
Clinical significance and changes to the immune microenvironment of colorectal cancer patients with liver metastasis
Highlight box
Key findings
• The immunosuppressive condition of liver metastasis in colorectal cancer patients was related to poor prognosis.
What is known and what is new?
• The tumor microenvironment phenotype is the key factor determining the effect of immunotherapy and chemotherapy.
• This study clarified relationship between the changes and clinical significance of the immune microenvironment in colorectal cancer patients with liver metastasis.
What is the implication, and what should change now?
• This study showed that the immunosuppressive condition of liver metastatic cancer tissues in colorectal cancer patients was related to poor prognosis. However, further studies are needed to explore the molecular mechanism.
Introduction
The liver is one of the most common metastatic sites of advanced solid tumors (1-4). Hematological metastasis is the main cause of liver metastasis in colorectal cancer. Colorectal cancer contains very rich blood vessels. Most colorectal cancer cell metastasize to the liver along the portal vein after invading the small veins. Liver metastasis often involves multiple and wide-ranging diseases that cannot be treated surgically. In clinical practice, it is often observed that the target lesion of an advanced solid tumor can be controlled following systematic treatment, such as chemotherapy and targeted therapy; however, the size and number of liver metastatic tumors may still increase, resulting in clinical problems. Immunotherapy covers a wide range of tumor types and is safer than traditional radiotherapy and chemotherapy. Yet, its overall effective rate is only 20–40% (5). The tumor microenvironment phenotype is the key factor determining the effect of immunotherapy and chemotherapy. And immunotherapy and chemotherapy can also affect the tumor microenvironment phenotype (6). Meanwhile, tumor proliferation and invasiveness depend on the internal characteristics of tumor cells as well as the microenvironment (7,8). Tumor microenvironments at different anatomical locations are specific, regulate tumor growth and progression, and affect the therapeutic response. Therefore, it is crucial to explore the immune microenvironment changes and its clinical significance in the liver metastasis of colorectal cancer. Given the lack of relevant research that is currently available, we designed the present study. We present the following article in accordance with the STARD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1169/rc).
Methods
General information
We retrospectively collected the data of 50 colorectal cancer patients with liver metastasis treated in Chongqing University Cancer Hospital from January 2017 to January 2019. Liver metastatic cancer tissues and normal liver tissues were also collected. The inclusion criteria were as follows: (I) the primary lesion was pathologically diagnosed as colorectal cancer accompanied by solitary resectable liver nodules (diameter ≥1 cm), and the postoperative pathological diagnosis was liver metastatic cancer from colorectal cancer; (II) aged 18–80 years old; (III) no lung metastasis, bone metastasis, brain metastasis, etc.; and (IV) patients who received simultaneous resection of colorectal cancer and liver metastasis from which we could obtain tissue samples and complete clinical records. The exclusion criteria were as follows: (I) recurrent colorectal cancer; (II) primary liver cancer; (III) complicated with other malignant tumors; (IV) patients with abnormal portal vein and mesenteric artery and vein structure; (V) those with hepatic insufficiency; (VI) patients who received special treatments, such as radiotherapy, chemotherapy, immunotherapy, and targeted therapy, preoperatively; and (VII) patients with other major diseases. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Chongqing University Cancer Hospital (No. 20220174), and owing to its retrospective nature, the requirement for written informed consent from the participants was waived.
Detection methods
Intraoperatively, liver metastatic cancer tissue samples and normal liver tissues (liver tissues more than 1 cm from the tumor edge) were taken to prepare the cell suspensions. Lymphocytes in the tissues were separated by density gradient centrifugation, washed with phosphate-buffered solution (PBS), added with corresponding monoclonal antibodies, and reacted at room temperature for 30 minutes. The Epics XL flow cytometer (Beckman Coulter, USA) was used to detect the level of T helper cell 1/T helper cell 2 (Th1/Th2) and regulatory T cells within 60 minutes.
Observation indicators
(I) General data: age, gender, body mass index (BMI), diabetes, and hypertension; (II) pathological characteristics: location of the primary lesion, maximum diameter of the primary lesion, T stage, lymph node metastasis, liver metastasis diameter, pathological type, and degree of differentiation; (III) prognosis: the recurrence and mortality rates at 3 years postoperatively were followed up through outpatient visits and telephone interviews.
Statistical analysis
SPSS 26.0 (IBM, Chicago, USA) was used to complete the data analysis in this study, and a two-tailed P value <0.05 was considered statistically significant. All measurement data conformed to a normal distribution, and the paired t-test was used to analyze the T-cell subset differences between the liver metastatic cancer tissues and normal liver tissues, which were expressed as the mean ± standard deviation (SD). The counting data were expressed as n (%). A receiver operating characteristic (ROC) curve was used to analyze the predictive value of T-cell subpopulations for the survival and recurrence of colorectal cancer patients with liver metastasis after surgery.
