
Establishment and validation of a predictive model of recurrence in primary hepatocellular carcinoma after resection
Highlight box
Key findings
• This model had high value in predicting the recurrence of primary hepatocellular carcinoma (HCC) after resection.
What is known and what is new?
• Single biological indicators have limited value in predicting patient prognosis.
• This study aimed to analyze the risk factors of recurrence in patients with primary HCC after surgical resection and establish a prediction model according to the relevant risk factors.
What is the implication, and what should change now?
• This model had high value in predicting the recurrence of primary HCC after surgical resection. This model could assist clinicians to assess the prognosis of patients. Intensive treatments for high-risk patients might improve patient prognosis.
Introduction
Primary hepatocellular carcinoma (HCC) is a common malignant tumor. Hepatitis B is the main cause of primary HCC in China. Primary HCC is aggressive with a high incidence rate and mortality (1). Postoperative recurrence and metastasis are the most important causes of death. Identifying the risk factors of postoperative recurrence could help clinicians to assess the prognosis of patients. Targeted interventions according to the relevant risk factors could also help to reduce the postoperative recurrence rate.
A previous study confirmed that multiple foci, large tumor size, portal vein tumor thrombus, and vascular invasion are risk factors for the early recurrence of primary HCC within 1 year of surgery (2); however, the follow-up time of the study was insufficient, and thus its value is limited. In addition, single biological indicators have limited predictive value for prognosis. Nomogram model could assist clinicians to assess the prognosis of patients and identify the high risk of recurrence patients. Some scholars have tried to use nomogram prediction models to predict the prognosis of various diseases in recent years, and research have shown that prediction models often have higher diagnostic value than a single biological indicator (3-5). However, studies in primary HCC are limited. Thus, it is necessary to further explore the risk factors of recurrence of primary HCC after surgical resection and establish a prediction model according to the relevant risk factors to assess the prognosis of primary HCC patients after surgery. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1303/rc).
Methods
General information
The data of 424 patients with primary HCC treated at the Wuhan Third Hospital were retrospectively collected. All the patients were treated with surgery. The patients were divided into the recurrence group (n=189) and control group (n=235) according to whether the cancer recurred after surgery. Inclusion criteria: (I) have primary HCC; (II) be aged ≥18 years; (III) have undergone surgical treatment; and (IV) have complete clinical data. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had metastatic liver cancer; (II) had other malignant tumors; (III) had unresectable liver cancer; (IV) had undergone palliative surgical resection for advanced liver cancer; (V) had perioperative liver failure leading to death; and/or (VI) were lost to follow-up. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Wuhan Third Hospital (No. 202200172), and the requirement for individual informed consent was waived.
Treatment strategy
The patients were required to undergo hepatectomy within a limited time after admission, and were given symptomatic support treatment, such as liver protection, anti-inflammatory treatment, and nutritional support. The diagnosis of primary HCC was confirmed by postoperative pathology. The patients were followed-up for 5 years after surgery through outpatient visits, and their abdominal computed tomography or magnetic resonance imaging and α-fetoprotein (AFP) results were reviewed regularly to observe whether there was a suspected recurrence after operation. If there is a suspected recurrence, we used liver puncture biopsy to determine whether it was a recurrence or not.
Observation indicators
The observation indicators were as follows: age at the time of diagnosis, gender, body mass index, smoking history, drinking history, tumor size, systemic immune-inflammation (SII) index, number of lesions, tumor differentiation degree, ascites, vascular invasion, portal vein tumor thrombus, tumor site, liver function Child-Pugh grade, surgical method, the integrity of the tumor capsule, and the preoperative AFP level.
Establishment and verification of the model
This study used R4.0.3 statistical software for the statistical analysis. The data set was randomly divided into the training set and verification set (according to the principle of completely random number table). The sample size of the training set was 297, and the sample size of the verification set was 127. According to the selected independent variables, the prediction model was established, and a decision curve analysis was conducted using the data from the training set. The calibration curve and receiver operating characteristic (ROC) curve were drawn using the data from the validation set. The Hosmer-Lemeshow Goodness-of-Fit Test was used to test the model with the validation set to evaluate the predictive value of the model.
