
Imaging findings of hepatocellular carcinoma with portal vein tumor thrombosis secondary to hepatic portal vein collateral circulation: a cross-sectional study
Highlight box
Key findings
• Common hepatic collateral branches in patients with PVTT mainly include the biliary collateral branch, gastric collateral branch, mesenteric collateral branch, and accessory portal vein system.
What is known and what is new?
• MSCT image can show the collateral branch of portal vein.
• The biliary collateral branch is mostly found in patients with obstruction of the right portal branch and main trunk. A gastric collateral branch mostly occurs in patients with obstruction of the left portal vein and main trunk. A mesenteric collateral branch mostly occurs in patients with PVTT extending to the SMV. An accessory portal vein system mostly occurs in patients with obstruction of the portal vein trunk.
What is the implication, and what should change now?
• This study provides a basis for determining when TACE should be applied in patients with PVTT, and the indications of TACE in these patients should be changed accordingly.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world, the fourth leading cause of cancer-related death in the world. Hepatocellular carcinoma (HCC) accounts for 90% of liver cancers (1,2). According to the latest data, among all the malignant tumors reported in China, the incidence and mortality of liver cancer ranked fourth and third, respectively (1,2). Because of the biological characteristics of liver cancer and the anatomical characteristics of liver, HCC can easily invade the blood vessels in the liver, especially the portal vein system. The incidence of portal vein tumor thrombosis (PVTT) is 44–62.2% (3). Once PVTT occurs, it often progresses rapidly and can cause portal hypertension, hepatocellular jaundice, intractable ascites and portal vein thrombosis, The treatment of portal thrombosis is very difficult, which can seriously affect the prognosis of the patients (4). The median survival time of patients with HCC and PVTT is reported to be only 2.7 months (3). A recent study suggested that magnetic resonance imaging (MRI)-guided tumor tracking hypofractionated radiotherapy (HFRT) and stereotactic body radiotherapy (SBRT) are feasible, effective, and safe treatment options in patients with HCC with tumor thrombosis in the main trunk or first branch of the portal vein (5), and an accurate diagnosis of PVTT can be made by radiomics (6). The presence of PVTT plays an important role in the prognosis and clinical staging of HCC. According to the Chinese Society of Clinical Oncology (CSCO) Guidelines for Diagnosis and Treatment of HCC (2022 edition), transarterial chemoembolization (TACE) can also be used for patients with type Ⅲa liver cancer, incomplete obstruction of the main portal vein, or formation of compensatory collateral vessels between the hepatic artery and portal vein despite complete obstruction. Therefore, a correct understanding of the formation of collateral vessels of the hepatic portal vein is of great significance for deciding the interventional treatment plan of HCC.
When the PVTT completely blocks the main portal vein or its branches, most PVTT patients have portal vein collateral circulation. The appearance of hepatic collateral circulation plays an important role in maintaining the perfusion of the hepatic portal vein. However, at present, there is little research on collateral circulation of the hepatic portal vein.
This study aimed to examine the imaging manifestations, classification, and incidence of collateral circulation of the hepatic portal vein through multiphase enhanced compute tomography (CT) and axial images, coronal multiplanar reconstruction (MPR) images, and maximum intensity projection (MIP) images. To provide scientific basis for TACE or not of HCC with PVTT, but this study only studied the collateral circulation of portal vein, and did not further study the intrahepatic perfusion of these collateral vessels. Future research can be carried out according to this aspect. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-45/rc).
Methods
Study design
Our database was retrospectively established and prospectively maintained. The study was approved by the Human Investigation Committee of The Fourth Hospital of Hebei Medical University(2021KY170). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent for the use of their data and images.
Setting
This study retrospectively analyzed 431 cases of Hepatocellular carcinoma (HCC) with PVTT diagnosed with enhanced CT examination of the upper abdomen in our hospital from May 2020 to October 2021.
Participants
The inclusion criteria for patients were the following: (I) ultimately diagnosed with HCC, (II) accompanied by complete obstruction of the main portal vein or left/right branches, and (III) with collateral circulation of the hepatic portal vein established.
The exclusion criteria were the following: (I) a final diagnosis other than malignant tumor of the liver, (II) PVTT without complete blockage of the main portal vein or the left/right branches, and (III) without collateral circulation of the hepatic portal vein established.
A total of 71 patients were eligible for enrollment. There were 63 males and 8 females in the study group. The patients ranged in age from 33 to 78 years, with an average age of 59.2 (SD 8.3) years. All cases were diagnosed in clinic with an alpha-fetoprotein (AFP) test, imaging examinations (CT, ultrasound, MRI), and other means, and met the diagnostic criteria of HCC. The tumor occurred in the right lobe for 40 patients, in the left lobe for 9 patients, and in both lobes for 22 patients.
Variables
The grouping of this study was based on an improved classification of PVTT with the following parameters: type 0, presence of tumor thrombus formation under microscopy; type I: tumor thrombosis involving the portal vein branches of grade 2 and above; type II, tumor thrombus involving the first grade portal vein branch; type III, tumor thrombus involving the main portal vein; and type IV, tumor thrombosis involving the superior mesenteric vein (SMV) or inferior vena cava. In this study, the invasion of the portal vein branch by the cancer thrombosis in the PVTT classification was updated to include a completely blocked portal vein branch because the complete blockage of the portal vein trunk or branch is a necessary condition for the establishment of hepatic collateral circulation. Subsequently, all patients with PVTT were divided into the following 4 groups according to the position where the tumor thrombosis completely blocked the portal vein: group IIa, patients whose tumor thrombosis only invaded the left portal vein and completely blocked it; group IIb, patients whose tumor thrombosis only invaded the right branch of the portal vein and completely blocked it; group Ⅲ, patients with tumor thrombosis invading the main portal vein and completely blocking it; and group Ⅳ, patients with cancer thrombosis extending along the portal vein to the splenic vein or SMV and completely blocking one of them.
Measurement
All patients were examined by multislice spiral CT with enhanced scanning of the upper abdomen. Patients were required to fast for 4–6 hours before the examination and drink 1,000 mL of drinking water 15 minutes before the examination to fill the gastrointestinal tract. The informed consent form for administration of the contrast agent was signed by the family members. Before the examination, the technician instructed the patient to breathe 2–3 times.
