
Pembrolizumab combined with paclitaxel and platinum as induction therapy for locally advanced esophageal squamous cell carcinoma: a retrospective, single-center, three-arm study
Highlight box
Key findings
• Pembrolizumab combined with paclitaxel and platinum can be used as induction therapy for locally advanced ESCC and lays the foundation for subsequent surgery or radiotherapy.
What is known and what is new?
• Pembrolizumab confers benefits in prolonging PFS and OS for patients with ESCC.
• Radical surgery for patients with locally advanced ESCC could be taken after induction therapy based on pembrolizumab, which has a higher ORR, MPR rate, pCR rate.
What is the implication, and what should change now?
• Pembrolizumab worth further exploration in the field of immunotherapy for locally advanced ESCC.
Introduction
Esophageal cancer has the seventh highest incidence of all malignancies worldwide and has the sixth worst prognosis because of its aggressiveness and poor survival rates (1). The incidence and mortality rates for esophageal cancer in China account for more than half those worldwide. More than 90% of Chinese esophageal cancer patients have esophageal squamous cell carcinoma (ESCC), and 70% of patients are diagnosed at an advanced stage and require comprehensive treatment (2). The CROSS and NEOCRTEC-5010 trials laid the foundation for neoadjuvant chemoradiotherapy (nCRT) as the standard treatment modality for locally advanced esophageal cancer (3,4). However, the rise of immune checkpoint inhibitors (ICIs) has provided a new treatment modality for locally advanced esophageal cancer.
Programmed cell death-1 (PD-1) is an inhibitory costimulatory molecule. PD-1 is mainly expressed in cluster of differentiation (CD)4, CD8 T cells, natural killer cells, B cells, and monocytes when these cells are activated (5). Programmed cell death-ligand 1 (PD-L1) is a transmembrane protein that is one of the primary ligands of PD-1 and is expressed in many types of tumor cells and antigen-presenting cells (6). PD-L1 can combine with PD-1 to reduce the proliferation of PD-1-positive cells, inhibit their cytokine secretion, and induce apoptosis (7,8). Thus, PD-L1 can attenuate the host immune response to tumor cells. ICIs targeting PD-1/PD-L can permit the T-cell-mediated death of tumor cells by blocking the PD-1/PD-L1 pathway. Recently, immunotherapy based on the inhibition of PD-1/PD-L1 has achieved encouraging therapeutic results in locally advanced ESCC, and the application of induction therapy in locally advanced ESCC deserves in-depth study and exploration.
Currently, the standard treatment for locally advanced ESCC is still based on nCRT/chemotherapy combined with surgery, and the mode of clinical investigation of PD-1 inhibitors combined with neoadjuvant therapy is still based on concurrent chemoradiotherapy. Pembrolizumab is a humanized monoclonal immunoglobulin G4 antibody that targets against PD-1 and is a PD-1 inhibitor. The KEYSTONE-001 study showed that the use of pembrolizumab in combination with chemotherapy as neoadjuvant therapy for resectable ESCC had a high major pathological response (MPR) rate, pathological complete response (pCR) rate, and R0 resection rate, and acceptable tolerability (9). In the KEYNOTE-590 study, pembrolizumab plus chemotherapy significantly improved overall survival (OS) and the objective response rate (ORR) compared to chemotherapy alone as a first-line treatment for patients with locally advanced and metastatic ESCC (10). Thus, pembrolizumab was approved by the Food and Drug Administration (FDA) as a first-line treatment for patients with locally advanced and metastatic ESCC.
To date, no retrospective studies of pembrolizumab combined with chemotherapy and surgical treatment for locally advanced or metastatic potentially resectable ESCC have been conducted. Immunotherapy based on pembrolizumab would make a chance for patients with locally advanced and potentially resectable ESCC to take radical surgery. So the present study sought to evaluate the efficacy and safety of pembrolizumab combined with paclitaxel and platinum at our center, and focused on immune-related response evaluation and postoperative pathology results. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1196/rc).