Statistical analysis
R4.0.3 statistical software and SPSS26.0 software were used to complete the data analysis of this study. A 2-tailed P<0.05 indicated that the difference was statistically significant. The data that conformed to a normal distribution are presented as the mean ± standard deviation, and the independent sample t-test was used to analyze the differences between the two groups. The data that did not conform to a normal distribution are presented as the median [interquartile range (IQR)], and the non-parametric test was used to analyze the differences between the two groups. The counting data are presented as the number (percentage), and the Chi-Square test was used to analyze the differences between the two groups. The predictive value of different indicators on postoperative recurrence of liver cancer patients was analyzed by using the ROC curve; multivariate logistic regression analysis was used to explore the risk factors of postoperative recurrence in patients with primary HCC.
Results
Comparison of the clinicopathological features
There were significant statistical differences between the two groups in terms of the tumor size, SII index, the number of lesions, tumor differentiation degree, ascites, vascular invasion, and portal vein tumor thrombus (P<0.05) (Table 1).
Table 1
| Category | Recurrence group (n=189) | Control group (n=235) | t/χ2/Z value | P value |
|---|---|---|---|---|
| Age at the time of diagnosis (years), mean ± SD | 50.12±8.31 | 50.78±9.02 | 0.775 | 0.438 |
| Gender, n (%) | 0.138 | 0.711 | ||
| Male | 132 (69.84) | 168 (71.49) | ||
| Female | 57 (30.16) | 67 (28.51) | ||
| Body mass index (kg/m2), mean ± SD | 24.26±2.17 | 24.62±1.98 | 1.783 | 0.075 |
| Smoking history, n (%) | 21 (11.11) | 25 (10.64) | 0.024 | 0.876 |
| Drinking history, n (%) | 18 (9.52) | 29 (12.34) | 0.843 | 0.358 |
| Hepatitis B, n (%) | 150 (79.37) | 180 (76.60) | 0.466 | 0.495 |
| Tumor size (cm), median (25% quantile–75% quantile) | 4.61 (2.84–6.32) | 2.51 (1.59–3.68) | 9.480 | 0.000 |
| SII index, median (25% quantile–75% quantile) | 488.65 (297.65–710.44) | 382.67 (236.14–528.26) | 5.184 | 0.000 |
| Number of lesions, n (%) | 12.880 | 0.000 | ||
| Single lesion | 161 (85.19) | 224 (95.32) | ||
| Multiple foci | 28 (14.81) | 11 (4.68) | ||
| Differentiation degree of tumor, n (%) | 15.705 | 0.000 | ||
| Poorly differentiated | 81 (42.86) | 58 (24.68) | ||
| Moderately and well differentiated | 108 (57.14) | 177 (75.32) | ||
| Ascites, n (%) | 17 (8.99) | 7 (2.98) | 7.100 | 0.008 |
| Vascular invasion, n (%) | 20 (10.58) | 6 (2.55) | 11.731 | 0.001 |
| Portal vein tumor thrombus, n (%) | 18 (9.52) | 7 (2.98) | 8.087 | 0.004 |
| Tumor site, n (%) | 2.016 | 0.1561 | ||
| Left liver | 77 (40.74) | 80 (34.04) | ||
| Right liver | 112 (59.26) | 155 (65.96) | ||
| Liver function Child-Pugh grade, n (%) | 0.096 | 0.757 | ||
| Grade A | 126 (66.67) | 160 (68.09) | ||
| Grade B | 63 (33.33) | 75 (31.91) | ||
| Surgery, n (%) | 0.034 | 0.853 | ||
| Open | 23 (12.17) | 30 (12.77) | ||
| Laparoscopic | 166 (87.83) | 205 (87.23) | ||
| Integrity of tumor capsule, n (%) | 1.556 | 0.212 | ||
| Yes | 146 (77.25) | 193 (82.13) | ||
| No | 43 (22.75) | 42 (17.87) | ||
| Preoperative AFP level (μg/L), mean ± SD | 462.46±89.46 | 448.64±90.47 | 1.571 | 0.117 |
SD, standard deviation; SII, systemic immune-inflammation index; AFP, α-fetoprotein.
