Development and validation of an XGBoost model to predict 5-year survival in elderly patients with intrahepatic cholangiocarcinoma after surgery: a SEER-based study
Highlight box
Key Findings
• The developed XGBoost model exhibited some predictive capacity, and performed better than the AJCC system in predicting the postoperative 5-year survival of elderly ICC patients.
What is known and what is new?
• The AJCC system is the most frequently used staging system for ICC, but it is more applicable to a cohort of patients as opposed to individual patients.
• Compared with the AJCC system, the XGBoost model exhibited a better predictive performance.
What is the implication, and what should change now?
• The XGBoost model may be employed to predict 5-year survival in elderly patients with ICC after surgery, and may subsequently be used to promote individualized treatment.
Introduction
Intrahepatic cholangiocarcinoma (ICC), the second most common primary liver cancer, is a highly fatal hepatobiliary neoplasm originating from the epithelial cells of the intrahepatic bile ducts (1,2). The incidence and mortality rates of ICC continue to increase worldwide (3,4). Although surgery remains the optimal modality to extend survival in ICC patients, their prognosis remains unfavorable, with a 5-year overall survival after surgery of 30–35% (5-7). Notably, the incidence of ICC increases with age, as the incidence in older patients is almost twice as high as that in younger patients (8). Most patients are between 55 and 75 years old (2), suggesting that the elderly account for the majority of cases. Hence, effective tools to predict the postoperative prognosis of elderly patients with ICC are urgently needed.
Currently, the American Joint Commission on Cancer (AJCC) system (9,10) is the most frequently used staging system for ICC. However, it is more applicable to a cohort of patients as opposed to individual patients, and many other factors, such as age, tumor number, margin status, and treatment, should be considered in addition to tumor size, lymph node metastasis, and distant metastasis (5,11-14). Older age, larger tumor size, multiple tumors, lymph node metastasis, and vascular invasion were reported as predictors for shorter overall survival in ICC (6). Sahara et al. included sum of the number and largest tumor size >7, N1 disease, R1 resection, poor/undifferentiated tumor grade, major vascular invasion, and adjuvant chemotherapy to establish an online calculator to estimate 5-year survival following hepatectomy in ICC patients, with a concordance index (C-index) of 0.696 in the training set and 0.672 in the testing set (15). A prediction model of overall survival in resectable ICC was constructed with an immune signature for ICC, exhibiting a C-index of 0.719 in the derivation cohort and 0.667 in the validation cohort (16). A clinical-radiologic-radiomics (CRR) model was used to predict postsurgical overall survival in mass-forming ICC (C-index =0.71) (17). Nomograms have been established to predict survival in individual ICC patients after surgical resection or in elderly patients (18-20), with C-indexes around 0.7. However, these prediction tools had limited predictive abilities, and there are no models that predict survival following surgery in the elderly with ICC. Besides, Sahara et al. (15) indicated that nomograms had limited applicability and clinical utility because they are cumbersome and cannot be easily utilized in a simple, real clinical setting with varying clinical and pathological factors. Recently, artificial intelligence models on the basis of machine learning (ML) algorithms have attracted increasing attention in clinical practice. Extreme gradient boosting (XGBoost), a typical boosting algorithm, is an integrated technology that can be applied to adjust the errors generated by existing models (21,22). XGBoost models have been used for effective and precise survival prediction in several cancers, including breast cancer (23), osteosarcoma (24), and non-small-cell lung cancer (25); however, their applicability to ICC is unknown.
This study intended to develop and validate an XGBoost model to predict 5-year survival in elderly ICC patients after surgery, utilizing data in the Surveillance, Epidemiology, and End Results (SEER) program. Moreover, predictive performances of the XGBoost model and the AJCC staging system were compared. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1238/rc).