Methods
Participants and treatments
We collected the clinical data of clinical stage III–IV ESCC patients at the Fujian Provincial Hospital from August 2019 to June 2022. To be eligible for inclusion in this study, patients had to meet the following inclusion criteria: (I) have been diagnosed by pathology or cytology; (II) have clinical stage III or stage IV ESCC according to the tumor, node, metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC; 8th edition), and have a chance of resectability after induction therapy; (III) have received ≥4 cycles of pembrolizumab combined with paclitaxel and platinum; and (IV) have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0–2 before treatment with pembrolizumab. Patients were excluded from the study if they met any of the following exclusion criteria: (I) had ESCC combined with other tumors; (II) had previously undergone chemotherapy or radiotherapy before treatment with pembrolizumab; (III) had a response that could not be evaluated after treatment with pembrolizumab; and/or (IV) had severe functional damage to important internal organs.
Research design: The research study was divided into 3 stages. In the first stage, the patients received 4 cycles of pembrolizumab (200 mg on day 1) in combination with paclitaxel (135 mg/m2 on day 1) and nedaplatin (100 mg/m2 on day 1) according to standard treatment and underwent imaging to assess efficacy. In the second stage, the patients were divided into the operation (group A), radical radiotherapy (group B), and observation (group C) groups according to their treatment wishes, and efficacy was assessed at the end of the treatment. In the third stage, the patients received maintenance therapy with pembrolizumab every 3–4 weeks for at least 1 year. Treatment was discontinued if a participant had progressive disease (PD), unacceptable toxicity, or requested discontinuation. The evaluation was performed at the last follow-up date, or when PD was found, or death occurred.
The surgical approach in group A was video-assisted thoracoscopic esophagectomy and esophagogastric cervical anastomosis, and 2/3 field lymph nodes were dissected. The total dose of radiotherapy in group B was 40–45 Gy, which was administered in 20–22 fractions (5 fractions per week of 2–2.12 Gy per fraction). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Research Ethics Board of the Fujian Provincial Hospital (No. 2021-031-04). Individual consent for this retrospective analysis was waived.
Clinicopathological characteristics
The medical records of all the patients were reviewed retrospectively, and the clinical characteristic data were gathered (including sex, age, tumor metastasis status, clinical staging, PS score, expression of PD-1, and surgical outcome). To determine the expression of PD-L1, hematoxylin and eosin (H&E) staining of the pathological sections of the tumors or metastatic lymph nodes was performed. PD-L1 was evaluated by immunohistochemistry staining using the SP142 (Burning Rock Biotech, Guangzhou, China) anti-PD-L1 antibody. The percentage of tumor cells positive for PD-L1 was determined using the combined positive score (CPS) (11). CPS is the ratio of the number of all PD-L1-expressing cells (tumor cells, lymphocytes, and macrophages) to the number of all tumor cells. The tumor cells had to show partial or complete membrane staining (≥1+) to be counted as “stained”, while the immune cells were counted if there was any staining.
Follow-up protocol and efficacy assessment
For the follow-up, all the patients’ electronic medical records were collected or telephone interviews were conducted. The follow-up data included clinicopathological characteristics, treatment-related adverse events (AEs), disease progression, and survival. The tumor assessment was performed before the first dose of pembrolizumab, and the response evaluation was performed by a computed tomography (CT) scan following every 2 cycles of treatment.
Efficacy was assessed at the first and second stages, and the last follow-up date. The therapeutic response was defined according to the immune-related Response Evaluation Criteria in Solid Tumors (iRECIST) (12). Each response was categorized as immune partial response (iPR), immune complete response (iCR), immune stable disease (iSD), or immune progressive disease (iPD). The R0 resection, MPR, and pCR rates were also calculated for the patients who underwent surgery.