Risk factors for the recurrence of primary HCC after surgical resection
The multivariate regression analysis showed that multiple foci, poorly differentiated tumors, ascites, vascular invasion, and portal vein tumor thrombus were risk factors for the recurrence of primary HCC in patients after surgical resection (P<0.05) (Table 2).
Table 2
| Variables | B | S.E. | Wald | P | Relative risk (95% CI) |
|---|---|---|---|---|---|
| Multiple foci | 1.432 | 0.385 | 13.824 | 0.000 | 4.187 (1.968–8.906) |
| Poorly differentiated tumors | 0.915 | 0.222 | 16.906 | 0.000 | 2.496 (1.614–3.860) |
| Ascites | 0.969 | 0.486 | 3.977 | 0.046 | 2.635 (1.017–6.829) |
| Vascular invasion | 1.686 | 0.491 | 11.787 | 0.001 | 5.398 (2.062–14.134) |
| Portal vein tumor thrombus | 1.166 | 0.479 | 5.937 | 0.015 | 3.209 (1.256–8.199) |
| Constant | –11.466 | 1.890 | 36.814 | 0.000 | 0.000 |
HCC, hepatocellular carcinoma; S.E., standard errors; CI, confidence interval.
Establishment and validation of a model of recurrence of primary HCC after resection
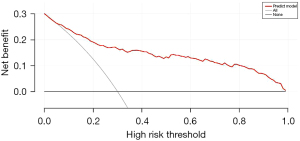
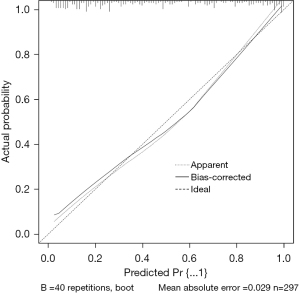
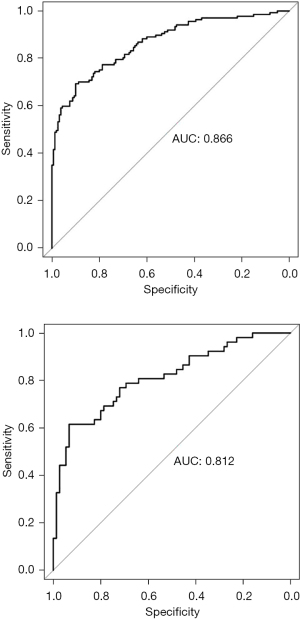
The data set was randomly divided into the training set and the verification set. The sample size of the training set was 297, and the sample size of the verification set was 127. The following factors were included in the model: tumor size, SII index, multiple foci, poorly differentiated tumors, ascites, vascular invasion, and portal vein tumor thrombus. The nomogram was established, and a decision curve analysis was conducted (Figures 1,2). The calibration and ROC curves were drawn using the data of the validation set (Figures 3,4). The area under the ROC curve of the training set was 0.866 [95% confidence interval (CI): 0.824–0.907], and the area under the ROC curve of the validation set was 0.812 (95% CI: 0.734–0.890). The Hosmer-Lemeshow Goodness-of-Fit Test was used to test the model with the validation set (χ2=11.243, P=0.188), and the results indicated that the model had high value in predicting the recurrence of primary HCC after surgical resection.
