
Life-threatening “hyper-progression” on immunotherapy revealed as pseudo-progression in DNA mismatch repair deficiency: a case report
Highlight box
Key findings
• Life-threatening medical complications that met all applicable definitions of hyper-progression was revealed to be pseudo-progression in a patient who received PD-1 blockade therapy for deficient DNA mismatch repair (dMMR) gastric cancer.
What is known and what is new?
• Hyper-progression and pseudo-progression are two atypical patterns of response to immune checkpoint inhibitors (ICI). Tools that are currently available to clinicians to distinguish pseudo-progression from true cancer progression or hyper-progression are limited.
• This case report demonstrates that defining hyper-progression or pseudo-progression by changes in tumor size and/or clinical status only may be inadequate.
What is the implication, and what should change now?
• Considering mismatch repair status in deciding the aggressiveness of clinical management during apparent “hyper-progression”, given the substantially higher rate of durable response to ICI in dMMR tumors, is important.
Introduction
Immune checkpoint inhibitors (ICI), alone or in combination with chemotherapy, have become standard therapies for the treatment of many solid tumors. ICIs are particularly effective in patients whose tumors exhibit deficient DNA mismatch repair (dMMR), which is observed in >10% of gastric tumors and 2–4% of other tumors (1,2). These tumors have inactivated or suppressed expression of MMR proteins secondary to genetic aberrations at the germline or somatic level leading to decreased ability to correct replication errors in microsatellites and predispose carriers to DNA damage and development of cancer (3). dMMR can be detected by immunohistochemistry (IHC) to assess lost expression of at least one (out of four) MMR proteins (i.e., MLH1, PMS2, MSH2 and MSH6) or polymerase chain reaction-based test that quantifies the frequency of changes in alleles in the microsatellite region (4). Next-generation sequencing has recently emerged to become another diagnostic tool for this purpose (5). ICIs are believed to be more efficacious in dMMR [vs. MMR-proficient (pMMR)] tumors due to the presence of hypermutations that leads to neoantigen generation and immune stimulation (6). Pseudo-progression is an ICI-related phenomenon characterized by radiographic enlargement of cancer metastases or the appearance of new lesions—believed to represent infiltration by immune cells—followed by their radiographic regression (7,8). The possibility that true cancer progression may actually represent pseudo-progression complicates clinical decision-making because tools that distinguish the two disparate situations are lacking. Although the reported rate of pseudo-progression is generally <10% (9), failure to identify it can profoundly impact patient survival and quality-of-life. Hyper-progression (i.e., rapid tumor worsening) is another pattern of response to ICI that occurs in 4–29% of patients (10-12). It is generally established by ruling out pseudo-progression (13), but this has not been extensively examined. Whether MMR status should be considered in situations of potential hyper-/pseudo-progression is not well-defined.
Here we report the first case, to our knowledge, of life-threatening hyper-progression which was revealed to be pseudo-progression in a patient with dMMR gastric cancer, which was found to have loss of MLH1 and PMS2 expression based on IHC testing. We present the following case in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-709/rc).
Case presentation
A 62-year-old man with gastroesophageal junctional (GEJ) adenocarcinoma was initially diagnosed with T3NxM0 disease and received curative-intent concurrent chemoradiation with carboplatin plus paclitaxel (14). Six weeks after completion of chemoradiation, he was found to have new hepatic metastases and was started on treatment with 5-fluorouracil, leucovorin plus oxaliplatin (FOLFOX). He then experienced a best radiographic response of stable disease, and his dysphagia worsened and weight declined. At that point, results from tumor testing returned revealing loss of MLH1 and PMS2 protein expression, signifying dMMR. Given emerging data that 86% of patients with dMMR gastric/GEJ adenocarcinomas may experience durable response lasting more than 10 months with pembrolizumab (15), treatment was switched to this anti-programmed death 1 (PD-1) agent.
After 2 doses of pembrolizumab (200 mg every 3 weeks), the patient noted significant improvement in dysphagia and calorie intake. A week after the third dose, he presented to a local emergency department with copious melena, hematochezia, and hematemesis. He was profoundly hypotensive (blood pressure 66/48) and had a 2-point drop in hemoglobin within 24 hours. Upper endoscopy showed a large, diffusely ulcerated, friable mass in the lower esophagus and clotted blood in the stomach. After receiving 5 units of red blood cells (RBCs), he was transferred to the intensive care unit. Computed tomography (CT) imaging showed wall irregularity of the splenic artery that was concerning, although not definitive, for erosion from the gastric primary, as well as a marked increase in the number, size and conspicuity of hepatic masses (Figure 1). His serum tumor marker, carcinoembryonic antigen (CEA), had also increased.