In addition, the efficacy parameters included the ORR, progression-free survival (PFS), and OS. The ORR was defined as CR plus PR. The disease control rate (DCR) was defined as the ORR plus stable disease (SD). PFS was defined as the time from the first dose of pembrolizumab therapy to the date of disease progression or death. OS was defined as the time from the first dose of pembrolizumab to death from any cause. Efficacy assessments were made every 2 cycles. The follow-up deadline was June 30, 2022, and the median follow-up time was 14 months.
Safety assessment
During each medical visit, routine physical and laboratory examinations (mainly including hematological, biochemical, and thyroid function tests) were performed on all the patients. All the AEs were graded according to the Common Terminology Criteria for Adverse Events version 5.0.
Statistical analysis
Most of the analyses in this study are descriptive statistics, and the enumeration data are presented as the number (n) and percentage (%).
The data analysis was performed using SPSS 26 Statistics software (IBM, Herrenberg, Germany). Kaplan-Meier survival curves were generated and analyzed using GraphPad Prism version 9.3 (GraphPad Software Inc., La Jolla, CA, USA). Fisher’s exact test was used to compare the differences between groups A and B in terms of the incidence of AEs. A P value of 0.05 was considered statistically significant.
Results
Baseline characteristics of all participants
A total of 39 patients (33 male and 6 female) with a median age of 64 years (42–80 years) were included in this study. Among the patients, 13 had a PS score of 2, and 26 had a PS score of 0–1. In terms of T stage, 34 patients were diagnosed with stage T3, and 5 patients were diagnosed with stage T4. In terms of clinical stage, 30 patients were diagnosed with stage III, and 9 patients were diagnosed with stage IV. The patients received a median of 8 cycles (range: 4–32 cycles) of pembrolizumab therapy. The baseline characteristics of the 39 patients after induction therapy are shown in Table 1.
Table 1
| Characteristic | Group A (n=22) | Group B (n=9) | Group C (n=8) |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 19 (86.4) | 8 (88.9) | 6 (75.0) |
| Female | 3 (13.6) | 1 (11.1) | 2 (25.0) |
| Median age [range] (years) | 57 [50–72] | 70 [42–80] | 69 [56–80] |
| ECOG PS, n (%) | |||
| 0–1 | 16 (72.7) | 4 (44.4) | 6 (75.0) |
| 2 | 6 (27.3) | 5 (55.6) | 2 (25.0) |
| Tumor location, n (%) | |||
| Up | 1 (4.5) | 1 (11.1) | 1 (12.5) |
| Middle | 15 (68.2) | 8 (88.9) | 6 (75.0) |
| Lower | 6 (27.3) | 0 (0) | 1 (12.5) |
| cT category (AJCC-TNM8th), n (%) | |||
| T3 | 19 (86.4) | 8 (88.9) | 7 (87.5) |
| T4 | 3 (13.6) | 1 (11.1) | 1 (12.5) |
| Stage (AJCC-TNM8th), n (%) | |||
| III | 17 (77.3) | 8 (88.9) | 5 (62.5) |
| IV | 5 (22.7) | 1 (11.1) | 3 (37.5) |
| Period of pembrolizumab therapy, cycles | |||
| Median [range] | 8 [4–26] | 8 [4–20] | 10 [4–32] |
| PD-L1 CPS, n (%) | |||
| <10 | 20 (90.9) | 5 (55.6) | 4 (50.0) |
| ≥10 | 2 (9.1) | 4 (44.4) | 4 (50.0) |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; AJCC-TNM: tumor, node, metastasis staging system of the American Joint Committee on Cancer; PD-L1, programmed cell death-ligand 1; CPS, combined positive score.