Methods
Data source and study population
Data on elderly patients with ICC were collected from the SEER database [SEER 18 Regs Custom Data (with additional treatment fields), Nov 2018 Sub (1975–2016 varying)], which comprises 18 population-based registries and covers approximately 30% of the US population (26). Institutional review board approval was exempted for this study as data from the SEER database are publicly available. This retrospective cohort study only involved patients with microscopically confirmed primary ICC aged 60 years or older and undergoing cancer-directed surgery (surgery of primary site codes 20–80). Patients who had missing data on lymphadenectomy, the pathologic examination of lymph nodes, AJCC stage, tumor size, and follow-up were excluded. The median follow-up time was 20 (Q1, Q3: 8, 39) months. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Study variables
The outcome variable was 5-year survival after surgery in elderly ICC patients. Other variables included the primary site labeled, age, gender, race, marital status, tumor size (mm), multiple (satellite) tumors/nodules (no/yes), more than one lobe invaded (no/yes), major vascular invasion (no/yes), gallbladder invasion (no/yes), direct invasion of adjacent organs (extrahepatic bile ducts, gallbladder, ligament, diaphragm; no/yes), AJCC (7th edition) stage, T stage, N stage, M stage, resection and transplant (wedge or segmental resection, (extended) lobectomy without transplant, transplant), lymph nodes removed, radiotherapy (no/unknown/yes), chemotherapy (no/unknown/yes), and survival months.
Multiple (satellite) tumors/nodules included satellitosis, multifocal tumors and intrahepatic metastases. Major vascular invasion referred to an invasion of the branches of the main portal vein (right or left portal vein, excluding sectoral or segmental branches) or an invasion of one or more of the three hepatic veins (right, middle or left) [https://staging.seer.cancer.gov/cs/input/02.05.50/liver/extension/?breadcrumbs=(~schema_list~),(~view_schema~,~liver~)].
Construction and evaluation of the XGBoost model
The study population was classified as training and testing sets at a ratio of 7:3 in a random manner. The training set was utilized to develop a model, and the testing set was employed to internally validate the model. XGBoost (21), a gradient tree boosting algorithm, was adopted to construct a prediction model for 5-year survival after surgery in elderly ICC patients in the training set. The variables were directly screened using the XGBoost model via multivariate analysis.
The predictive performance of the model was assessed by the area under the receiver operating characteristic (ROC) curve (AUC), sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The patients were grouped in accordance with their 5-year survival predicted by the XGBoost model, and the Kaplan-Meier curve was constructed and compared using the log-rank test to assess the model’s ability to distinguish survival status. Cox regression analysis was carried out to assess the risk of death in the predicted populations, and hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. Furthermore, the predictive ability of the XGBoost model and the AJCC system (7th edition) were also compared.
Statistical analysis
Continuous data with normal distribution were illustrated as the mean ± standard deviation (SD); the independent sample t-test was applied to make inter-group comparisons. Continuous data with skewed distribution were reported by the median and quartiles [M (Q1, Q3)]; between-group comparisons were subject to the Mann-Whitney U rank sum test. Categorical data were presented as the number of cases and the composition ratio [n (%)]; inter-group comparisons was conducted using the Chi-square test or the Fisher’s exact test. Samples with missing values were deleted. The threshold value of AUC for a good prediction model was 0.8. Feature importance analysis was conducted in the XGBoost model. All statistical tests were two-sided. P<0.05 denoted statistical significance. XGBoost modeling was conducted with Python 3.8 (Python Software Foundation, Delaware, USA), and other analyses were completed with SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Totally 1,055 elderly patients with ICC who underwent cancer-directed surgery were enrolled in this study after excluding patients without data on follow-up (n=1), AJCC stage (n=98), and tumor size (n=97). The patient selection flow chart is shown in Figure 1. The average age was 70.49 years. White people (83.22%) accounted for the majority of cases. Based on their vital status, these patients were classified into a survival group (n=512) and a death group (n=543). The median follow-up times of the death and survival groups were 15 and 30 months, respectively. Tumor size in the death group was significantly greater than that in the survival group (P<0.001). Patients in the survival group had fewer multiple (satellite) tumors/nodules than those in the death group (P<0.001). The following factors were markedly better in the survival group compared to the death group: more than one lobe invaded, major vascular invasion, direct invasion of adjacent organs, AJCC stage, T stage, N stage, and M stage (all P<0.05). Table 1 presents the basic characteristics of the enrolled patients.