Results
Clinical and pathological data of colorectal cancer patients with liver metastasis
A total of 50 patients were enrolled, including 29 males and 21 females, with an average age of 54.62±8.12 years old. The average BMI of the included patients was 26.72±2.88 kg/m2. Six patients were complicated with diabetes and five patients were complicated with hypertension. Twenty-eight patients were diagnosed with colon cancer and 22 patients were diagnosed with rectal cancer. The average maximum diameter of the primary lesions was 4.36±1.22 cm, of which 30 cases were T3 stage and 20 cases were T4 stage. Moreover, there were 41 cases of lymph node metastasis. The average diameter of the liver metastases was 2.42±0.86 cm. There were 45 cases of adenocarcinoma and five cases of non-adenocarcinoma, including 35 cases with moderately and poorly differentiated adenocarcinomas and 15 cases with well-differentiated tumors. A total of 36 cases recurred 3 years after surgery, and 26 patients had died at 3 years postoperatively (Table 1).
Table 1
| Variable | Number (n=50) |
|---|---|
| Age (years) (mean ± SD) | 54.62±8.12 |
| Gender, n (%) | |
| Male | 29 (58.00) |
| Female | 21 (42.00) |
| BMI (kg/m2) (mean ± SD) | 26.72±2.88 |
| Diabetes, n (%) | |
| Yes | 6 (12.00) |
| No | 44 (88.00) |
| Hypertension, n (%) | |
| Yes | 5 (10.00) |
| No | 45 (90.00) |
| Location of primary lesion, n (%) | |
| Colon cancer | 28 (56.00) |
| Rectal cancer | 22 (44.00) |
| Maximum diameter of primary lesion (cm) (mean ± SD) | 4.36±1.22 |
| T stage, n (%) | |
| T3 | 30 (60.00) |
| T4 | 20 (40.00) |
| Lymph node metastasis, n (%) | |
| Yes | 41 (82.00) |
| No | 9 (18.00) |
| Diameter of liver metastasis (cm) (mean ± SD) | 2.42±0.86 |
| Pathological type, n (%) | |
| Adenocarcinoma | 45 (90.00) |
| Non-adenocarcinoma | 5 (10.00) |
| Degree of differentiation, n (%) | |
| Moderately and poorly differentiated | 35 (70.00) |
| Well differentiated | 15 (30.00) |
| Recurrence 3 years after operation, n (%) | 36 (72.00) |
| Death 3 years after operation, n (%) | 26 (52.00) |
SD, standard deviation; BMI, body mass index.
Differences of T-cell subsets between liver metastatic cancer tissues and normal liver tissues
Compared with the normal liver tissues, the Th1/Th2 levels in the liver metastatic cancer tissues were significantly decreased (0.88±0.24 vs. 1.34±0.27, P=0.000), while the regulatory T-cell levels were markedly increased (8.57±2.31 vs. 6.89±1.71, P=0.000) (Table 2).
Table 2
| Variable | n | Th1/Th2 | Regulatory T cells |
|---|---|---|---|
| Liver metastatic cancer tissue (mean ± SD) | 50 | 0.88±0.24 | 8.57±2.31 |
| Normal liver tissue (mean ± SD) | 50 | 1.34±0.27 | 6.89±1.71 |
| t value | – | 9.481 | 4.396 |
| P value | – | 0.000 | 0.000 |
SD, standard deviation; Th1/Th2, T helper cell 1/T helper cell 2.
Predictive value of the Th1/Th2 level in liver metastatic cancer tissues for the recurrence and survival of patients 3 years after surgery
The Th1/Th2 level in liver metastatic cancer tissue exhibited a good predictive value for the recurrence and survival of patients 3 years after surgery, and the areas under the curves were 0.783 (95% confidence interval: 0.649–0.916, P=0.002) and 0.763 (95% confidence interval: 0.628–0.898, P=0.001), respectively (Figures 1,2).
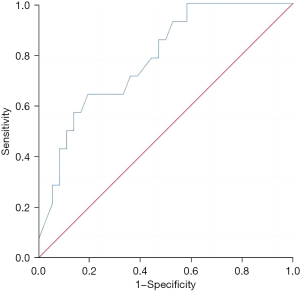
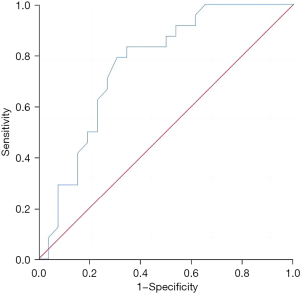
Predictive value of the regulatory T-cell level in liver metastatic cancer tissues for the recurrence and survival of patients 3 years after surgery
The regulatory T-cell level in liver metastatic cancer tissue had a good predictive value for the recurrence and survival of patients 3 years after surgery, and the areas under the curves were 0.788 (95% confidence interval: 0.656–0.919, P=0.002) and 0.763 (95% confidence interval: 0.628–0.897, P=0.001), respectively (Figures 3,4).