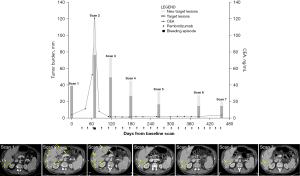
Palliative care and interventional radiology were consulted to assist in weighing the benefits and risks of possible interventions, and a less aggressive management plan was pursued for multiple reasons. The patient had seemingly unequivocal tumor progression symptomatically and radiographically and met all applicable definitions of hyper-progression (i.e., doubled tumor size or growth rate within 2 months of initiating treatment) (10,11), suggesting that pseudo-progression was exceedingly unlikely or impossible. Typical survival of metastatic gastric cancer patients who hyper-progress on immunotherapy is 20–65 days (16). The source of bleeding was unclear and would require further workup. Supportive care without aggressive intervention could translate into comfort care measures only if hemodynamic instability developed.
Forty-eight hours later, the patient’s hemoglobin dropped to 6.4 after recurrent melena. He received 1 unit of RBCs and medical oncology was consulted to explore all options including comfort measures only. The patient insisted on full-code status citing prior advice from an oncologist that increases in tumor size, particularly one that is dMMR, could represent pseudo-progression, and a more aggressive management plan was pursued.
Overnight, he developed hypotensive shock after further melena and hematemesis. Vasopressor support with norepinephrine was started, and he received 6 units of RBCs, 2 units of fresh frozen plasma, and 1 pool of platelets. CT angiogram revealed protrusion of the splenic artery into the wall of the GEJ tumor, a new infarction in the spleen, and new hyperdense material in the stomach likely representing interval hemorrhage. Embolization of the splenic artery was performed. His condition stabilized and he was discharged home after 7 days of hospitalization.
In the outpatient setting, he was determined to be a candidate for further anticancer therapy, and the initial plan was to discontinue immunotherapy and switch to cytotoxic therapy. However, repeat CEA showed an interval decrease, prompting repeat CT imaging. Surprisingly, the new scan showed marked interval response with decrease in the size and number of hepatic metastases (Figure 1), indicating that the earlier expansion of hepatic lesions actually represented pseudo-progression. Pembrolizumab was resumed. His tumor lesions responded further, including at his most recent follow-up 13 months later. He is physically active and has no cancer symptoms.
All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This case challenges our current approach to assessing progression on ICI by demonstrating that even life-threatening “hyper-progression” can represent pseudo-progression. If not for this patient’s strong and clearly expressed wish for aggressive care, it is possible that comfort measures only could have been pursued with subsequent death.
Hyper-progression and pseudo-progression are two patterns of atypical response to ICI. The former is generally understood as rapid, true disease progression while the later indicates delayed response with initial, radiographic tumor growth followed by tumor shrinkage (9,17). The concept of hyper-progression was examined independently by three groups of researchers and common to the proposed criteria is rapid and/or accelerated tumor growth within 2 months of initiation of treatment that was seen respectively in 9%, 4% and 29% of patients in their studies (10-12). Only Champiat et al. included 13 patients with gastrointestinal cancers (8 colorectal cancer, 2 cholangiocarcinoma, 2 gastric/esophageal cancer and 1 pancreatic cancer) and 2 patients (1 colorectal cancer and 1 with cholangiocarcinoma) were found to have hyper-progression, which was defined by a twofold or greater increase of tumor growth rate in the first cycle of immunotherapy compared to the growth rate during the wash-out, off-therapy period (10). Pseudo-progression was first observed in 9.7% (22 of 227) of patients with melanoma who received ipilimumab (18). A systematic review comprising 19 clinical trials across melanoma, non-small-cell lung carcinoma and renal cell carcinoma reported that 6% of patients developed pseudo-progression following treatment with nivolumab or pembrolizumab (19). Overall, the rates of hyper-/pseudo-progression in gastrointestinal cancers have not been analyzed systematically to date. Our patient’s target lesions grew from 35 to 75 mm with development of multiple new hepatic metastases after 7 weeks of ICI. Although he met all proposed definitions of hyper-progression that was associated with poor survival (10-12), he was found to have pseudo-progression with durable treatment response, suggesting that defining hyper- or pseudo-progression by changes in tumor size only may be inadequate.