Table 1
| Variable | Total (n=1055) | Survival group (n=512) | Death group (n=543) | χ2/t/Z | P |
|---|---|---|---|---|---|
| Primary site labeled, n (%) | 2.699 | 0.100 | |||
| C22.0-liver | 218 (20.66) | 95 (18.55) | 123 (22.65) | ||
| C22.1-intrahepatic bile duct | 837 (79.34) | 417 (81.45) | 420 (77.35) | ||
| Age, mean ± SD | 70.49±6.42 | 70.16±6.30 | 70.79±6.52 | −1.60 | 0.109 |
| Gender, n (%) | 2.813 | 0.094 | |||
| Female | 518 (49.10) | 265 (51.76) | 253 (46.59) | ||
| Male | 537 (50.90) | 247 (48.24) | 290 (53.41) | ||
| Race, n (%) | 0.545 | 0.762 | |||
| Asian | 112 (10.62) | 56 (10.94) | 56 (10.31) | ||
| White | 878 (83.22) | 422 (82.42) | 456 (83.98) | ||
| Other | 65 (6.16) | 34 (6.64) | 31 (5.71) | ||
| Marital status, n (%) | 4.524 | 0.210 | |||
| Separated | 232 (21.99) | 108 (21.09) | 124 (22.84) | ||
| Married | 681 (64.55) | 328 (64.06) | 353 (65.01) | ||
| Unmarried | 102 (9.67) | 59 (11.52) | 43 (7.92) | ||
| Other | 40 (3.79) | 17 (3.32) | 23 (4.24) | ||
| Tumor size, M (Q1, Q3) | 51.00 (33.00, 75.00) | 48.00 (30.00, 70.00) | 55.00 (35.00, 76.00) | −3.690 | <0.001 |
| Multiple (satellite) tumors/nodules, n (%) | 27.240 | <0.001 | |||
| No | 908 (86.07) | 470 (91.80) | 438 (80.66) | ||
| Yes | 147 (13.93) | 42 (8.20) | 105 (19.34) | ||
| More than one lobe invaded, n (%) | 6.644 | 0.010 | |||
| No | 1,035 (98.10) | 508 (99.22) | 527 (97.05) | ||
| Yes | 20 (1.90) | 4 (0.78) | 16 (2.95) | ||
| Major vascular invasion, n (%) | 4.861 | 0.027 | |||
| No | 1,024 (97.06) | 503 (98.24) | 521 (95.95) | ||
| Yes | 31 (2.94) | 9 (1.76) | 22 (4.05) | ||
| Gallbladder invasion, n (%) | 0.072 | 0.789 | |||
| No | 1,031 (97.73) | 501 (97.85) | 530 (97.61) | ||
| Yes | 24 (2.27) | 11 (2.15) | 13 (2.39) | ||
| Direct invasion of adjacent organs, n (%) | 16.243 | <0.001 | |||
| No | 924 (87.58) | 470 (91.80) | 454 (83.61) | ||
| Yes | 131 (12.42) | 42 (8.20) | 89 (16.39) | ||
| AJCC stage, n (%) | 93.765 | <0.001 | |||
| I | 390 (36.97) | 256 (50.00) | 134 (24.68) | ||
| II | 255 (24.17) | 125 (24.41) | 130 (23.94) | ||
| III | 196 (18.58) | 54 (10.55) | 142 (26.15) | ||
| IV | 214 (20.28) | 77 (15.04) | 137 (25.23) | ||
| T, n (%) | 82.860 | <0.001 | |||
| T1 | 440 (41.71) | 277 (54.10) | 163 (30.02) | ||
| T2 | 343 (32.51) | 157 (30.66) | 186 (34.25) | ||
| T3 | 171 (16.21) | 55 (10.74) | 116 (21.36) | ||
| T4 | 101 (9.57) | 23 (4.49) | 78 (14.36) | ||
| N, n (%) | 33.802 | <0.001 | |||
| N0 | 860 (81.52) | 454 (88.67) | 406 (74.77) | ||
| N1 | 195 (18.48) | 58 (11.33) | 137 (25.23) | ||
| M, n (%) | 8.140 | 0.004 | |||
| M0 | 1,001 (94.88) | 496 (96.88) | 505 (93.00) | ||
| M1 | 54 (5.12) | 16 (3.13) | 38 (7.00) | ||
| Resection and transplant, n (%) | 3.413 | 0.182 | |||
| Wedge or segmental resection | 370 (35.07) | 184 (35.94) | 186 (34.25) | ||
| (Extended) lobectomy without transplant | 503 (47.68) | 251 (49.02) | 252 (46.41) | ||
| Transplant | 182 (17.25) | 77 (15.04) | 105 (19.34) | ||
| Lymph nodes removed, n (%) | 1.382 | 0.501 | |||
| 0 | 503 (47.68) | 249 (48.63) | 254 (46.78) | ||
| 1–3 | 313 (29.67) | 155 (30.27) | 158 (29.10) | ||
| >3 | 239 (22.65) | 108 (21.09) | 131 (24.13) | ||
| Radiotherapy, n (%) | 0.539 | 0.463 | |||
| No/unknown | 921 (87.30) | 443 (86.52) | 478 (88.03) | ||
| Yes | 134 (12.70) | 69 (13.48) | 65 (11.97) | ||
| Chemotherapy, n (%) | 0.019 | 0.891 | |||
| No/unknown | 676 (64.08) | 327 (63.87) | 349 (64.27) | ||
| Yes | 379 (35.92) | 185 (36.13) | 194 (35.73) | ||
| Survival months, M (Q1, Q3) | 20.00 (8.00, 39.00) | 30.00 (13.00, 63.00) | 15.00 (5.00, 28.00) | 10.192 | <0.001 |
SD, standard deviation; AJCC, the American Joint Commission on Cancer.