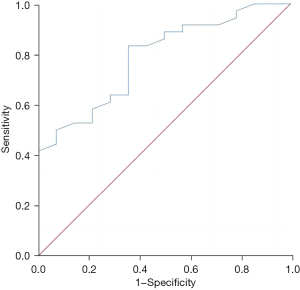
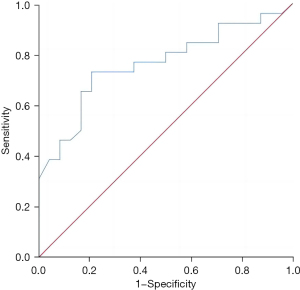
Discussion
Previous studies have sought to identify novel tumor biological indicators, provide new targets for treatment, and accurately predict the prognosis of patients (9-11). The immune microenvironment is not only a key factor in the occurrence and development of tumors, but also has an important impact on the effectiveness of immunotherapy. The present study explored the changes and clinical significance of T-cell subsets in the immune microenvironment of colorectal cancer patients with liver metastasis. The results showed that the Th1/Th2 level was significantly reduced and the regulatory T cell level was substantially increased in colorectal cancer patients with liver metastasis, indicating that liver metastasis is an immunosuppressive condition. In addition, we found that the decreased Th1/Th2 level and the increased regulatory T-cell level in liver metastasis were related to the poor prognosis of colorectal cancer patients.
The liver is composed of 80% parenchymal cells and 20% non-parenchymal cells and has a dual blood supply from the portal vein and inferior vena cava. It is an important organ for carbohydrate and lipid metabolism in the human body and also participates in immune regulation. Due to long-term exposure to a large number of harmless antigens and the need to maintain tolerance to these antigens, the liver is normally in an immunosuppressive condition. The special immune microenvironment of the liver is closely related to its specific cellular components and immunosuppressive cells. Therefore, the immune microenvironment of liver tumor tissues is also different from that of tumor tissues in other organs. A previous study in patients with primary liver cancer showed that the balance of peripheral blood Th1/Th2 in patients shifted to Th2, indicating that the Th1/Th2 ratio decreased (12). At this time, the killing ability of the body to tumor cells is reduced, then the tumor cells can rapidly proliferate and metastasize, which was related to poor patient prognosis. Th1 is the main T cell responsible for killing tumor cells in the human body. The decrease in the Th1/Th2 ratio indicates that Th1 function is inhibited, suggesting that the patient is in an immunosuppressive condition, which may eventually lead to a poor prognosis. In addition, regulatory T cells are immunosuppressive cells; down-regulation of regulatory T cells can improve the prognosis of patients, while elevated levels of regulatory T cells are indicative of a poor prognosis (13,14). In this study, the increase of regulatory T cell levels in liver metastatic cancer tissues inhibited the infiltration of immune cells into the tumor tissue, which promoted the growth and metastasis of tumor cells, and ultimately led to the poor prognosis of patients. The role of regulatory T cells has also been confirmed in the studies of other malignant tumors, further supporting the findings of the present study (15-20).
Limitations
The present study had some limitations that should be noted. This was a retrospective clinical study, and the sample size was relatively insufficient to further explore the molecular mechanism of poor prognosis caused by changes in the immune microenvironment of liver metastasis. Moreover, we did not dynamically monitor the changes of immune microenvironment of liver metastases. Finally, we failed to study the local immune microenvironment in this retrospective study.
Conclusions
This study showed that the immunosuppressive condition of liver metastatic cancer tissues in colorectal cancer patients was related to poor prognosis. However, further studies are needed to explore the molecular mechanism.
Acknowledgments
Funding: This work was funded by Laboratory Open Fund of Chongqing University Cancer Hospital (Grant No. cstc2020jxjl0113), the Chongqing Science and Health Joint Project (Grant No. 2022MSXM131), and the Chongqing Natural Science Foundation (Grant No. cstc2018jcyjAX0814).