The scanning range was from the top of the diaphragm to the lower edge of the liver. The specific scanning parameters are as follows: tube voltage 120 kV, automatic tube current, a rotation time of 0.5 s/r, a layer thickness and layer spacing of 5 mm, a pitch of 0.9, a detector collimation of 128×0.6 mm, and a matrix of 512×512. Image reconstruction proceeded as follows: After scanning, thin-layer reconstruction of the B10 soft tissue algorithm was performed on the original data. The thickness of the reconstructed layer was 1 mm, and the interval between the layers was 1 mm. Patients underwent an abdominal enhancement scan after the routine plain scan. Intravenous injection of iohexol (300 mgI/mL) was carried out from the median elbow vein via a high-pressure syringe, with an injection dose of 1.5 mL/kg and an injection rate of 3 mL/s. Arterial phase scanning was performed 35 seconds after the contrast agent injection, and venous phase scanning was performed 70 seconds after the contrast agent injection. The axial reconstruction images of the portal vein of patients were transmitted to the workstation. First, the central position of the tumor and the position where the portal vein tumor thrombus had invaded and completely blocked all branches of the portal vein were observed, and the tumor thrombus classification of each patient was recorded according to the modified PVTT classification standard. Then, image postprocessing was conducted, and the reconstruction methods adopted MPR and MIP. After image reconstruction, the collateral vessels of the hepatic portal vein from various veins directly communicating with portal vein [such as the gallbladder vein, left gastric vein (LGV), right gastric vein (RGV), SMV, pancreaticoduodenal vein, splenic vein] were observed on the coronal plane of the reconstructed image. The reconstructed images were interpreted by 3 senior radiologists with experience in liver CT diagnosis. Each doctor diagnosed the CT image independently, and then the group discussed and decided upon the final imaging diagnosis. In this way, the diagnostic accuracy concerning the collateral vessels of the hepatic portal vein in all directions of CT was ensured.
Bias considerations
All patients enrolled in this study had a history of hepatitis B-related cirrhosis. Patients with a history of cirrhosis tend to have obvious portal hypertension, which might have certain impacts on this study.
Study size
This study is a cross-sectional study. Based on the imaging database of the research team, 71 cases eligible for inclusion were extracted. Their age, gender, and cancer stage distribution are consistent with the demographic characteristics of HCC.
Qualitative variables were used in this study, so there was no separation of quantitative variables.
Statistical methods
The data were analyzed using SPSS version 22.0 for Windows (SPSS Inc., Chicago, IL, USA). Normally distributed continuous variables were expressed as mean ± standard deviation. For non-normally distributed variables, median and interquartile ranges (IQR) were used, as appropriate. Categorical data were summarized as frequencies and percentages. The Chi-squared test, for categorical variables, was used to examine differences between the groups. Statistical significance was set as a two-sided P value <0.05.
Results
Participants
This study retrospectively analyzed 431 cases of primary liver cancer (HCC) with PVTT diagnosed with enhanced CT examination of the upper abdomen in our hospital from May 2020 to October 2021. In all, 95 cases conformed to the inclusion criteria, but 24 of these were excluded due the inability to identify the collateral circulation vessels from the MPR and MIP images. The study flowchart patient enrollment is shown in Figure 1. A total of 71 patients were ultimately eligible for the study, and their characteristics are summarized in Table 1.
Table 1
| Characteristic | Data |
|---|---|
| Gender | |
| Male | 63 (88.7) |
| Female | 8 (11.3) |
| Age, years | |
| Range | 33–78 |
| Average age (mean ± SD) | 59.2±8.3 |
| Tumor location | |
| Left lobe | 9 (12.7) |
| Right lobe | 40 (56.3) |
| All | 22 (31.0) |
| HBV-DNA | |
| ≥1,000 | 55 (77.5) |
| <1,000 | 16 (22.5) |
| AFP | |
| ≥400 ng/mL | 42 (59.1) |
| <400 ng/mL | 29 (40.9) |
| Group | |
| IIa | 16 (22.5) |
| IIb | 9 (12.7) |
| III | 27 (38.0) |
| IV | 9 (26.8) |
AFP, alpha-fetoprotein; HBV-DNA, hepatitis B virus-DNA.
Outcome data
Imaging manifestations of PVTT formation
Direct signs for PVTT formation
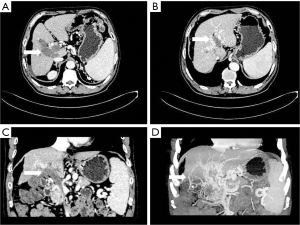
The boundary between the hepatocellular carcinoma focus and the adjacent intrahepatic portal vein branches was blurred, and the distance between the tumor focus and the portal vein was usually less than 2 cm. The portal vein in the affected segment was dilated or enlarged, and the density in the cavity was changed, with a filling defect being apparent (see Figure 2). There was typically localized low density of the hepatic segment or hepatic lobe portal phase, but the well-established collateral circulation to the hepatic portal vein sometimes did not show low density changes.
Indirect signs and accompanying manifestations for PVTT
Indirect signs and accompanying manifestations for PVTT included compensatory dilatation and thickening of the hepatic artery, spleen enlargement, ascites formation, and the formation of collateral circulation (see Table 2).
Table 2
| Variables | Number | Percentage (%) |
|---|---|---|
| Ascites | ||
| Yes | 35 | 49 |
| No | 36 | 51 |
| Splenomegaly | ||
| Yes | 54 | 76 |
| No | 17 | 24 |
| Esophagogastric varices | ||
| Yes | 53 | 75 |
| No | 18 | 25 |
| Sponge degeneration of the portal vein | ||
| Yes | 31 | 44 |
| No | 40 | 56 |
The manifestations of collateral circulation to hepatic portal vein
This study found that the collateral circulation to hepatic portal vein could be divided into 5 types, which are described in the following sections.
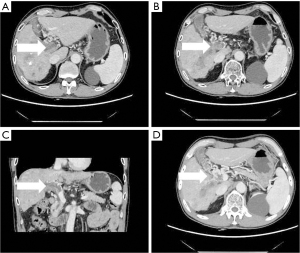
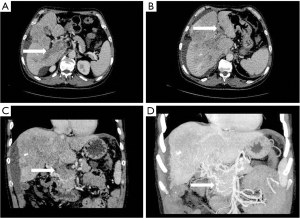
- Collateral branch of the hepatic portal vein, including collateral branch of hepatic portal vein from the cystic vein and paracholedochal venous plexus (PCVP). Collateral circulation of the hepatic portal vein from the cystic vein (CV) appeared on MIP image as 1 or 2 tortuous collateral vessels around the gallbladder wall, which passed through the hepatic capsule near the gallbladder fossa and directly entered the liver, connecting some branches of the right portal vein in the right lobe of the liver (see Figure 2). On MIP images, the hepatic collateral branches from the PCVP appeared to ascend to the hilum along the tortuous vascular plexus around the extrahepatic bile duct and then entered the liver from the hilum (see Figure 3).