Construction of the XGBoost model
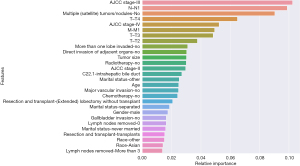
The study population was randomly divided into 738 patients in the training set and 317 patients in the testing set. Feature importance analysis was performed in the XGBoost model, as illustrated in Figure 2. AJCC stage III, N1 stage, multiple (satellite) tumors/nodules, T4 stage, AJCC stage IV, M1 stage, T3 stage, T2 stage, more than one lobe invaded, direct invasion of adjacent organs, tumor size, radiotherapy, and AJCC stage II were found to be features of relative importance. This model was tested and adjusted repeatedly, and the best parameters were determined. The parameter settings of this XGBoost model were as follows: n_estimators =2,000, learning_rate =0.0001, subsample =0.5, and colsample_bytree =0.3.
Evaluation of the XGBoost model
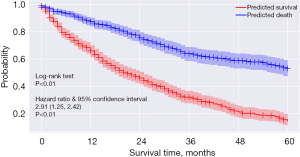
The predictive performance of the XGBoost model was assessed by the AUC, sensitivity, specificity, PPV, NPV, and Kaplan-Meier curve using a cutoff value of 0.507. The XGBoost model exhibited an AUC of 0.811 (95% CI: 0.781–0.841), a sensitivity of 0.573 (95% CI: 0.524–0.623), a specificity of 0.890 (95% CI: 0.858–0.923), and a PPV of 0.849 (95% CI: 0.805–0.893) in the training set. For internal validation in the testing set, the model showed an AUC of 0.713 (95% CI: 0.656–0.769), a sensitivity of 0.478 (95% CI: 0.401–0.555), a specificity of 0.814 (95% CI: 0.753–0.875), and a PPV of 0.726 (95% CI: 0.642–0.811) (Table 2). These findings indicated that the XGBoost model showed some predictive ability. Moreover, there was a significant difference between the survival curves of patients predicted to survive and those predicted to die (P<0.01), and the 5-year mortality risk of patients predicted to die was 2.91 times that of patients predicted to survive (HR =2.91, 95% CI: 2.42–3.50, P<0.01) (Figure 3), suggesting that the XGBoost model exhibited a good capacity to distinguish survival status.
Table 2
| Dataset | AUC | Sensitivity | Specificity | PPV | NPV | Cut-off |
|---|---|---|---|---|---|---|
| Our model | ||||||
| Training set | 0.811 (0.781–0.841) | 0.573 (0.524–0.623) | 0.890 (0.858–0.923) | 0.849 (0.805–0.893) | 0.660 (0.618–0.703) | 0.507 |
| Testing set | 0.713 (0.656–0.769) | 0.478 (0.401–0.555) | 0.814 (0.753–0.875) | 0.726 (0.642–0.811) | 0.602 (0.536–0.668) | – |
| AJCC system | ||||||
| Training set | 0.696 (0.640–0.751) | 0.547 (0.470–0.623) | 0.795 (0.732–0.858) | 0.733 (0.654–0.812) | 0.629 (0.562–0.697) | 0.633 |
| Testing set | 0.651 (0.613–0.689) | 0.500 (0.450–0.550) | 0.722 (0.675–0.768) | 0.659 (0.604–0.713) | 0.574 (0.528–0.619) | – |
AJCC, the American Joint Commission on Cancer; AUC, area under the receiver operating characteristic curve; PPV, positive predictive value; NPV, negative predictive value.