Tools that are currently available to clinicians to distinguish pseudo-progression from true progression or hyper-progression are limited for both dMMR and pMMR tumors (20). Serum CEA has been proposed for this purpose. One case report showed that the CEA level decreased at the time of pseudo-progression in a patient receiving anti-PD-1 for colorectal cancer (21), and another study suggested that CEA levels increase more in patients with true disease progression compared to those with pseudo-progression (107% vs. 32%) (22). However, our patient demonstrates that a twofold or greater increase in CEA can be unreliable in identifying true progression as CEA levels can be elevated due to non-malignant factors such as inflammation and reduced hepatic clearance. Clinical stability (versus deterioration) in association with radiographic progression has also been considered an important parameter to distinguish pseudo-progression from true progression based on Immune Response Evaluation Criteria in Solid Tumors (iRECIST) (7,9,13). Radiographic progression in most cases of pseudo-progression is relatively mild but the degree of change can vary in pseudo-progression patients with gastric cancer according to the case series reported by Michalarea et al. (23). In addition, circulating tumor DNA (ctDNA) may be a potential tool for identifying true progression (24,25). Radiographic confirmation of true progression with a recommended minimal interval of 4 weeks as proposed by iRECIST (7) is not feasible in urgent situations.
In this patient, the key feature that led the patient to advocate for aggressive care and continued immunotherapy was the dMMR status of the patient’s tumor. While the predictive value of dMMR has been established, its importance in assessing pseudo-progression has not been previously appreciated. Available data suggests that the rates of hyper-progression do not appear to differ between dMMR and pMMR tumors but the rates of true progression as best overall response are significantly higher in pMMR tumors (26). Accordingly, this case report suggests that consideration of the tumor’s MMR status may be helpful in determining the aggressiveness of providing supportive care to a patient with apparent “hyper-progression”. In a study of 18 patients with dMMR gastric cancer, pembrolizumab monotherapy led to an overall response rate of 55.6% (27) and another study that enrolled 61 patients with metastatic gastric cancer reported a response rate of 85.7% in 7 patients with MSI-H tumors (15). Although neither study documented hyper-progression or pseudo-progression, the high response rate of dMMR gastric cancer to ICI support consideration of an aggressive approach before discontinuing ICI.
The clinical management of true hyper-progression on ICI alone (without concurrent cytotoxic chemotherapy) is not clear. Options include discontinuation of ICI potentially followed immediately by cytotoxic therapy. Another potential option is to add cytotoxic therapy to ICI, or to have potentially avoided hyper-progression from the outset by having administered ICI in combination with concurrent cytotoxic chemotherapy. The benefits of this latter approach is suggested by recent results from phase 3 clinical trials in gastroesophageal cancer that compared ICI alone versus chemotherapy alone (28-30), which showed early progression and death in the ICI alone arm, as compared with the chemotherapy alone arm. This early progression/death was not evident in the arm in which ICI was given concurrently with chemotherapy.
Research is ongoing to further identify predictive markers of hyper-/pseudo-progression (16,20,22,24,25,31). Recent evidence indicates that PD-1 blockade can paradoxically upregulate immunosuppression in the tumor microenvironment and potentially lead to hyper-progression. Proliferative PD-1-expressing regulatory T cells (Tregs; FoxP3hiCD45-CD4+) were found to be increased in patients with hyper-progression but reduced in those without hyper-progression after treatment with anti-PD-1 in gastric cancer (16). Further, a higher ratio of tumor-infiltrating PD-1+ cytotoxic T cells (CD8+) to PD-1+ regulatory T cells (CD4+) was associated with favorable treatment response and survival to anti-PD-1 (32). These data underscore the importance of further delineating the function and phenotype of tumor-infiltrating immune cells to understand the pathogenesis of hyper-/pseudo-progression. In addition, inter-tumoral heterogeneity among dMMR and pMMR tumors has been shown to be associated with clinical response to ICI (1,33-35), suggesting that heterogeneity between dMMR tumors may further contribute to differential immune reactions to ICI.
Conclusions
This case challenges our current approach to assessing progression on immunotherapy. First, it demonstrates that even life-threatening hyper-progression can represent pseudo-progression. Second, clinical parameters previously implicated as being able to distinguish pseudo- from true progression (e.g., stable/declining CEA, clinical stability) may be unreliable (our patient’s CEA increased, clinical status deteriorated). Other tools, including ctDNA and radiographic confirmation four weeks later, are not feasible in urgent situations. Third, this case highlights the potential importance of considering MMR status in deciding the aggressiveness of clinical management during apparent hyper-progression, given the substantially higher rate of durable response in dMMR tumors. While the value of dMMR in predicting response to ICIs has been established, its importance in assessing pseudo-progression has not been previously appreciated. These considerations have implications for decision-making across a spectrum of specialties that may be acutely consulted.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-709/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-709/coif). HHY reports consulting/advisory fees from ALX Oncology, Amgen, Astellas Scientific, Medical Affairs Inc., AstraZeneca, BeiGene, Bristol Myers Squibb, OncXerna, Macrogenics, Merck, Novartis, and Zymeworks; and grants from Boston Biomedical, Lilly/ImClone, Merck, and Roche/Genentech outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hause RJ, Pritchard CC, Shendure J, et al. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med 2016;22:1342-50. [Crossref] [PubMed]
- Bonneville R, Krook MA, Kautto EA, et al. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis Oncol 2017;2017:PO.17.00073.