Footnote
Reporting Checklist: The authors have completed the STARD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1169/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1169/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1169/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of Chongqing University Cancer Hospital (No. 20220174), and owing to its retrospective nature, the requirement for written informed consent from the participants was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirokawa F, Ueno M, Nakai T, et al. Treatment strategy for resectable colorectal cancer liver metastases from the viewpoint of time to surgical failure. Langenbecks Arch Surg 2022;407:699-706. [Crossref] [PubMed]
- Lang H, Baumgart J, Roth W, et al. Cancer gene related characterization of patterns and point of recurrence after resection of colorectal liver metastases. Ann Transl Med 2021;9:1372. [Crossref] [PubMed]
- Tago T, Katsumata K, Udou R, et al. Significance of Radiofrequency Ablation for Unresectable Colorectal Cancer With Liver Metastases. Anticancer Res 2021;41:5539-47. [Crossref] [PubMed]
- Ho WW, Gomes-Santos IL, Aoki S, et al. Dendritic cell paucity in mismatch repair-proficient colorectal cancer liver metastases limits immune checkpoint blockade efficacy. Proc Natl Acad Sci U S A 2021;118:e2105323118. [Crossref] [PubMed]
- Sasaki K, Kobayashi S, Kudo M, et al. Hypothyroidism and hypopituitarism as immune-related adverse events due to lenvatinib plus pembrolizumab therapy in the immediate postoperative period after laparoscopic hepatectomy for liver metastases from gastric cancer: a case report. Surg Case Rep 2021;7:267. [Crossref] [PubMed]
- Wu Y, Yang S, Ma J, et al. Spatiotemporal Immune Landscape of Colorectal Cancer Liver Metastasis at Single-Cell Level. Cancer Discov 2022;12:134-53. [Crossref] [PubMed]
- Moretto R, Corallo S, Belfiore A, et al. Prognostic impact of immune-microenvironment in colorectal liver metastases resected after triplets plus a biologic agent: A pooled analysis of five prospective trials. Eur J Cancer 2020;135:78-88. [Crossref] [PubMed]
- Ciner AT, Jones K, Muschel RJ, et al. The unique immune microenvironment of liver metastases: Challenges and opportunities. Semin Cancer Biol 2021;71:143-56. [Crossref] [PubMed]
- Chen Y, Wang J, Zhang X, et al. Correlation between apparent diffusion coefficient and pathological characteristics of patients with invasive breast cancer. Ann Transl Med 2021;9:143. [Crossref] [PubMed]
- Qi A, Li Y, Yan S, et al. Effect of postoperative chemotherapy on blood glucose and lipid metabolism in patients with invasive breast cancer. Gland Surg 2021;10:1470-7. [Crossref] [PubMed]
- Teng D, Xia S, Hu S, et al. miR-887-3p Inhibits the Progression of Colorectal Cancer via Downregulating DNMT1 Expression and Regulating P53 Expression. Comput Intell Neurosci 2022;2022:7179733. [Crossref] [PubMed]
- Chen X, Zhu F, Wang B, et al. Clinical Effect of Iodine-125 Seed Implantation in Patients with Primary Liver Cancer and Its Effect on Th1/Th2 Cells in Peripheral Blood. J Oncol 2021;2021:6199732. [Crossref] [PubMed]
- Chen TT. Dahuang Zhechong pills inhibit liver cancer growth in a mouse model by reversing Treg/Th1 balance. Chin J Nat Med 2022;20:102-10. [Crossref] [PubMed]
- Ning T, Li J, He Y, et al. Exosomal miR-208b related with oxaliplatin resistance promotes Treg expansion in colorectal cancer. Mol Ther 2021;29:2723-36. [Crossref] [PubMed]
- Osman A, Yan B, Li Y, et al. TCF-1 controls Treg cell functions that regulate inflammation, CD8+ T cell cytotoxicity and severity of colon cancer. Nat Immunol 2021;22:1152-62. [Crossref] [PubMed]
- Tian EM, Yu MC, Feng M, et al. RORγt agonist synergizes with CTLA-4 antibody to inhibit tumor growth through inhibition of Treg cells via TGF-β signaling in cancer. Pharmacol Res 2021;172:105793. [Crossref] [PubMed]
- Lam JH, Hong M, Koo SL, et al. CD30+OX40+ Treg is associated with improved overall survival in colorectal cancer. Cancer Immunol Immunother 2021;70:2353-65. [Crossref] [PubMed]
- Xu R, Wu M, Liu S, et al. Glucose metabolism characteristics and TLR8-mediated metabolic control of CD4+ Treg cells in ovarian cancer cells microenvironment. Cell Death Dis 2021;12:22. [Crossref] [PubMed]
- Zhao Y, Zhang Z, Lei W, et al. IL-21 Is an Accomplice of PD-L1 in the Induction of PD-1-Dependent Treg Generation in Head and Neck Cancer. Front Oncol 2021;11:648293. [Crossref] [PubMed]
- Zhao S, Zheng X, Zhu X, et al. Surgical Trauma-induced CCL2 Upregulation Mediates Lung Cancer Progression by Promoting Treg Recruitment in Mice and Patients. Cancer Invest 2022;40:91-102. [Crossref] [PubMed]
(English Language Editor: A. Kassem)