Comparison of the XGBoost model and AJCC staging system
To evaluate the advantages of the XGBoost model, we compared it to the AJCC staging system (7th edition). According to Table 2, the cutoff value of the AJCC system was 0.633, and it had an AUC of 0.696 (95% CI: 0.640–0.751), a specificity of 0.795 (95% CI: 0.732–0.858), and a PPV of 0.733 (95% CI: 0.654–0.812) in the training set. In the testing set, the AUC, specificity, and PPV of the AJCC system were 0.651 (95% CI: 0.613–0.689), 0.722 (95% CI: 0.675–0.768), and 0.659 (95% CI: 0.604–0.713), separately. Compared with the AJCC staging system, the XGBoost model had a better predictive performance in terms of the AUC, specificity, and PPV, both in the training and testing sets.
Discussion
At present, there is a pressing need to accurately predict the 5-year survival of elderly ICC patients after surgery, as it may affect treatment planning and patient decision-making. This study established and validated an XGBoost model to predict the postoperative 5-year survival of elderly ICC patients. This XGBoost model achieved an AUC of 0.811, specificity of 0.890, and a PPV of 0.849 in the training set, and an AUC of 0.713, specificity of 0.814, and a PPV of 0.726 in the internal validation set, indicating some predictive capacity. In contrast to the AJCC system (7th edition), our model exhibited a better predictive performance, which may be employed to predict 5-year survival in elderly patients with ICC after surgery, and may subsequently be used to promote individualized treatment.
As far as we know, this study developed a prognostic model for elderly ICC patients after surgery based on an ML algorithm in a large-scale cohort for the first time. Previous studies focused on the survival prediction of ICC patients undergoing surgery (15,18,19). Hyder et al. (18) proposed a nomogram to estimate the long-term survival of ICC patients following resection, with a C-index of 0.692. The C-index of the nomogram developed by Wang et al. (19) for predicting survival among patients with ICC receiving partial hepatectomy was 0.74. Another prognostic tool on the basis of the metro-ticket paradigm was used to predict the 5-year overall survival following liver resection for ICC, with C-indexes of 0.725 and 0.724 in the training and validation sets, respectively (15). Regarding older adults suffering from ICC, the predictive nomogram for OS by Zhu et al. (20) had C-indexes of 0.725 and 0.724 in the training and validation cohorts, respectively. Since most ICC patients are elderly, the number of elderly patients diagnosed with ICC is growing, and surgery remains the optimal treatment for ICC patients (2,7,27). Therefore, we focused on elderly ICC patients undergoing surgery. Notably, prior prediction models were developed with Cox regression or logistic regression (conventional algorithms) which are replaceable by more advanced algorithms. Recently, artificial intelligence models based on ML algorithms have attracted increasing attention in clinical practice. Most models, such as random forests (RF), support vector machines (SVM), Bayesian networks and XGBoost, are developed on the basis of traditional ML algorithms (28). XGBoost, a typical boosting algorithm, can adjust the errors generated by existing models, which is efficient, flexible and portable (22). These advantages ensure the superior performance of XGBoost to other models in ML competitions (29). Thus, an XGBoost model was constructed in this study and showed some predictive ability in both the training and validation sets. Its performance in predicting the 5-year survival after surgery in elderly ICC patients was also confirmed by comparison with the AJCC system. Likewise, Ali et al. (30) reported that the AJCC (7th edition) did not make a precise prediction for survival in ICC patients.