Efficacy assessment
Efficacy was evaluated after systemic induction therapy with 4 cycles of pembrolizumab combined with chemotherapy in the first stage. Of the patients, 34 (87.2%) achieved iPR, and 5 (12.8%) achieved iSD. The ORR was 87.2% (34/39), and the DCR was 100%. Of the patients, 35 met the surgically resectable conditions after induction therapy, and the conversion rate was 89.7%. However, only 22 patients elected to undergo surgery in the second stage (group A), while 9 patients received radical radiotherapy because they refused surgery, or had an unresectable tumor (group B), and 8 patients did not undergo radical therapy and received maintenance treatment with pembrolizumab (group C).
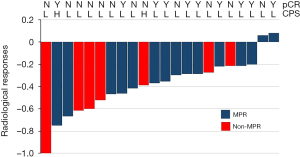
In the second stage, all the patients in group A underwent R0 resection. The surgical data are set out in Table 2. The median operation time was 362.5 min, the median blood loss during the operation was 150 mL, the median number of lymph node dissections was 33, and the median length of postoperative hospital stay was 12 days. Postoperative pathology showed that the MPR and pCR rates were 68.2% (15/22) and 45.5% (10/22), respectively. One patient had lymph node metastasis confirmed by postoperative pathology, and disease progression occurred in the sixth month after surgery. Downstaging occurred in 81.8% (18/22) of patients after pembrolizumab treatment. In addition, the subgroup analyses of the MPR and pCR showed that MPR or pCR occurred regardless of PD-L1 expression status (Figure 1).
Table 2
| Characteristic | Value |
|---|---|
| Median operation time [range] (min) | 362.5 [200–470] |
| Median blood loss [range] (mL) | 150 [100–500] |
| Number of LN dissection | |
| Median [range] | 33 [20–52] |
| Postoperative hospital stay days | |
| Median [range] | 12 [10–36] |
| Residual tumor, n (%) | |
| R0 | 22 (100) |
| R1–2 | 0 (0) |
| ypStage (AJCC-TNM8th), n (%) | |
| ypT0 (pCR) | 10 (45.5) |
| ypStage I | 16 (72.7) |
| ypStage II | 2 (9.1) |
| ypStage III | 4 (18.2) |
| Pathological response, n (%) | |
| pCR | 10 (45.5) |
| MPR | 15 (68.2) |
LN, lymph node; AJCC-TNM: tumor, node, metastasis staging system of the American Joint Committee on Cancer; pCR, pathological complete response; MPR, major pathological response.
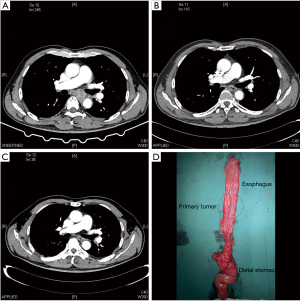
The symptoms of several patients who had an inability to eat or dysphagia were significantly relieved after treatment. The ECOG PS score of these patients was 3 before treatment, but 2 of the patients improved rapidly after treatment. Another 2 patients with dysphagia completed induction therapy in the first stage and surgery in the second stage with enteral nutrition support, and both had postoperative pathology results suggestive of pCR (the radiological results confirmed that the tumor shrank significantly after 2 cycles of neoadjuvant therapy). Radical surgery was performed after 4 cycles of neoadjuvant therapy, and no obvious tumor residue was observed by the naked eye (Figure 2).
In group B, 9 patients completed the second-stage radical treatment, and all achieved SD in the efficacy evaluations and then shifted to third-stage maintenance consolidation therapy.
In the third stage, the patients were able to maintain good compliance to undergo the treatment as scheduled, which shows the advantages of the policy for the drug delivery of pembrolizumab. Efficacy was assessed at the last follow-up date. Follow-up information was available for all patients, who had a median follow-up of 14 months (3–34 months).
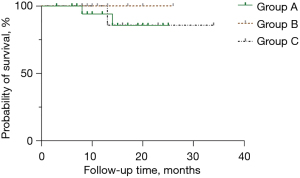
In group A, 2 patients had PD, and 1 died due to the tumor. In group C, 1 patient died due to PD. The median OS and PFS in each group were not reached. The Kaplan-Meier curves for PFS are presented in Figure 3.