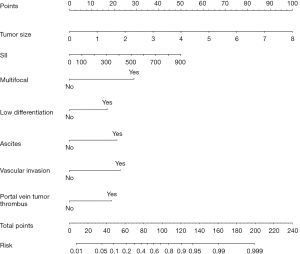
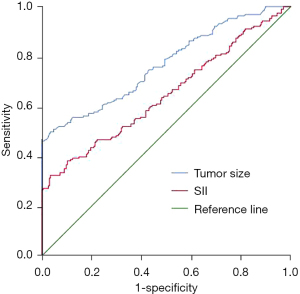
The value of tumor size and the SII index in predicting the recurrence of primary HCC after surgical resection
Tumor size had certain value in predicting the recurrence of primary HCC after surgical resection, and the area under the curve (AUC) was 0.768 (95% CI: 0.722–0.814, P=0.000). The SII index had certain value in predicting the recurrence of primary HCC after surgical resection, and the AUC was 0.646 (95% CI: 0.593–0.700, P=0.000). Thus, tumor size and the SII index had certain value in predicting the recurrence of primary HCC after surgical resection, but the predictive value was not as good as that of the nomogram model (Figure 5).
Discussion
Cancer is a common fatal disease. The effective identification of factors for a poor prognosis would not only help clinicians to assess the prognosis of patients (6-8) but would also help clinicians to identify high-risk patients according to risk factors, and thus reduce mortality (9-12). This study explored the risk factors of recurrence of primary HCC in patients after surgical resection. The results showed that tumor size, the SII index, multiple foci, poorly differentiated tumors, ascites, vascular invasion, and portal vein tumor thrombus were related to recurrence of primary HCC in patients after surgical resection. According to the relevant factors, a prediction model was also established. This prediction model was found to have a high value in predicting the recurrence of primary HCC in patients after surgical resection. The AUC was as high as 0.866 (95% CI: 0.824–0.907), which is higher than the prediction value of single prediction indicators, such as tumor size and the SII index.
Lymph node metastasis is rare in HCC; thus, in clinical practice, the prognosis of patients is mainly evaluated based on tumor size and number of lesions. At present, tumor size and the number of lesions have been confirmed as risk factors for the postoperative recurrence, metastasis, and death of patients with primary liver cancer (13-15), which is further supported by the findings of this study. The poor differentiation of liver cancer refers to the high degree of malignancy. The higher the degree of malignancy, the faster the growth of liver cancer cells, and the more likely they are to have intrahepatic metastasis and distant metastasis in the early stage, which in turn leads to the poor prognosis of patients (16-18).
The ascites in patients with liver cancer are mainly caused by chronic liver function impairment, which leads to a decrease in albumin synthesis, a decrease in plasma colloid osmotic pressure, or an imbalance in intracellular and extracellular water metabolism caused by portal hypertension. The flow of intracellular fluid out of the cell causes ascites. Ascites in patients with liver cancer generally indicates that the tumor has reached an advanced stage and that patient prognosis is poor (19,20).
Liver cancer patients with vascular invasion and portal vein tumor thrombus are more likely to have intrahepatic metastasis. As liver cancer cells are not sensitive to chemotherapy, such patients are more likely to have recurrence and metastasis (21-23). In addition, this study found that the SII index was also valuable in predicting recurrence in patients with primary HCC after surgical resection. The SII index is calculated using the following formula: SII index = (the platelet count + neutrophil count)/the lymphocyte count.
Neutrophils are inflammatory cells. An increase in the level of neutrophils means that the level of systemic inflammation is increased. An inflammatory state is conducive to tumor angiogenesis and promotes the proliferation and metastasis of liver cancer cells. Lymphocytes are immune cells of the body, and mainly include T lymphocytes, B lymphocytes and natural-killer cells, which are the main cells of the body that kill tumor cells. When the number of lymphocytes decreases, it indicates that the patient’s immune function is low. Platelets participate in the development of tumor growth, tumor cell exosmosis, and tumor metastasis. Thus, an increase in the SII index suggests that the internal environment is conducive to the growth and metastasis of tumor cells. The SII index has been proven to be associated with the long-term prognosis of colorectal cancer patients with liver metastasis (24,25). However, there is still a lack of relevant research in patients with primary liver cancer. This study found that the SII index is a new marker of poor prognosis in patients with liver cancer.