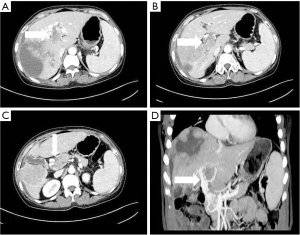
- Stomach branch of hepatic portal vein, including the lateral branch of hepatic portal vein emanating from LGV and RGV. On MIP images (see Figure 4), the lateral branch of hepatic portal vein from the LGV appeared as a branch of the hepatic vein extending directly from the gastric coronary vein on the side of the small gastric curvature, which passed through the hepatic capsule near the second segment of the left hepatic lobe and directly entered the liver, connecting some branches of the left portal vein of the left hepatic lobe. The hepatic collateral branch from the RGV appeared on MIP images as a hepatic venous vessel directly emanating from the RGV on the side of the small gastric curve, directly passing through the hepatic capsule and entering the liver near the segment IV of liver (left lobe of the liver) and connecting some branches of the left portal vein of the left lobe of the liver.
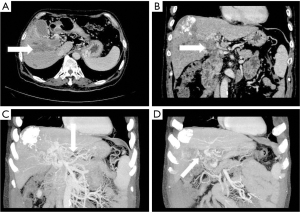
- Lateral mesenteric branch of the hepatic portal vein. On MIP images, the collateral branch of hepatic portal vein directly emanated from the SMV, which appeared as a tortuous vein shadow directly emanating from the main trunk of SMV, directly passing through the hepatic capsule into the left lobe or right lobe of the liver and connecting with the small branch of the portal vein (see Figure 5). Occasionally, the mesenteric branch of hepatic portal vein originated from the pancreaticoduodenal vein, passed through the hepatic capsule near the dorsal part of hepatic segment IV of the left lobe of liver, and directly entered the liver, connecting some branches of the left portal vein of the left lobe of liver.
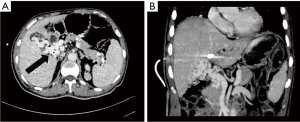
- Accessory portal vein system. The accessory portal vein system is the collateral vessel to the liver from the main portal vein itself. On MIP images, it appeared as a collateral vessel emanating from the main portal vein, bypassing the PVTT obstruction, and entering the hepatic portal collateral of the left or right lobe of the liver, often occurring in the area around the hilus (see Figure 6).
- Other rare hepatic portal collateral vessels. In this study, only 1 case was found of the collateral branch of the hepatic portal vein directly emanating from the splenic vein. On MIP images, it appeared as a tortuous vein shadow directly emanating from the splenic vein, which entered the liver in the area around the portal vein.
Principal results
In this study, a total of 71 patients with PVTT were enrolled. A total of 125 collateral hepatic portal vein vessels were observed in MPR and MIP reconstruction images. Among them, the branch of the biliary tract to hepatic portal vein was the most common, with a total of 55 cases, accounting for 77.5% of all the enrolled patients, and accounting for 44% of all the blood vessels with collateral circulation of the hepatic portal vein. The gastric branch collateral to the hepatic portal vein was the second most common vessel, with a total of 26 cases, accounting for 36.6% of all the enrolled patients and 20.8% of all the vessels collateral to the hepatic portal vein. There were 23 cases of the mesenteric branch collateral to the hepatic portal vein, accounting for 32.4% of all enrolled patients, and accounting for 18.4% of all vessels collateral to the hepatic portal vein. There were 20 cases of the accessory portal vein system collateral to the hepatic portal vein, accounting for 28.2% of all the enrolled patients and 16% of all the collateral hepatic portal vessels found. Finally, the incidence of the collateral circulation vessels of the hepatic portal vein was extremely low, with a total of 1 case, the incidence of which was 1.41%, accounting for 0.8% of all the collateral vessels of the hepatic portal vein (see Table 3).
Table 3
| Lateral branch of the hepatic portal vein | Occurrence, n | Percentage (%) | Incidence (%) |
|---|---|---|---|
| Collateral biliary tract | 55 | 44.0 | 77.5 |
| Stomach branch | 26 | 20.8 | 36.6 |
| Collateral mesentery | 23 | 18.4 | 32.4 |
| Accessory portal vein system | 20 | 16.0 | 28.2 |
| Other | 1 | 0.8 | 1.41 |
| Total | 125 | 100 |
A total of 71 patients were enrolled in this study. The incidence rate of hepatic portal collateral vessels of various types is the number of patients with this collateral vessel divided by the total number of patients enrolled.
Among all the enrolled patients with PVTT, the incidence and proportion of tumor thrombosis in the vessels with collateral circulation from the hepatic portal vein for types Ⅱa, Ⅱb, Ⅲ, and Ⅳ are shown in Table 4.
Table 4
| Type | Classification of the hepatic portal vein collateral | ||||
|---|---|---|---|---|---|
| Biliary branch | Gastric branch | Lateral branch of the mesentery | Accessory portal vein system | Other | |
| Type IIa | 7 (12.7%) | 7 (26.9%) | 1 (4.3%) | 3 (15%) | 0 (0%) |
| Type IIb | 16 (29%) | 0 (0%) | 3 (13.0%) | 5 (25%) | 0 (0%) |
| Type III | 23 (41.8%) | 15 (57.7%) | 16 (69.6%) | 8 (40%) | 1 (100%) |
| Type IV | 9 (16.4%) | 4 (15.4%) | 3 (13.0%) | 4 (20%) | 0 (0%) |
| Total | 55 (100%) | 26 (100%) | 23 (100%) | 20 (100%) | 1 (100%) |
Other analyses
There was statistical significance between the incidence of biliary collateral branches and gastric branches of the hepatic portal vein and the different positions of portal vein branches completely blocked by PVTT (P<0.05). There was no statistical significance between the incidence of hepatic collateral circulation originating from mesenteric vein and the accessory portal vein system and the different positions where portal vein branches were completely blocked by PVTT (P>0.05; Table 5). The incidence of cavernous transformation of the portal vein was statistically different among tumor thrombus types Ⅱa, Ⅱb, Ⅲ, and Ⅳ (P<0.05; Table 6 and Figure 7).