Safety assessment
No fatal treatment-related AEs occurred during the period of immunotherapy or follow-up. The common AEs were mainly grade 1–2, and mostly included rash and hypothyroidism. The incidence of severe AEs (grade ≥3) was 15.4% (6/39). One patient stopped treatment after 4 cycles of induction therapy due to a severe rash. The main chemotherapy-related AEs were hematologic adverse effects, including a white blood cell count decrease (33.3%, 13/39) and a platelet count decrease (17.9%, 7/39). The only grade 3 AE in group A was anastomotic leakage, and because the leak was <5 mm and the patient did not experience symptoms of systemic infection, the patient resumed feeding after 4 weeks of nutritional support combined with local wound dressing changes. In the radiotherapy group, 1 grade 3 AE was rash. The AEs in groups A and B are shown in Table 3, and there was no significant difference in the incidence of AEs between groups A and B. All the AEs were manageable, and no AE-related deaths occurred.
Table 3
| Adverse events | Group A | Group B | P | |||
|---|---|---|---|---|---|---|
| Grade 1–2, n (%) | Grade 3, n (%) | Grade 1–2, n (%) | Grade 3, n (%) | |||
| Decreased white blood cell count | 7 (31.8) | 1 (4.5) | 3 (33.3) | 0 (0.0) | >0.999 | |
| Anemia | 3 (13.6) | 0 (0.0) | 1 (11.1) | 0 (0.0) | >0.999 | |
| Decreased platelet count | 4 (18.2) | 0 (0.0) | 2 (22.2) | 0 (0.0) | >0.999 | |
| Gastrointestinal disorders | 10 (45.5) | 0 (0.0) | 4 (44.4) | 0 (0.0) | 0.695 | |
| Creatinine increased | 2 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 | |
| Pneumonia | 1 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 | |
| Transaminases increased | 4 (18.2) | 0 (0.0) | 1 (11.1) | 0 (0.0) | >0.999 | |
| Heart failure | 1 (4.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | >0.999 | |
| Skin disorders | 3 (13.6) | 0 (0.0) | 0 (0.0) | 1 (11.1) | >0.999 | |
| Hypothyroidism | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | >0.999 | |
| Anastomotic leakage | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | >0.999 | |
| Gastrostomy | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | >0.999 | |
| Jejunostomy | 0 (0.0) | 1 (4.5) | 0 (0.0) | 0 (0.0) | >0.999 | |
Discussion
This study sought to investigate the efficacy and safety of pembrolizumab combined with paclitaxel and platinum as induction therapy for locally advanced ESCC. This induction therapy showed a powerful conversion rate to surgical resection for patients. Additionally, clinical benefits were observed in patients who refused surgical therapy and sought non-invasive radical radiotherapy or even maintenance therapy with pembrolizumab, which allowed this study to explore the effects of pembrolizumab combination therapy in different clinical scenarios.
The CROSS study represents a milestone in the treatment of esophageal cancer and established nCRT as the standard treatment for locally advanced esophageal cancer. The NEOCRTE5010 clinical trial focused on Chinese ESCC patients (4), and showed that preoperative nCRT contributed to tumor downstaging, increased the R0 resection rate, and improved patient prognosis. However, tissue fibrosis caused by concurrent chemoradiotherapy and other side effects could affect perioperative safety, intraoperative dissection and separation, and the blood supply to the esophagogastric anastomosis. This effect may mask or reduce the survival benefits derived from nCRT. Thus, more scholars have begun to explore the use of immunotherapy in the neoadjuvant field of esophageal cancer.