In addition, this study found that single biological indicators, such as tumor size and the SII index, had some value in predicting the recurrence of primary HCC in patients after surgical resection, but their predictive value was not high. Nomogram models integrate multiple related indicators. Nomogram prediction models have been shown to successfully predict the prognosis of many diseases (26-30). This study established a prediction model and found that the prediction value of this model was high. The model had an AUC as high as 0.866 (95% CI: 0.824–0.907), which was higher than the prediction value of any single prediction indicators, such as tumor size and the SII index.
Limitations
First, it was a single-center retrospective clinical study. Second, the verification of the model was completed using an internal validation method.
Conclusions
Our model was shown to have high value in predicting the recurrence of primary HCC after surgical resection. The model might assist clinicians to assess the prognosis of patients. Intensive treatments for high-risk patients might also improve patient prognosis.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1303/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1303/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1303/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Wuhan Third Hospital (No. 202200172), and the requirement for individual informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Xia W, Peng T, Guan R, et al. Development of a novel prognostic nomogram for the early recurrence of liver cancer after curative hepatectomy. Ann Transl Med 2021;9:1541. [Crossref] [PubMed]
- Yue Y, Liang J, Wu Y, et al. A Nomogram for Predicting Liver Metastasis of Lymph-Node Positive Luminal B HER2 Negative Subtype Breast Cancer by Analyzing the Clinicopathological Characteristics of Patients with Breast Cancer. Technol Cancer Res Treat 2022;21:15330338221132669. [Crossref] [PubMed]
- Li M, Zhang F, Lu SX, et al. A Novel Thoracoabdominal Aorta CTA-based Nomogram Model to Identify Ideal Candidates for Transradial Approach Chemoembolization in Patients with Liver Cancer. J Cancer 2022;13:2863-71. [Crossref] [PubMed]
- Yang D, Su Y, Zhao F, et al. A Practical Nomogram and Risk Stratification System Predicting Cancer-Specific Survival for Hepatocellular Carcinoma Patients With Severe Liver Fibrosis. Front Surg 2022;9:920589. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- He XC, Chen HY, Qiu Y, et al. Associations of iron status with breast cancer risk factors in adult women: Findings from National Health and Nutrition Examination Survey 2017-2018. J Trace Elem Med Biol 2021;68:126867. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Qi A, Li Y, Sun H, et al. Incidence and risk factors of sexual dysfunction in young breast cancer survivors. Ann Palliat Med 2021;10:4428-34. [Crossref] [PubMed]
- Liu X, Yang W, Petrick JL, et al. Higher intake of whole grains and dietary fiber are associated with lower risk of liver cancer and chronic liver disease mortality. Nat Commun 2021;12:6388. [Crossref] [PubMed]
- Chen Y, Qi A, Teng D, et al. Probiotics and synbiotics for preventing postoperative infectious complications in colorectal cancer patients: a systematic review and meta-analysis. Tech Coloproctol 2022;26:425-36. [Crossref] [PubMed]
- Kamphues C, Andreatos N, Kruppa J, et al. The optimal cut-off values for tumor size, number of lesions, and CEA levels in patients with surgically treated colorectal cancer liver metastases: An international, multi-institutional study. J Surg Oncol 2021;123:939-48. [Crossref] [PubMed]