Table 5
| Lateral branch of the hepatic portal vein | IIa | IIb | III | IV | χ2 | P |
|---|---|---|---|---|---|---|
| Biliary branch | 15.744 | 0.001 | ||||
| Yes | 7 | 16 | 23 | 9 | ||
| No | 9 | 3 | 4 | 0 | ||
| Gastric branch | 13.763 | 0.003 | ||||
| Yes | 7 | 0 | 15 | 4 | ||
| No | 9 | 19 | 12 | 5 | ||
| Lateral branch of the mesentery | 4.429 | 0.202 | ||||
| Yes | 1 | 3 | 16 | 3 | ||
| No | 15 | 16 | 11 | 6 | ||
| Accessory portal vein system | 3.877 | 0.275 | ||||
| Yes | 3 | 5 | 8 | 4 | ||
| No | 13 | 14 | 19 | 5 | ||
| Other* | ||||||
| Yes | 0 | 0 | 1 | 0 | ||
*, There was only 1 case of other rare hepatic collateral portal vein in this study, so no statistical analysis was made.
Table 6
| Classification of tumor thrombosis | χ2 | P | ||||
|---|---|---|---|---|---|---|
| IIa | IIb | III | IV | |||
| Cavernous degeneration of the portal vein | 24.604 | 0.000 | ||||
| Yes | 2 | 3 | 19 | 7 | ||
| No | 14 | 16 | 8 | 2 | ||
Discussion
Multislice CT portal observation of the collateral circulation to hepatic portal vein
Multislice CT portal has the advantages of fast scanning speed, high spatial resolution, and high density resolution. However, conventional axial CT is not conducive to showing the beginning, end, and shape of the collateral branch of the hepatic portal vein. Among its powerful postprocessing functions, CT vascular reconstruction of the portal vein is mainly based on coronal or sagittal MIP, and can be supplemented with volume rendering (VR) images of various angles and thicknesses. The image can be rotated from multiple angles, which can clearly show the invasion of the portal vein by the tumor thrombus in the main portal vein and the adjacent tumors, and can intuitively outline the collateral vessels of the portal vein, showing good anatomical display of the intrahepatic and extrahepatic branches of the portal vein. Moreover, the 3-dimensional reconstruction technology is helpful for showing the continuous tracking and adjacent relationship of the start and end of the blood vessels, especially for the imaging display and diagnosis of the portal vein collateral vessels secondary to the portal vein tumor thrombosis.
Multi-slice spiral CT portal vein and the collateral image postprocessing technology
MPR can display any section of the scanned image, which can significantly reduce background interference and overlapping shadows of the surrounding blood vessels. However, the 3-dimensional sense of space is not strong. MIP can reconstruct a 2-dimensional image by performing the highest density mathematical beam perspective projection on the observed CT scanning volume or a certain thickness plane, which can clearly show the branches of the portal vein and the collateral circulation vessels established around the portal vein. Its disadvantages are heavy background interference and many overlapping shadows of the blood vessels around the portal vein. VR makes use of all relevant data of the image, and can better reflect the spatial relationship between the portal vein and its collateral vessels by adjusting the transparent threshold and different false colors. However, due to the enhancement of organs around the portal vein (such as the liver and spleen) in the portal phase and when the portal vein main trunk and its branches are completely blocked by portal vein tumor thrombus, the perfusion of the portal vein contrast agent is poor, which reduces the edge sharpness of the blood vessels and the image quality of the VR blood vessels. As we known, that an MIP image is superior to a VR image in displaying the hepatic artery, portal vein, and its collateral circulation. The VR image is superior to the MIP image in displaying normal portal vein branches. In this study, MPR and MIP were generally used for image reconstruction, and VR postprocessing technology was used as necessary.
Imaging manifestations of PVTT
Clinically, portal vein tumor thrombus can be divided into two types, one is microscopic tumor thrombus and the other is macroscopic tumor thrombus. Microscopic tumor thrombus refers to the tumor thrombus that can only be observed under a microscope or pathological examination and usually involves only the branches of portal vein above grade 3. Macroscopic tumor thrombus, which was the main focus of this study, refers to the tumor thrombus that can be observed in ultrasound, CT, MRI, and other imaging examinations and operations. It usually occurs in the main portal vein and the grade 2 or 3 branches. Imaging signs of hepatocellular carcinoma involving portal vein include the following: (I) Direct signs: The boundary between hepatocellular carcinoma focus and adjacent intrahepatic portal vein branches is blurred, the distance between the location of hepatocellular carcinoma and portal vein is usually less than 2 cm, the portal vein in the affected segment is dilated or enlarged, the density in the cavity changes, and a filling defect appears (7-9). There is no low density change in those who have localized low density in the hepatic segment or portal phase and have good circulation to the hepatic collateral. (II) Indirect signs and accompanying manifestations: The indirect signs and manifestations include compensatory thickening of the hepatic artery, spleen enlargement, ascites formation, and the formation of collateral. It is worth noting that the early tumor thrombus enhancement phenomenon and the small linear or striped tumor blood vessels in the tumor thrombus are found in the arterial phase images, which is an important basis for distinguishing PVTT from PVT. Sometimes it is difficult to judge the specific degree and location of the portal vein invasion by the cancer thrombus only using conventional axial images. MPR-reconstructed images can more comprehensively observe the position of the tumor thrombus blocking the portal vein from 3 angles—sagittal, coronal, and axial—and its image can also be reconstructed from multiple angles. It can clearly show the invasion of the portal vein by the tumor thrombus in the main branch of the portal vein and adjacent tumors at the best angle, and the obtained diagnostic information is better than that of conventional axial CT images in the portal phase.