PD-1/PD-L1 inhibitors restore the antitumor activity of T cells by blocking the signaling pathway between PD-1 and PD-L1 (13), and are gradually being used at various stages of tumor progression. The KEYNOTE-181 study compared pembrolizumab monotherapy with chemotherapy in locally advanced or metastatic esophageal cancer (14), and the results showed that pembrolizumab monotherapy significantly improved OS compared to chemotherapy [P=0.004, hazard ratio (HR) =0.64, 95% confidence interval (CI): 0.46–0.90], which established immunotherapy as a second-line treatment in advanced esophageal cancer.
The KEYNOTE-590 study enrolled patients with locally advanced, unresectable, or metastatic esophageal cancer (including adenocarcinoma, squamous cell carcinoma, and Siewert type I adenocarcinoma of the esophagogastric junction) (10), and the results showed that pembrolizumab combined with cisplatin and 5-fluorouracil (the CF regimen) improved PFS compared to chemotherapy (P<0.001, HR =0.65, 95% CI: 0.54–0.70) and significantly reduced the risk of disease progression and death. Thus, pembrolizumab was approved by the FDA in March 2021 for the first-line treatment of locally advanced or metastatic esophageal cancer.
In addition, given the recent clinical benefits of paclitaxel in head and neck squamous cell carcinoma and ESCC populations, it has been conjectured that paclitaxel sensitizes tumor cells to T cells, disturbs immunosuppressive mechanisms, and has a strong synergistic effect with PD-1/PD-L1 inhibitors. Thus, in 2 prospective, single-arm, phase-II clinical trials, TD-NICE (15) and KEYSTONE-001 (9), researchers used PD-1 inhibitors in combination with paclitaxel and platinum in patients with resectable locally advanced ESCC. The results showed that the R0 resection rates were 97.2% and 100%, the MPR rates were 72% and 72.4%, and the pCR rates were 50% and 41.1%, respectively. These 2 small prospective studies showed the potential benefit of the application of PD-1 inhibitors and paclitaxel in the treatment of locally advanced ESCC.
In the present study, we mainly explored individualized patterns of combination therapy with PD-1 inhibitors and retrospectively analyzed the data of 39 patients with locally advanced ESCC who received combination therapy with pembrolizumab in different clinical scenarios between 2019 and 2022 at the Fujian Provincial Hospital. The results showed that pembrolizumab combined with paclitaxel and nedaplatin achieved an impressive therapeutic effect in locally advanced ESCC with an ORR of 87.1% and a DCR of 100%. After induction therapy with pembrolizumab plus paclitaxel, the rate of surgical conversion was 89.7%. However, while only 56.4% of the patients ultimately underwent surgery for various reasons, all achieved R0 resection, and the pCR and MPR rates were similar to those reported in other studies. The patients who underwent radical radiotherapy also achieved excellent disease control. Among the patients who did not receive radical treatment, only 1 experienced disease progression while on maintenance therapy and died. Only 6 grade 3 AEs were observed, and all the AEs were consistent with those previously reported in clinical trials. Due to the short follow-up time, the median OS and PFS in each group were not reached. However, 23 patients were still alive after >12 months of follow-up, and the longest PFS time was 34 months.
This study suggests that the use of pembrolizumab combined with paclitaxel and platinum as induction therapy for locally advanced and potentially resectable ESCC results in a powerful conversion rate to surgical resection for patients. However, only half of the patients underwent surgical treatment because of their age, psychological quality, or family opposition, which is consistent with the current clinical treatment. Nevertheless, a clinical benefit was observed in patients who refused surgical therapy and sought non-invasive radical radiotherapy or even maintenance therapy with pembrolizumab, which allowed this study to explore the significance of pembrolizumab combination therapy in different clinical scenarios. However, a longer observation period and larger sample size are needed to confirm its efficacy and safety.
