
The role of adjuvant chemotherapy after neoadjuvant chemotherapy or chemoradiotherapy plus esophagectomy in patients with esophageal cancer: a retrospective cohort study
Highlight box
Key findings
• For patients with locally advanced esophageal cancer who have residual disease after surgery, adjuvant therapy after neoadjuvant chemoradiotherapy and surgery may be a better treatment. Adjuvant chemotherapy may improve the OS of patients with residual disease.
What is known and what is new?
• For patients with locally advanced esophageal cancer, neoadjuvant therapy plus surgery is better than single surgery;
• The results of this study showed that in addition to pCR patients, adjuvant chemotherapy had better OS after neoadjuvant therapy plus surgery.
What is the implication, and what should change now?
• Patients with residual disease after neoadjuvant therapy plus surgery should be treated with adjuvant chemotherapy.
Introduction
Cancers of the esophagus account for over 500,000 cancer deaths each year, accounting for 5.3% of all cancer deaths worldwide (1). East Asian and Middle Eastern countries, such as China, Iran, and Turkmenistan, have a higher prevalence of esophageal squamous cell carcinoma, whereas Western countries have a higher prevalence of adenocarcinoma (2).
Surgery has been the mainstay of treatment for resectable locally advanced esophageal cancer, but esophagectomy alone has a poor overall prognosis (2). The long-term overall survival (OS) rate was shown to be only 28.5–58.5%. The local and regional recurrence rate was from 17.0% to 51.8%. Even after radical surgery, the rate of distant metastasis is 31–55% (3). Therefore, many studies have begun to explore adjuvant and neoadjuvant treatment options. The following are some of the benefits of neoadjuvant treatments: Tumor downstaging makes the surgical procedure more accessible and increases the rate of radical resection; patients can complete the treatment plan more easily before surgery; before the local blood supply is destroyed, chemotherapeutic drugs can reach the target (4). The results of the OE02 trial in the UK and CROSS trial in the Netherlands showed that the OS of patients was better than that of patients undergoing surgery alone, regardless of whether preoperative neoadjuvant chemotherapy (NAC) or preoperative neoadjuvant chemoradiotherapy was used (5,6). However, the efficacy of adjuvant chemotherapy (AC) after neoadjuvant treatment plus surgery remains unclear. With the increasing experience of multimodal therapy, AC has become another interesting topic. Some studies have shown that patients with residual diseases still have a high risk of local or remote metastasis after new adjuvant treatment and surgery. Compared with single observation, adjuvant systemic therapy is expected to provide better results (7). At present, there is no guideline to recommend AC for patients with esophageal cancer after receiving neoadjuvant therapy and esophagectomy (8). Considering that patients face additional risk of adverse events, not all patients are suitable for AC.
Therefore, this study aims to find out what kind of people can benefit from AC, and provide therapeutic strategies for patients with locally advanced esophageal cancer. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1008/rc).
Methods
Patient selection
We performed a single-center retrospective study between December 2014 and November 2020. Data were collected from patients who received NAC or neoadjuvant chemoradiotherapy plus surgery with complete survival information at the First Affiliated Hospital of Zhengzhou University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University (No. 2020-KY-277). The Ethics Committee of the First Affiliated Hospital of Zhengzhou University waived the informed consent requirement for this study. The inclusion criteria were as follows: esophageal cancer was diagnosed by pathological biopsy; NAC; neoadjuvant chemoradiotherapy; R0 resection. The exclusion criteria were as follows: NAC combined with targeted therapy or immunotherapy; adjuvant radiotherapy, adjuvant chemoradiotherapy, immunotherapy, or targeted therapy; dead within 90 days of surgery; nonradical (R1/R2) resection; one cycle of postoperative chemotherapy (Figure 1).