The manifestations and significance of PVTT patients’ collateral circulation to the hepatic portal vein
Collateral branch of the biliary tract opening to the hepatic portal vein
According to the relevant research (10,11), the biliary collateral branches of the portal vein are the preset venous channels in the gallbladder bed. Under normal circumstances, they are usually very small. However, once the portal vein is completely blocked, they will be used as drainage veins for congestion and expansion, forming a portal blood flow that compensates for the blockage of the hepatic collateral pathways. The biliary collateral branch of the hepatic portal vein is mainly composed of the CV and PCVP. In this study, it was found that the CV of patients with portal vein tumor thrombus appeared as tortuous and expanded enhanced blood vessel along the gallbladder wall during the portal vein enhancement phase, extending from the right branch of the portal vein along the cystic duct to the gallbladder, and the gallbladder wall was obviously depressed and enhanced. The hepatic collateral branches from the CV appeared on CT images as 1 or 2 tortuous and dilated collateral vessels around the gallbladder wall, which passed through the hepatic capsule near the gallbladder fossa and directly entered the liver, connecting some branches of the right portal vein of the right lobe of the liver (Figure 1). The venous plexus beside the common bile duct drains the veins of the common bile duct (CBD). It is arranged in 2 clusters, one located around the extrahepatic bile duct and the other parallel to the common bile duct. The branch of the portal vein in caudate lobe or segment iv communicates with the CV. This venous plexus is connected with the posterior superior pancreaticoduodenal vein, the gastrocolic trunk (GCT), and the RGV. The SMV is located below it, and the branch of the intrahepatic portal vein is located above it (11). These venous plexi around the common bile duct constitute the collateral branch of the hepatic portal vein, which expands when the portal vein is completely blocked and moves upward along the tortuous vascular plexus around the extrahepatic bile duct to the hilum, where it enters the liver (Figure 2). As a recent Doppler ultrasound study by De Gaetano et al. (12) showed, the collateral biliary tract of portal vein, as a common hepatic portal pathway, transports the blood flow of abdominal vein to the liver, which is consistent with the collateral biliary tract of hepatic portal vein observed under enhanced CT in this study. In this study, a total of 55 patients were found to have collateral circulation in the branch of the hepatic portal bile duct, with an incidence of 77.5%, accounting for 44% of all the vessels found. It is the most frequent collateral branch of the hepatic portal vein among patients with PVTT. According to the classification of tumor thrombus types Ⅱa, Ⅱb, Ⅲ, and Ⅳ, the incidence of collateral circulation of the hepatic portal bile duct in each group was 7 cases, 16 cases, 23 cases, and 9 cases respectively, and the incidence in the 55 patients with established hepatic portal bile duct collateral circulation was 43.8%, 84.2%, 85.2%, and 100%, respectively. There was a statistical significance in the opening rate of the collateral branch of biliary tract to the hepatic portal vein among the different tumor thrombus groups (P<0.05). The data of this group indicate that in all the types of PVTT, except type IIA (complete obstruction of tumor thrombus in the left portal vein), the incidence of the collateral circulation for the branch of the biliary tract from the hepatic portal vein was extremely high. It can be seen that the incidence of collateral biliary branches from the hepatic portal vein is related to the complete obstruction of right branch and/or trunk of the portal vein. When the right branch and/or the main branch of the portal vein is completely blocked, the lateral branch of the hepatic portal vein is more likely to open.
Gastric branch of hepatic portal vein
In this study, there were 26 patients with portal vein tumor thrombus establishing hepatic portal vein gastric branch, the incidence rate was 36.6%, which was the second most frequent among all hepatic portal vein collateral branches. The gastric branches of hepatic portal vein mainly originate from the LGV and the RGV.
Left gastric vein
The LGV is also known as the gastric coronary vein. Under normal circumstances, the LGV drains from both gastric surfaces, rises to the left along the small gastric curve, and connects with the esophageal vein at the esophageal opening. It descends, merges with several short gastric veins, and then connects with the portal vein, splenic vein, or the confluence of both at the right rear of the omental sac (13). In this study, it was found that when the tumor thrombus caused the portal vein branch to be completely blocked, an abnormal collateral vessel extending along the hepatogastric ligament to the left side of the portal vein and directly flowing into the left portal vein would emanate from the LGV (Figure 3). The lateral branch of the LGV expands and receives the blood flow from the spleen and stomach to the left branch of the portal vein, which acts as the hepatic blood supply branch to compensate for the portal vein. Contrast-enhanced CT often shows the relatively enhanced area at the early stage of the posterior edge of the left lobe II and III of the liver because the contrast medium flows into the liver from the collateral branch established by the LGV in the early stage compared with the drainage of the SMV back to the liver (14). In our study, among the tumor thrombus types Ⅱa, Ⅱb, Ⅲ, and Ⅳ, the opening rate to the collateral hepatic portal vein was statistically significant (P<0.05). There were 7 cases, 0 cases, 15 cases, and 4 cases of hepatic portal vein opening to the gastric branch, and the incidence rates were 43.8%, 0%, 55.6%, and 44.4% respectively. Therefore, when the tumor thrombus invaded and completely blocked the left portal vein, the main portal vein extended to the SMV and splenic vein, with the incidence of a gastric branch collateral to the hepatic portal vein was about 50%. However, when the left portal vein remained unobstructed, the establishment of hepatic portal vein gastric branch was very rare (Ⅱb is a tumor thrombus in the right portal vein only, which is completely blocked, and the opening rate to hepatic portal vein gastric branch was 0% in this study).
Right gastric vein
The RGV is also known as pyloric vein. As a continuation of the LGV, under normal circumstances, the RGV extends to the right along the pyloric part of the small gastric curvature and terminates at the main portal vein. The opening of the RGV (into the portal vein is usually 1–2 cm lower than the opening of the left LGV) Before it flows into the portal vein, it is connected by the preduodenal pyloric vein (PPV) and the pyloric duodenal vein (PDV), PPV rises in front of the pylorus, and the PDV sends out some branches to the PCVP (15). In this study, it was found that when the main or branch of portal vein was completely blocked by PVTT, the hepatic portal collateral branch established by the RGV directly crossed the hepatic capsule and connected with the branch of the left portal vein, which drained the gastric venous blood into the liver. Contrast-enhanced CT often shows that a relatively high enhancement area is formed at the posterior edge of liver IV segment, which is usually wedge-shaped, and the enhancement mechanism is the same as the abnormal perfusion of LGV drainage (14). The pyloric vein can also provide blood flow to the hepatic portal vein for the PCVP when the tumor thrombus completely blocks the portal vein and can compensate for the lost blood flow of the portal vein.