In the current study, AJCC stage, multiple (satellite) tumors/nodules, tumor-node-metastasis (TNM) stage, more than one lobe invaded, direct invasion of adjacent organs, tumor size, and radiotherapy were relatively important features for survival prediction in elderly ICC patients after surgery. The effect of tumor number on the postoperative survival of ICC patients was corroborated by prior studies, and multiple (satellite) tumors/nodules were associated with a greater risk of death in ICC (31-34). TNM stage was also identified as an independent predictor for survival in patients with ICC undergoing surgery and in elderly ICC patients (20,35). Consistently, direct invasion of adjacent organs was related to 5-year survival following ICC resection (36). Greater tumor size was related to malignant pathological factors, like worse tumor differentiation and vascular invasion, and tumor size was an independent prognostic factor for solitary ICC following resection (6,37,38). In this study, tumor size and age were expressed as continuous variables rather than categorical variables, which meant that 5-year survival could be predicted for a specific patient instead of a group of patients, indicating personalized prediction. Additionally, all of the variables in the basic characteristics of elderly ICC patients were considered in the survival prediction, which may help to provide accurate predictions.
Several limitations need to be considered in interpreting our results. Firstly, this was a retrospective study. Missing data could not be obtained at the time of the study. Some variables that may affect prognosis, such as nutritional status and comorbidities, were not available in the SEER database. Secondly, the prediction model had some predictive ability based on its AUC, specificity and PPV in the training and testing sets despite low sensitivity values, which necessitates more studies to improve the model in 5-year survival prediction of elderly ICC patients after surgery. Thirdly, although our model was developed and internally verified in the American population, external validation is required for applicability assessment.
Conclusions
The XGBoost model was developed to predict the postoperative 5-year survival of elderly ICC patients and exhibited some predictive performance based on the SEER database. Compared with the AJCC staging system, this model had a better predictive ability. Future studies are warranted to externally validate the applicability of our model.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1238/rc
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1238/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268-89. [Crossref] [PubMed]
- Zhang H, Yang T, Wu M, et al. Intrahepatic cholangiocarcinoma: Epidemiology, risk factors, diagnosis and surgical management. Cancer Lett 2016;379:198-205. [Crossref] [PubMed]
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int 2019;39:19-31. [Crossref] [PubMed]
- Bertuccio P, Malvezzi M, Carioli G, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol 2019;71:104-14. [Crossref] [PubMed]
- de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol 2011;29:3140-5. [Crossref] [PubMed]
- Mavros MN, Economopoulos KP, Alexiou VG, et al. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg 2014;149:565-74. [Crossref] [PubMed]
- Cillo U, Fondevila C, Donadon M, et al. Surgery for cholangiocarcinoma. Liver Int 2019;39:143-55. [Crossref] [PubMed]
- Vitale A, Spolverato G, Bagante F, et al. A multi-institutional analysis of elderly patients undergoing a liver resection for intrahepatic cholangiocarcinoma. J Surg Oncol 2016;113:420-6. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Spolverato G, Yakoob MY, Kim Y, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2015;22:4020-8. [Crossref] [PubMed]
- Bagante F, Spolverato G, Merath K, et al. Intrahepatic cholangiocarcinoma tumor burden: A classification and regression tree model to define prognostic groups after resection. Surgery 2019;166:983-90. [Crossref] [PubMed]
- Raoof M, Dumitra S, Ituarte PHG, et al. Development and Validation of a Prognostic Score for Intrahepatic Cholangiocarcinoma. JAMA Surg 2017;152:e170117. [Crossref] [PubMed]
- Dhanasekaran R, Hemming AW, Zendejas I, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep 2013;29:1259-67. [Crossref] [PubMed]
- Sahara K, Tsilimigras DI, Mehta R, et al. A novel online prognostic tool to predict long-term survival after liver resection for intrahepatic cholangiocarcinoma: The "metro-ticket" paradigm. J Surg Oncol 2019;120:223-30. [Crossref] [PubMed]
- Tian M, Liu W, Tao C, et al. Prediction of overall survival in resectable intrahepatic cholangiocarcinoma: IS(ICC) -applied prediction model. Cancer Sci 2020;111:1084-92. [Crossref] [PubMed]
- Park HJ, Park B, Park SY, et al. Preoperative prediction of postsurgical outcomes in mass-forming intrahepatic cholangiocarcinoma based on clinical, radiologic, and radiomics features. Eur Radiol 2021;31:8638-48. [Crossref] [PubMed]
- Hyder O, Marques H, Pulitano C, et al. A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014;149:432-8. [Crossref] [PubMed]
- Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013;31:1188-95. [Crossref] [PubMed]
- Zhu H, Ji K, Wu W, et al. Describing Treatment Patterns for Elderly Patients with Intrahepatic Cholangiocarcinoma and Predicting Prognosis by a Validated Model: A Population-Based Study. J Cancer 2021;12:3114-25. [Crossref] [PubMed]
- Chen, T. and C. Guestrin. XGBoost: A Scalable Tree Boosting System. in the 22nd ACM SIGKDD International Conference. 2016.