We also found that the difficulty of operation in patients was significantly reduced after pembrolizumab, which mainly manifested as significantly reduced tumor invasion, thickened and toughened tissue, but less bleeding. We considered the following possibilities: (I) after combined chemotherapy with a PD-1 inhibitor, the tumor shows centripetal shrinkage, which differs to the eccentric shrinkage of chemotherapy or chemoradiotherapy, and significantly reduces the infiltration to surrounding organs and tissues; (II) tissue spaces around the tumor are relatively loose and easy to separate; and (III) induction therapy occludes the tumor-supporting vessels and leads to less bleeding during the operation.
Combination therapy with PD-1 inhibitors results in high rates of surgical conversion; however, further research needs to be conducted to determine the benefits (if any) of PD-1 inhibitors or chemotherapy. According to this study and several previously reported small-sample phase-II prospective studies (9,16), the MPR rate was approximately 60–70% and the pCR rate was approximately 40–50%, which means that 40–50% of the patients did not benefit from treatment with PD-1 inhibitors. The selection of immunotherapy-sensitive populations is a difficult problem in clinical settings.
In TD-NICE, researchers found no obvious genes indicative of a survival benefit through next-generation sequencing screening, and that the PD-L1 CPS score was not associated with the prediction of postoperative MPR or pCR. Sharma et al. found that a proportion of patients developed primary drug resistance or did not respond to PD-1/PD-L1 inhibitors, and some responded initially but subsequently developed acquired drug resistance (17). Primary drug resistance may result from the tumor immunologic microenvironment (TIME) hindering the tumor clearance process by T cells (18). Lu et al. suggested that assessment of the TIME based on multiplex immunohistochemistry has higher accuracy in evaluating immunotherapy efficacy (19). Thus, screening the beneficiary of PD-1/PD-L1 inhibitors by checking the TIME is a research direction for immunotherapy for esophageal cancer.
The role of radiotherapy in the induction therapy of esophageal cancer has also been debated. At our center, concurrent chemoradiotherapy is applied to only 20% of patients in clinical practice due to the toxic side effects of radiotherapy limit patient acceptance. However, radiotherapy, as a powerful local treatment, has obvious mitigation advantages for large tumor foci. Thus, in several classic clinical studies, the pCR of local lesions was high, but controversy remains as to whether the control of regional lymph nodes results in any survival benefits. In the JCOG 1109NExT study (20), the pCR rate of nCRT was as high as 36.7%, but the pCR rate of the docetaxel, cisplatin, and 5-fluorouracil (DCF) group was only 18.6%. However, regardless of the OS or PFS, the DCF group maintained a better relapse-free survival benefit than concurrent chemoradiotherapy group. The JCOG 1109NExT study did not conduct a direct comparison of DCF group and concurrent chemoradiotherapy group, but the benefit of hazard ratio (HR) also confirmed the limitations of radiotherapy as a local treatment measure, and showed that benefit of systemic therapy in the induction phase contributed more to the prolongation of PFS and long-term survival.
The sequential therapy of neoadjuvant radiotherapy during the induction phase for patients who do not respond well to PD-1 inhibitors combined with chemotherapy could help improve surgical conversion rates, which is the assumption that we propose a individualized treatments. From the retrospective data, the three-arm retrospective study we designed also conformed to clinical practical application. No comparison has yet been conducted to determine the benefits of each method; however, radical treatment for patients whose tumors are unresectable or who are unwilling to undergo surgical treatment is also recommended. PD-1 inhibitors combined with chemotherapy in patients with locally advanced ESCC achieved a benefit similar to that observed in advanced ESCC. Pembrolizumab combined with paclitaxel and platinum can be used as induction therapy for patients with locally advanced and potentially resectable ESCC and lays the foundation for subsequent surgery or radiotherapy, while maintenance therapy with pembrolizumab also confers benefits in prolonging PFS and OS for patients.
Our study had some limitations. Because it was a retrospective, single-center, three-arm study with a small sample size and a short follow-up period, further confirmation of the efficacy and safety of pembrolizumab combined with neoadjuvant chemotherapy for locally advanced and potentially resectable ESCC was not possible.