Collateral mesenteric branch of the hepatic portal vein
The mesenteric vein is composed of the SMV and the inferior mesenteric vein (IMV). The SMV runs in the small mesentery, collects venous blood from the duodenum to the intestine above the left curvature of the colon that is part of the stomach and pancreas, and merges with the splenic vein at the back of the neck of the pancreas to form the portal vein. The IMV is a branch of the splenic vein, which collects venous blood from the area where the arteries of the spleen are distributed, including the left colic vein, sigmoid colon vein, and the inferior rectal vein. It goes up the left side of duodenojejunostomy and Treitz ligament, and enters the splenic vein behind the pancreatic head, sometimes into the SMV. When the PVTT extends from the main portal vein to the SMV or splenic vein and completely blocks them, the established hepatic collateral circulation preferentially bypasses the blocked site and flows back to the liver, instead of directly forming an abdominal portosystemic shunt (16). In this study, it was found that when the tumor thrombus caused the portal vein to be completely blocked, the SMV would emit an abnormal collateral vessel extending along the mesentery to the right side of the portal vein and directly flowing into the right branch of the portal vein (Figure 4). The collateral branch of the mesenteric vein expands to the hepatic portal vein and receives the blood flow collected from the abdominal intestine to the main portal vein, which acts as the collateral branch of the liver supplying blood to compensate the portal vein. In this study, there were 23 cases (32.4%) of hepatic portal collateral vessels established through the mesentery vein, accounting for 18.4% of all the collateral hepatic portal vessels found. The mesenteric branches of the hepatic portal vein mainly include the GCT, the first jejunal (FJV), and posterior superior pancreaticoduodenal vein (PSPDV) (17). The incidence of collateral mesentery in cancer embolus types Ⅱa, Ⅱb, Ⅲ, and Ⅳ was 1 case, 3 cases, 16 cases, and 3 cases, respectively, and the incidence rates were 6.3%, 15.8%, 59.3%, and 33.3%, respectively. There was no statistical significance among the 4 groups (P>0.05). Although there was no statistical difference in the incidence of this liver type collateral branch among the 4 groups of cancer thrombus, it is not difficult to see that the incidence of this collateral branch in PVTT type II is obviously lower than that in the other 2 groups, which may be due to the fact that patients with PVTT cannot easily establish this collateral branch when the PVTT does not invade the main portal vein and mesenteric vein.
In patients with PVTT, the pancreaticoduodenal vein is an important branch to the mesenteric branch of the hepatic portal vein. The pancreaticoduodenal vein is drained at the pancreatic head and duodenum by 2 anterior and posterior venous arches. The posterior arch is composed of the PSPDV and posterior inferior pancreaticoduodenal vein (PIPDV), and the anterior arch is composed of anterior superior pancreaticoduodenal vein (ASPDV) and anterior inferior pancreaticoduodenal vein (AIPDV). In this study, it was found that PSPDV connected with the paracholedochal vein plexus, and the hepatic artery behind the pancreatic head moved vertically upward with the common bile duct in the hepatoduodenal ligament. When the portal vein thrombus is completely blocked, the PSPDV is usually used to drain the venous blood in the abdominal mesentery to the liver, usually within 2 cm of the portal vein confluence (18). The ASPDV, AIPDV, and PIPDV are connected with the SMV or its 2 main branches. The 2 main tributaries of the SMV are the GCT and the FJV, which connect to the SMV on roughly the same level, but the 2 sides are opposite. The AIPDV and PIPDV are usually connected to the FJV independently or jointly. The FJV joins the SMV at the level of pancreatic uncinate process. The ASPDV forms the GCT together with the right gastroepiploic vein and the right colic vein. Bandali et al. (16) found that the detection rate of enhanced CT in the PSPDV was 61–65% in patients with primary HCC and PVTT, but only 13% in patients with cirrhosis and portal hypertension without tumor thrombus. In this study, it was found that when extrahepatic portal vein occlusion (EHPOV) occurred, the hepatic drainage of the duodenal vein was from the duodenal bulb to the liver via the PCVP. Because the PSPDV connects with the PCVP, it is also an important hepatic portal collateral branch when the PVTT is completely blocked.
Accessory portal vein system
In this study, the accessory portal vein system actually appeared to be a hepatic collateral branch established by the portal vein itself when the portal vein was cancerous. When the tumor thrombus invades and completely blocks the main or branch of the portal vein, the portal vein itself will generate a collateral hepatic portal vein, which bypasses the blocked part of the tumor thrombus and drains the blood collected by abdominal organs to the main portal vein and flows into the liver, serving as the collateral hepatic blood supply to compensate for the portal vein, as shown in Figure 5. Portal-systemic collateral vessels refers to one part of portal vein system entering another part of the portal vein system to realize a compensatory function of the liver portal vein system itself (19). In this study, there were 20 cases of hepatic portal collateral vessels established by the accessory portal vein system, accounting for 28.2% of all the enrolled patients, and accounting for 16% of all the hepatic portal collateral vessels found. The incidences of an accessory portal vein system in tumor thrombosis types Ⅱa, Ⅱb, Ⅲ, and Ⅳ were 3 cases, 5 cases, 8 cases, and 4 cases, respectively, and the incidence rates were 18.8%, 26.3%, 29.6%, and 44.4%, respectively. There was no statistical significance among the 4 groups of tumor thrombus (P>0.05). When the portal vein tumor thrombosis only involves and completely blocks the unilateral portal vein branch, the portal vein blood flow of the contralateral hepatic lobe will also compensate for the hepatic blood flow of the blocked side, which also belongs to the collateral branch between the portal veins. The formation of the collateral portal vein indicates that the blood flow bypasses the blocked part of the portal vein system, and the collateral branches tend to flow to the hepatic portal vein system. Although the incidence of such collateral vessels was not statistically significant among the 4 groups, it mainly occurred in EHPVO.
Additional observations
Because PVTT leads to the formation of collateral hepatic portal vessels, which is a multistep, multifactor, and complex interaction process, there is currently no unified standard to classify all abnormal hepatic portal vein collateral circulation. In this study, in addition to the above typical classification of collateral hepatic portal circulation, we also found that the hepatic portal collateral directly emanated from the splenic vein and entered the liver in the area around the portal vein. There was only one such collateral vessel in all the patients enrolled, and the incidence rate was only 1.41%, accounting for 0.8% of all the hepatic portal collateral vessels found. This may be related to the vascular variation of patients’ abdominal veins or the operation involving the juxtaposition of the abdominal structure (drained by the systemic veins) and the intestinal tract (drained by the portal vein tributaries), all of which may lead to the formation of hepatic collateral circulation (20) in patients with PVTT at abnormal sites.
Rerecognition of the cavernous degeneration of the portal vein
Cavernous transformation of the portal vein (CTPV) means that after the main portal vein and/or its branches are completely blocked by different causes, a large number of tiny veins are formed around it, creating a bypass to the hepatic portal vein collateral vessel to compensate for the hepatic blood flow lost by the portal vein. It is a compensatory disease that ensures normal hepatic blood flow and liver function. We believe that CTPV is a general term representing a vague understanding of the collateral circulation of the portal vein to liver and cannot accurately reflect the real phenomenon of the disease. In this paper, hepatic collateral circulation was classified and explained in detail, making it easier to understand the significance of various types of collateral circulation. The system starts from the PSVP and the RGC, and the RGV sends out some branches to the PCVP through the PDV. The PSPDV is directly connected to the PDVP. Dividing at the hilum of the liver to form a venous network, the venous network branches flow into the segmental veins adjacent to the hepatic portal, including the caudate lobe, quadrate lobe, and segments near the hepatic portal (segments III, IV, and V) (21). In this study, it was found that this system is a collateral channel for venous blood collected from the stomach, duodenum, and pancreas of the portal vein to return to the venous plexus around the biliary tract of the liver. It not only compensates for the hepatic portal blood flow when the portal vein is blocked but also serves as a potential anastomosis between the left and right lobes of the liver. In this study, 31 patients were found to have cavernous degeneration of the portal vein, accounting for 44% of all the enrolled patients. The biliary collateral, gastric collateral, and mesenteric collateral of the hepatic portal vein described in this study all communicated directly or indirectly with the CTPV. Previously, some researchers (22) have observed that in about 41% cases of CTPV, the cystic veins were also connected with this venous system. Other researchers believe that cavernous degeneration of the portal vein is just a massive expansion of the hepatoduodenal ligament and the preset vascular structure in gallbladder bed caused by portal vein obstruction (23).
