied not only increased the number of LN dissections along the left RLN but also decreased the incidence of hoarseness, suggesting that our novel method for LN dissection along the left RLN in patients in the semi-prone position during McKeown MIE is safe and reliable.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1273/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1273/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1273/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The research protocol of this clinical study was approved by the Ethics Committee of the Sir Run Run Shaw Hospital (No. 20220314-33). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018;154:360-73. [Crossref] [PubMed]
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Pineros M. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer 2018. Available online: https://gco.iarc.fr/today, accessed [16 Feb 2019].
- Chen R, Zheng RS, Zhang SW, et al. Analysis of incidence and mortality of esophageal cancer in China, 2015. Zhonghua Yu Fang Yi Xue Za Zhi 2019;53:1094-7. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Yoshida N, Yamamoto H, Baba H, et al. Can Minimally Invasive Esophagectomy Replace Open Esophagectomy for Esophageal Cancer? Latest Analysis of 24,233 Esophagectomies From the Japanese National Clinical Database. Ann Surg 2020;272:118-24. [Crossref] [PubMed]
- Gottlieb-Vedi E, Kauppila JH, Malietzis G, et al. Long-term Survival in Esophageal Cancer After Minimally Invasive Compared to Open Esophagectomy: A Systematic Review and Meta-analysis. Ann Surg 2019;270:1005-17. [Crossref] [PubMed]
- Liu B, Li X, Yu MJ, et al. Utility of single-port laparoscopic retrograde gastric mobilization during McKeown esophagectomy for esophageal cancer: a 2-year experience with 120 cases in a single institution. J Thorac Dis 2022;14:3983-91. [Crossref] [PubMed]
- Chen Y, Xie Y, Zhang H, et al. Modified McKeown vs. traditional McKeown minimally invasive esophagectomy in improving short-term efficacy and the quality of life of esophageal cancers: a retrospective comparative cohort study. J Gastrointest Oncol 2022;13:1579-88. [Crossref] [PubMed]
- Leng X, He W, Yang H, et al. Prognostic Impact of Postoperative Lymph Node Metastases After Neoadjuvant Chemoradiotherapy for Locally Advanced Squamous Cell Carcinoma of Esophagus: From the Results of NEOCRTEC5010, a Randomized Multicenter Study. Ann Surg 2021;274:e1022-9. [Crossref] [PubMed]
- Akutsu Y, Kato K, Igaki H, et al. The Prevalence of Overall and Initial Lymph Node Metastases in Clinical T1N0 Thoracic Esophageal Cancer: From the Results of JCOG0502, a Prospective Multicenter Study. Ann Surg 2016;264:1009-15. [Crossref] [PubMed]
- Wang Z, Mao Y, Gao S, et al. Lymph node dissection and recurrent laryngeal nerve protection in minimally invasive esophagectomy. Ann N Y Acad Sci 2020;1481:20-9. [Crossref] [PubMed]
- Zhu ZY, Luo RJ, He ZF, et al. Learning Curve for Lymph Node Dissection Around the Recurrent Laryngeal Nerve in McKeown Minimally Invasive Esophagectomy. Front Oncol 2021;11:654674. [Crossref] [PubMed]
- Noshiro H, Iwasaki H, Kobayashi K, et al. Lymphadenectomy along the left recurrent laryngeal nerve by a minimally invasive esophagectomy in the prone position for thoracic esophageal cancer. Surg Endosc 2010;24:2965-73. [Crossref] [PubMed]
- Oshikiri T, Yasuda T, Harada H, et al. A new method (the "Bascule method") for lymphadenectomy along the left recurrent laryngeal nerve during prone esophagectomy for esophageal cancer. Surg Endosc 2015;29:2442-50. [Crossref] [PubMed]
- Zhang S, Zhang P, Guo S, et al. Comparative study of three types of lymphadenectomy along the left recurrent laryngeal nerve by minimally invasive esophagectomy. Thorac Cancer 2020;11:224-31. [Crossref] [PubMed]
- Tachimori Y. Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: efficacy of lymph node dissection according to tumor location. J Thorac Dis 2017;9:S724-30. [Crossref] [PubMed]
- Kumakura Y, Yokobori T, Yoshida T, et al. Elucidation of the Anatomical Mechanism of Nodal Skip Metastasis in Superficial Thoracic Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2018;25:1221-8. [Crossref] [PubMed]
- Jha S, D’hamija N, Kumar A, Rawat S. Robotic-assisted esophagectomy: A literature review and our experience at tertiary care centre. Laparosc Endosc Robot Surg 2020;3:74-9.
- Oshikiri T, Takiguchi G, Urakawa N, et al. Novel "Modified Bascule Method" for Lymphadenectomy Along the Left Recurrent Laryngeal Nerve During Robot-Assisted Minimally Invasive Esophagectomy. Ann Surg Oncol 2021;28:4918-27. [Crossref] [PubMed]
- Wong IYH, Zhang RQ, Tsang RKY, et al. Improving Outcome of Superior Mediastinal Lymph Node Dissection During Esophagectomy: A Novel Approach Combining Continuous and Intermittent Recurrent Laryngeal Nerve Monitoring. Ann Surg 2021;274:736-42. [Crossref] [PubMed]
- Niwa Y, Koike M, Hattori M, et al. The Prognostic Relevance of Subcarinal Lymph Node Dissection in Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2016;23:611-8. [Crossref] [PubMed]
- Tan Z, Ma G, Zhao J, et al. Impact of thoracic recurrent laryngeal node dissection: 508 patients with tri-incisional esophagectomy. J Gastrointest Surg 2014;18:187-93. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