Conclusions
Pembrolizumab in combination with paclitaxel and platinum as induction therapy for locally advanced and potentially resectable ESCC has a higher ORR, MPR rate, pCR rate, and surgical conversion rate, and a lower incidence of AEs, and was shown to have robust benefits in different clinical practice scenarios. This induction treatment paradigm is worth further exploration in the field of immunotherapy for locally advanced ESCC.
Acknowledgments
The authors would like to extend their sincere thanks to Mr. Peiyu Qiu from MSD Medical Affairs for his scientific comments on this manuscript.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1196/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1196/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1196/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Research Ethics Board of the Fujian Provincial Hospital (No. 2021-031-04). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer 2012;31:281-6. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Yang H, Liu H, Chen Y, et al. Long-term Efficacy of Neoadjuvant Chemoradiotherapy Plus Surgery for the Treatment of Locally Advanced Esophageal Squamous Cell Carcinoma: The NEOCRTEC5010 Randomized Clinical Trial. JAMA Surg 2021;156:721-9. [Crossref] [PubMed]
- Larsson M, Shankar EM, Che KF, et al. Molecular signatures of T-cell inhibition in HIV-1 infection. Retrovirology 2013;10:31. [Crossref] [PubMed]
- Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793-800. [Crossref] [PubMed]
- Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020;10:727-42. [PubMed]
- Zhou RM, Li Y, Wang N, et al. Association of programmed death-1 polymorphisms with the risk and prognosis of esophageal squamous cell carcinoma. Cancer Genet 2016;209:365-75. [Crossref] [PubMed]
- Shang X, Zhao G, Liang F, et al. Safety and effectiveness of pembrolizumab combined with paclitaxel and cisplatin as neoadjuvant therapy followed by surgery for locally advanced resectable (stage III) esophageal squamous cell carcinoma: a study protocol for a prospective, single-arm, single-center, open-label, phase-II trial (Keystone-001). Ann Transl Med 2022;10:229. [Crossref] [PubMed]
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 2021;398:759-71. [Crossref] [PubMed]
- Kulangara K, Zhang N, Corigliano E, et al. Clinical Utility of the Combined Positive Score for Programmed Death Ligand-1 Expression and the Approval of Pembrolizumab for Treatment of Gastric Cancer. Arch Pathol Lab Med 2019;143:330-7. [Crossref] [PubMed]
- Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143-52. [Crossref] [PubMed]
- Maimela NR, Liu S, Zhang Y. Fates of CD8+ T cells in Tumor Microenvironment. Comput Struct Biotechnol J 2019;17:1-13. [Crossref] [PubMed]
- Kojima T, Shah MA, Muro K, et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 2020;38:4138-48. [Crossref] [PubMed]
- Yan X, Duan H, Ni Y, et al. Tislelizumab combined with chemotherapy as neoadjuvant therapy for surgically resectable esophageal cancer: A prospective, single-arm, phase II study (TD-NICE). Int J Surg 2022;103:106680. [Crossref] [PubMed]
- Ma J, Zhang J, Yang Y, et al. Camrelizumab combined with paclitaxel and nedaplatin as neoadjuvant therapy for locally advanced esophageal squamous cell carcinoma (ESPRIT): A phase II, single-arm, exploratory research. Ann Oncol 2021;32:S1400. [Crossref]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Lei Q, Wang D, Sun K, et al. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front Cell Dev Biol 2020;8:672. [Crossref] [PubMed]
- Lu S, Stein JE, Rimm DL, et al. Comparison of Biomarker Modalities for Predicting Response to PD-1/PD-L1 Checkpoint Blockade: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:1195-204. [Crossref] [PubMed]
- Nakamura K, Kato K, Igaki H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. Jpn J Clin Oncol 2022;40:suppl 238.
(English Language Editor: L. Huleatt)