Song et al. (24) found that the CT imaging rate of biliary branches in patients with CTPV and non-CTPV patients was 83–94% and 0%, respectively. Because of the high obstruction degree of tumor thrombus in the HCC, the morphological changes of collateral vessels of the CTPV may be more significant than those caused by other causes. Under normal circumstances, only the proper hepatic artery can be seen around the portal vein in CT images, and in rare cases, there are 2–3 small blood vessel sections around the portal vein. Therefore, some scholars believe that more than 3 blood vessel sections around the portal vein can be used as the standard for confirming a collateral circulation opening (25), which is consistent with results of this study, as shown in Figure 6. Spongiform degeneration of the portal vein occurred in 2 cases, 3 cases, 19 cases, and 7 cases in the 4 groups of tumor thrombus classification, and the incidence rates were 12.5%, 15.8%, 70.4%, and 77.8% respectively. There was a statistical difference among the 4 groups (P<0.05). It may be that cavernous degeneration of portal vein is more likely to occur because the main portal vein is completely blocked, so the incidence of cavernous degeneration of the portal vein in tumor thrombus types III and IV is significantly higher than that in type II. Spongiform degeneration of the portal vein is a common and important collateral pathway to the hepatic portal vein in patients with PVTT.
Significance of collateral portal vein circulation in patients with PVTT
The liver receives a double blood supply, being fed from the hepatic artery and portal vein at the same time. The hepatic artery supplies 20–30% of the total blood supply of the liver, which is the nutritional blood vessel of the liver. The portal vein, which accounts for 70–80% of the total blood supply of the liver, is the functional blood vessel of the liver, and thus has a critical role in the liver’s blood supply. When it is invaded by tumor to form a tumor thrombus, this greatly reduces the normal blood supply of the liver. Even if the blood flow of hepatic artery is compensated for, this cannot guarantee the normal blood supply of liver and normal liver function. Common complications include upper gastrointestinal bleeding, ascites, hypersplenism, and hepatic encephalopathy, which eventually lead to liver failure and the death of patients. In this study, there were 35 patients with ascites, accounting for 49% of all patients; 54 patients with splenomegaly, accounting for 76%; and 53 patients with esophageal varices, accounting for 75%. When these complications arise, it is extremely important to establish the collateral circulation of the hepatic portal vein. A large number of hepatic venules are generated around the portal vein to compensate for the hepatic blood flow lost by the portal vein and to ensure the normal hepatic blood flow and liver function.
TACE is one of the most common techniques for treating unresectable liver cancer in patients with PVTT (26). Although TACE may help to prolong the overall survival time (4–7 months) of patients with HCC and PVTT type III and IV, the use of TACE in these patients is still controversial due to the risk of liver infarction and liver failure. At present, TACE is considered to be suitable for patients with PVTT (27) who have good liver function and have established enough hepatic portal collaterals around the blocked portal vein. Therefore, for patients with PVTT, the establishment of a collateral portal vein to the liver can not only compensate for the blood flow from the portal vein to the liver, but also provide more treatment methods for patients with advanced liver cancer.
It is easy for patients with primary liver cancer with PVTT to establish circulation to the hepatic portal collateral, and the degree of opening to the hepatic portal collateral will directly affect the blood flow compensation of the blocked portal vein. In this study, the anatomical imaging manifestation and incidence of these hepatic portal collateral vessels were studied by applying the multislice CT image postprocessing technology. The following common collateral vessels of hepatic portal vein were found: collateral vessels of biliary tract of the hepatic portal vein, hepatic portal vein gastric branch, lateral mesenteric branch of the hepatic portal vein, accessory portal vein system, and others. Among them, the collateral branch of the hepatic portal biliary tract was the most common.
Due to the insufficient sample size, this study can only roughly classify the collateral branches of the hepatic portal vein. In the future, we look forward to using larger sample sizes and generating more accurate classification standards. In this study, splenomegaly, ascites, collateral circulation formation, and other indicators reflected the symptoms of portal hypertension. In the future, a multimodal examination method can be used to measure portal vein pressure, and the direction of the collateral blood flow can be determined by color Doppler ultrasound. The blood supply of liver is a complex system, and factors such as cirrhosis, primary liver cancer, PVTT, and the formation of hepatic portal vein collaterals will affect the normal blood supply of the liver. At present, CT perfusion imaging technology can realize the quantitative analysis of hepatic blood flow, and this new examination technology can further explore the compensatory function of collateral vessels of the hepatic portal vein.
In summary, a better understanding of the anatomical classification and function of the hepatic portal collaterals can provide clinicians with more information for diagnosis and treatment planning.
Conclusions
- The application of multislice CT image postprocessing technology can clearly show the blood vessels communicating with the collateral branch of hepatic portal vein when portal vein tumor thrombosis occurs.
- Common hepatic collateral branches in patients with PVTT mainly include the biliary collateral branch, gastric collateral branch, mesenteric collateral branch, accessory portal vein system, and others. The hepatic collateral branch from the static branch of the gallbladder enters the area around the gallbladder fossa. The hepatic collateral branches from the LGV enter the second and third segments of the left hepatic lobe. The hepatic collateral branch from the RGV enters hepatic segment IV. The hepatic collateral branch from the pancreaticoduodenal vein enters the dorsal side of hepatic segment IV. The hepatic collateral branch from the accessory portal vein enters the area around the hepatic portal.
- A collateral branch of the hepatic portal bile duct is mostly found in patients with complete obstruction of the right portal branch and trunk. A hepatic gastric branch of the portal vein mostly occurs in patients with complete obstruction of the left portal vein and main trunk. A hepatic mesenteric branch of the portal vein mostly occurs in patients with PVTT extending to the SMV. An accessory portal vein system mostly occurs in patients with complete obstruction of the portal vein trunk.