- Ogunleye A, Wang QG. XGBoost Model for Chronic Kidney Disease Diagnosis. IEEE/ACM Trans Comput Biol Bioinform 2020;17:2131-40.
- Liu P, Fu B, Yang SX, et al. Optimizing Survival Analysis of XGBoost for Ties to Predict Disease Progression of Breast Cancer. IEEE Trans Biomed Eng 2021;68:148-60. [Crossref] [PubMed]
- Jiang J, Pan H, Li M, et al. Predictive model for the 5-year survival status of osteosarcoma patients based on the SEER database and XGBoost algorithm. Sci Rep 2021;11:5542. [Crossref] [PubMed]
- Huang Z, Hu C, Chi C, et al. An Artificial Intelligence Model for Predicting 1-Year Survival of Bone Metastases in Non-Small-Cell Lung Cancer Patients Based on XGBoost Algorithm. Biomed Res Int 2020;2020:3462363. [Crossref] [PubMed]
- Duggan MA, Anderson WF, Altekruse S, et al. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am J Surg Pathol 2016;40:e94-e102. [Crossref] [PubMed]
- Bartsch F, Baumgart J, Tripke V, et al. Resection of intrahepatic cholangiocarcinoma in elderly patients - is it reasonable? BMC Surg 2019;19:157. [Crossref] [PubMed]
- Kazem MA. Predictive models in cancer management: A guide for clinicians. Surgeon 2017;15:93-7. [Crossref] [PubMed]
- Cheng X, Li J, Xu T, et al. Predicting Survival of Patients With Rectal Neuroendocrine Tumors Using Machine Learning: A SEER-Based Population Study. Front Surg 2021;8:745220. [Crossref] [PubMed]
- Ali SM, Clark CJ, Mounajjed T, et al. Model to predict survival after surgical resection of intrahepatic cholangiocarcinoma: the Mayo Clinic experience. HPB (Oxford) 2015;17:244-50. [Crossref] [PubMed]
- Nathan H, Aloia TA, Vauthey JN, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol 2009;16:14-22. [Crossref] [PubMed]
- Uenishi T, Ariizumi S, Aoki T, et al. Proposal of a new staging system for mass-forming intrahepatic cholangiocarcinoma: a multicenter analysis by the Study Group for Hepatic Surgery of the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepatobiliary Pancreat Sci 2014;21:499-508. [Crossref] [PubMed]
- Jiang W, Zeng ZC, Tang ZY, et al. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol 2011;22:1644-52. [Crossref] [PubMed]
- Aherne EA, Pak LM, Goldman DA, et al. Intrahepatic cholangiocarcinoma: can imaging phenotypes predict survival and tumor genetics? Abdom Radiol (NY) 2018;43:2665-72. [Crossref] [PubMed]
- Lang H, Baumgart J, Heinrich S, et al. Liver Resection for Intrahepatic Cholangiocarcinoma-Single-Center Experience with 286 Patients Undergoing Surgical Exploration over a Thirteen Year Period. J Clin Med 2021;10:3559. [Crossref] [PubMed]
- Bagante F, Spolverato G, Weiss M, et al. Defining Long-Term Survivors Following Resection of Intrahepatic Cholangiocarcinoma. J Gastrointest Surg 2017;21:1888-97. [Crossref] [PubMed]
- Spolverato G, Ejaz A, Kim Y, et al. Tumor size predicts vascular invasion and histologic grade among patients undergoing resection of intrahepatic cholangiocarcinoma. J Gastrointest Surg 2014;18:1284-91. [Crossref] [PubMed]
- Kong J, Cao Y, Chai J, et al. Effect of Tumor Size on Long-Term Survival After Resection for Solitary Intrahepatic Cholangiocarcinoma. Front Oncol 2020;10:559911. [Crossref] [PubMed]
(English Language Editor: A. Kassem)