- Herrero de la Parte B, García-Alonso I, Mar-Medina C, et al. Ultrasound Tumor Size Assessment, Histology and Serum Enzyme Analysis in a Rat Model of Colorectal Liver Cancer. Ultrasound Med Biol 2020;46:1504-12. [Crossref] [PubMed]
- Ren Y, Cao Y, Ma H, et al. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: results of a single-center retrospective case control study. BMC Cancer 2019;19:983. [Crossref] [PubMed]
- Zhang X, Zhu XJ, Zhong Z, et al. Small Molecule-Induced Differentiation As a Potential Therapy for Liver Cancer. Adv Sci (Weinh) 2022;9:e2103619. [Crossref] [PubMed]
- Xu X, Zhang Z, Chen Q, et al. Predicting the prognosis of liver cancer patients based on cell differentiation trajectory and application of nanomaterials in treatment. Minerva Surg 2021; Epub ahead of print. [Crossref]
- Wei H, Wang J, Xu Z, et al. Hepatoma Cell-Derived Extracellular Vesicles Promote Liver Cancer Metastasis by Inducing the Differentiation of Bone Marrow Stem Cells Through microRNA-181d-5p and the FAK/Src Pathway. Front Cell Dev Biol 2021;9:607001. [Crossref] [PubMed]
- Gomaa AI, Al-Khatib A, Abdel-Razek W, et al. Ascites and alpha-fetoprotein improve prognostic performance of Barcelona Clinic Liver Cancer staging. World J Gastroenterol 2015;21:5654-62. [Crossref] [PubMed]
- Qureshi MO, Dar FS, Khokhar N. Cancer Antigen-125 as a marker of ascites in patients with liver cirrhosis. J Coll Physicians Surg Pak 2014;24:232-5.
- Zhang S, He L, Bo C, et al. Comparison of stereotactic body radiation therapy versus fractionated radiation therapy for primary liver cancer with portal vein tumor thrombus. Radiat Oncol 2021;16:149. [Crossref] [PubMed]
- Yang X, Zhu Y, Zhao X, et al. The Prognostic Comparison Between Hepatocellular Carcinoma with Portal Vein Tumor Thrombus and Bile Duct Cancer Thrombus After Liver Resection. Cancer Manag Res 2020;12:12077-86. [Crossref] [PubMed]
- Ye JZ, Wang YY, Bai T, et al. Surgical resection for hepatocellular carcinoma with portal vein tumor thrombus in the Asia-Pacific region beyond the Barcelona Clinic Liver Cancer treatment algorithms: a review and update. Oncotarget 2017;8:93258-78. [Crossref] [PubMed]
- Polk N, Budai B, Hitre E, et al. High Neutrophil-To-Lymphocyte Ratio (NLR) and Systemic Immune-Inflammation Index (SII) Are Markers of Longer Survival After Metastasectomy of Patients With Liver-Only Metastasis of Rectal Cancer. Pathol Oncol Res 2022;28:1610315. [Crossref] [PubMed]
- Lu Y, Xin D, Wang F. Predictive significance of preoperative systemic immune-inflammation index determination in postoperative liver metastasis of colorectal cancer. Onco Targets Ther 2019;12:7791-9. [Crossref] [PubMed]
- Ding X, Yang X, Wu D, et al. Nomogram predicting the cancer-specific survival of early-onset colorectal cancer patients with synchronous liver metastasis: a population-based study. Int J Colorectal Dis 2022;37:1309-19. [Crossref] [PubMed]
- Hao M, Li H, Wang K, et al. Predicting metachronous liver metastasis in patients with colorectal cancer: development and assessment of a new nomogram. World J Surg Oncol 2022;20:80. [Crossref] [PubMed]
- Zhao R, Dai Y, Li X, Zhu C. Construction and validation of a nomogram for non small cell lung cancer patients with liver metastases based on a population analysis. Sci Rep 2022;12:4011. [Crossref] [PubMed]
- Liu C, Hu C, Huang J, et al. A Prognostic Nomogram of Colon Cancer With Liver Metastasis: A Study of the US SEER Database and a Chinese Cohort. Front Oncol 2021;11:591009. [Crossref] [PubMed]
- Du Q, Wang Y, Guan S, et al. Retraction Note: The diagnostic nomogram of platelet-based score models for hepatic alveolar echinococcosis and atypical liver cancer. Sci Rep 2020;10:16405. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