- Cavernous degeneration of portal vein is a venous network composed of several hepatic portal vein collateral vessels.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-45/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-45/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-45/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-23-45/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Human Investigation Committee of The Fourth Hospital of Hebei Medical University (No. 2021KY170). This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All patients provided written informed consent for the use of their data and images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cheng S, Chen M, Cai J, et al. Chinese Expert Consensus on Multidisciplinary Diagnosis and Treatment of Hepatocellular Carcinoma with Portal Vein Tumor Thrombus (2018 Edition). Liver Cancer 2020;9:28-40. [Crossref] [PubMed]
- Chan SL, Chong CC, Chan AW, et al. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J Gastroenterol 2016;22:7289-300. [Crossref] [PubMed]
- Mähringer-Kunz A, Steinle V, Düber C, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: The more, the worse? Liver Int 2019;39:324-31. [Crossref] [PubMed]
- Yu Z, Zhou Y, Li Y, Xu L. Risk factors for postoperative portal vein thrombosis in patients with hepatitis B liver cancer and its impact on mortality—a retrospective study. Transl Cancer Res 2022;11:2858-65. [Crossref] [PubMed]
- Lee SJ, Kim M, Kwak YK, et al. MRI-guided radiotherapy for PVTT in HCC patients: evaluation of the efficacy and safety. J Cancer Res Clin Oncol 2022;148:2405-14. [Crossref] [PubMed]
- Tao ZW, Cheng BQ, Zhou T, et al. Management of hepatocellular carcinoma patients with portal vein tumor thrombosis: A narrative review. Hepatobiliary Pancreat Dis Int 2022;21:134-44. [Crossref] [PubMed]
- Bae JS, Lee JM, Yoon JH, et al. How to Best Detect Portal Vein Tumor Thrombosis in Patients with Hepatocellular Carcinoma Meeting the Milan Criteria: Gadoxetic Acid-Enhanced MRI versus Contrast-Enhanced CT. Liver Cancer 2020;9:293-307. [Crossref] [PubMed]
- Sereni CP, Rodgers SK, Kirby CL, et al. Portal vein thrombus and infiltrative HCC: a pictoral review. Abdom Radiol (NY) 2017;42:159-70. [Crossref] [PubMed]
- Elbanna KY, Khalili K, O'Malley M, et al. Imaging and implications of tumor thrombus in abdominal malignancies: reviewing the basics. Abdom Radiol (NY) 2020;45:1057-68. [Crossref] [PubMed]
- Ramesh Babu CS, Sharma M. Biliary tract anatomy and its relationship with venous drainage. J Clin Exp Hepatol 2014;4:S18-26. [Crossref] [PubMed]
- Hui CL, Loo ZY. Vascular disorders of the gallbladder and bile ducts: Imaging findings. J Hepatobiliary Pancreat Sci 2021;28:825-36. [Crossref] [PubMed]
- De Gaetano AM, Lafortune M, Patriquin H, et al. Cavernous transformation of the portal vein: patterns of intrahepatic and splanchnic collateral circulation detected with Doppler sonography. AJR Am J Roentgenol 1995;165:1151-5. [Crossref] [PubMed]
- Ohkubo M. Aberrant left gastric vein directly draining into the liver. Clin Anat 2000;13:134-7. [Crossref] [PubMed]
- Tian JL, Zhang JS. Hepatic perfusion disorders: etiopathogenesis and related diseases. World J Gastroenterol 2006;12:3265-70. [Crossref] [PubMed]
- Madhusudhan KS, Vyas S, Sharma S, et al. Portal vein abnormalities: an imaging review. Clin Imaging 2018;52:70-8. [Crossref] [PubMed]
- Bandali MF, Mirakhur A, Lee EW, et al. Portal hypertension: Imaging of portosystemic collateral pathways and associated image-guided therapy. World J Gastroenterol 2017;23:1735-46. [Crossref] [PubMed]
- Seeger M, Günther R, Hinrichsen H, et al. Chronic portal vein thrombosis: transcapsular hepatic collateral vessels and communicating ectopic varices. Radiology 2010;257:568-78. [Crossref] [PubMed]
- Moll R, von Lüdinghausen MH, Lackner K, et al. Collateral pathways in portal hypertension. Surg Radiol Anat 1990;12:127-33. [Crossref] [PubMed]
- Jang HJ, Khalili K, Yu H, et al. Perfusion and parenchymal changes related to vascular alterations of the liver. Abdom Imaging 2012;37:404-21. [Crossref] [PubMed]
- Mamone G, Caruso S, di Francesco F, et al. Unusual venous collateral pathways allow for reperfusion of the intrahepatic portal venous system in children with portal vein thrombosis after split liver transplantation: Clinical relevance and management implications. Pediatr Transplant 2019;23:e13539. [Crossref] [PubMed]
- Hourani R, Brant LJ, Rizk T, et al. Can proton MR spectroscopic and perfusion imaging differentiate between neoplastic and nonneoplastic brain lesions in adults? AJNR Am J Neuroradiol 2008;29:366-72. [Crossref] [PubMed]
- Liu Y, Hou B, Chen R, et al. Biliary collateral veins and associated biliary abnormalities of portal hypertensive biliopathy in patients with cavernous transformation of portal vein. Clin Imaging 2015;39:841-4. [Crossref] [PubMed]
- Gallego C, Velasco M, Marcuello P, et al. Congenital and acquired anomalies of the portal venous system. Radiographics 2002;22:141-59. [Crossref] [PubMed]
- Song B, Min P, Oudkerk M, et al. Cavernous transformation of the portal vein secondary to tumor thrombosis of hepatocellular carcinoma: spiral CT visualization of the collateral vessels. Abdom Imaging 2000;25:385-93. [Crossref] [PubMed]
- Qi X, Han G, Yin Z, et al. Cavernous vessels around a patent portal trunk in the liver hilum. Abdom Imaging 2012;37:422-30. [Crossref] [PubMed]
- Yang SB, Zhang JH, Fu YF, et al. TACE with portal vein radioactive seeds for HCC with portal vein tumor thrombus: a meta-analysis. Minim Invasive Ther Allied Technol 2022;31:856-64. [Crossref] [PubMed]
- Zhang Y, Wu JL, Li LQ. Efficacy comparison of optimal treatments for hepatocellular carcinoma patients with portal vein tumor thrombus. Ann Hepatol 2022;27:100552. [Crossref] [PubMed]
(English Language Editor: J. Gray)