- Ellegren H. Microsatellites: simple sequences with complex evolution. Nat Rev Genet 2004;5:435-45. [Crossref] [PubMed]
- Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248-57.
- Vanderwalde A, Spetzler D, Xiao N, et al. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med 2018;7:746-56. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Di Giacomo AM, Danielli R, Guidoboni M, et al. Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother 2009;58:1297-306. [Crossref] [PubMed]
- Chiou VL, Burotto M. Pseudoprogression and Immune-Related Response in Solid Tumors. J Clin Oncol 2015;33:3541-3. [Crossref] [PubMed]
- Champiat S, Dercle L, Ammari S, et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res 2017;23:1920-8. [Crossref] [PubMed]
- Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin Cancer Res 2017;23:4242-50. [Crossref] [PubMed]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 2017;28:1605-11. [Crossref] [PubMed]
- Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol 2020;21:e463-76. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Kim ST, Cristescu R, Bass AJ, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018;24:1449-58. [Crossref] [PubMed]
- Kamada T, Togashi Y, Tay C, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A 2019;116:9999-10008. [Crossref] [PubMed]
- Borcoman E, Nandikolla A, Long G, et al. Patterns of Response and Progression to Immunotherapy. Am Soc Clin Oncol Educ Book 2018;38:169-78. [Crossref] [PubMed]
- Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412-20. [Crossref] [PubMed]
- Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat Rev 2017;59:71-8. [Crossref] [PubMed]
- Ma Y, Wang Q, Dong Q, et al. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res 2019;9:1546-53.
- Trabjerg ND, Rask C, Jensen LH, et al. Pseudoprogression during treatment with pembrolizumab followed by rechallenge with chemotherapy in metastatic colorectal cancer: A case report. Clin Case Rep 2019;7:1445-9. [Crossref] [PubMed]
- Parseghian CM, Patnana M, Bhosale P, et al. Evaluating for Pseudoprogression in Colorectal and Pancreatic Tumors Treated With Immunotherapy. J Immunother 2018;41:284-91. [Crossref] [PubMed]
- Michalarea V, Fontana E, Garces AI, et al. Pseudoprogression on treatment with immune-checkpoint inhibitors in patients with gastrointestinal malignancies: Case series and short literature review. Curr Probl Cancer 2019;43:487-94. [Crossref] [PubMed]
- Lee JH, Long GV, Menzies AM, et al. Association Between Circulating Tumor DNA and Pseudoprogression in Patients With Metastatic Melanoma Treated With Anti-Programmed Cell Death 1 Antibodies. JAMA Oncol 2018;4:717-21. [Crossref] [PubMed]
- Bratman SV, Yang SYC, Iafolla MAJ, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer 2020;1:873-81. [Crossref] [PubMed]
- Hagi T, Kurokawa Y, Kawabata R, et al. Multicentre biomarker cohort study on the efficacy of nivolumab treatment for gastric cancer. Br J Cancer 2020;123:965-72. [Crossref] [PubMed]
- Kwon M, An M, Klempner SJ, et al. Determinants of Response and Intrinsic Resistance to PD-1 Blockade in Microsatellite Instability-High Gastric Cancer. Cancer Discov 2021;11:2168-85. [Crossref] [PubMed]
- Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022;603:942-8. [Crossref] [PubMed]
- Shitara K, Özgüroğlu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 2018;392:123-33. [Crossref] [PubMed]
- Doki Y, Ajani JA, Kato K, et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 2022;386:449-62. [Crossref] [PubMed]
- Basler L, Gabryś HS, Hogan SA, et al. Radiomics, Tumor Volume, and Blood Biomarkers for Early Prediction of Pseudoprogression in Patients with Metastatic Melanoma Treated with Immune Checkpoint Inhibition. Clin Cancer Res 2020;26:4414-25. [Crossref] [PubMed]
- Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 2020;21:1346-58. [Crossref] [PubMed]
- Yoon HH, Shi Q, Heying EN, et al. Intertumoral Heterogeneity of CD3(+) and CD8(+) T-Cell Densities in the Microenvironment of DNA Mismatch-Repair-Deficient Colon Cancers: Implications for Prognosis. Clin Cancer Res 2019;25:125-33. [Crossref] [PubMed]
- Mlecnik B, Bindea G, Angell HK, et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016;44:698-711. [Crossref] [PubMed]
- Schwitalle Y, Linnebacher M, Ripberger E, et al. Immunogenic peptides generated by frameshift mutations in DNA mismatch repair-deficient cancer cells. Cancer Immun 2004;4:14.