Variables
Basic clinical data of patients including sex, age, body mass index (BMI), Charlson Comorbidity Index, tumor location, neoadjuvant therapy, Eastern Cooperative Oncology Group (ECOG) score, minimally invasive surgery, histological subtype, ypTNM, ypT, ypN, pathological response, lymph nodes dissected, and recurrence were collected. Neoadjuvant therapy included NAC and neoadjuvant chemoradiotherapy. R0 resection was defined as microscopic complete resection. Pathological complete remission (pCR) indicated that the esophagus and the resected lymph nodes had no tumor cells under microscopy. However, pCR of esophageal cancer is rare, so patients with ypTIS and ypT0 were included in this study.
Staging and follow-up
All patients were clinically staged using the eighth edition of the tumor-node-metastasis (TNM) classification system (Union for International Cancer Control and American Joint Committee on Cancer). Preoperative comorbidities were recorded in accordance with the Charlson Comorbidity Index. The intervals of patients’ first year reexaminations were 1, 3, and 6 months. Thereafter, they were reviewed every 6 months. After 2 years, patients were rechecked once a year. The follow-up included: computed tomography (CT) of the head, chest, and whole abdomen, upper gastrointestinal radiography, whole-body bone imaging, blood tumor markers, and gastroscopy when necessary. We obtained the tumor recurrence and survival status of the patients through inpatient medical records, outpatient electronic medical records, and telephone follow-up of the First Affiliated Hospital of Zhengzhou University.
Treatment strategy
The NAC was a platinum-based drug-based double-drug combination in a 21-day cycle. The main regimens included paclitaxel and cisplatin, docetaxel and nedaplatin, and docetaxel and cisplatin. A total of 73 patients received 1 cycle of NAC, 185 received 2 cycles, 22 received 3 cycles, 17 received 4 cycles, 3 received 5 cycles, and 3 received 6 cycles.
Neoadjuvant chemoradiotherapy is the simultaneous administration of radiotherapy during chemotherapy, which starts within 24 hours of the first cycle of chemotherapy, with a total dose of 40.0 Gy, 2.0 Gy/d, 5 times a week. The 3 most common surgical approaches were the minimally invasive McKeown procedure, Sweet procedure, and Ivor Lewis procedure. All operations involved 2-field or 3-field lymph node dissection. The stomach was most commonly used for esophageal reconstruction. Anastomosis included manual and mechanical anastomosis. The AC regimen was the same as the NAC regimen, with most patients receiving 4 cycles or less.
Study endpoints
The primary endpoint of this study was OS and the secondary endpoint was disease-free survival (DFS). OS was defined as the time from surgery to death or the time to last follow-up. DFS was defined as the time from surgery to recurrence. The follow-up deadline was June 2022.
Statistical analysis
The baseline data were divided into AC(+) and AC(−) groups according to whether AC was performed. The consistent data with a normal distribution and homogeneity of variance are expressed as the mean [standard deviation (SD)], and the data is statistically analyzed using t-test. Data that do not conform to the normal distribution are expressed as the median value, and a nonparametric rank sum test (Mann Whitney U test) is used. Categorical variables were analyzed by the chi-square test or Fisher’s exact test. Kaplan-Meier survival curves were used to estimate recurrence-free survival (RFS) and OS between groups. Univariate Cox analysis was first performed on all variables. Then, variables with P<0.1 in the univariate analysis and clinically meaningful variables were included in the multivariate Cox analysis. Two-sided P values <0.05 were assessed as statistically significant. Those without follow-up information were excluded. Statistical analysis was performed using SPSS 21.0.
Results
Patient population
In total, 318 patients were included in the study, comprising 226 males (71.1%) and 92 females (28.9%), with a sex ratio of 2.5:1. Patients who received AC were younger (61.7 vs. 64.4 years) than those who did not receive AC. There were 42 upper thoracic lesions (13.2%), 195 middle thoracic lesions (61.3%), and 81 lower thoracic lesions (25.5%). The Charlson Comorbidity Index scores were as follows: score 0, n=205 (64.5%), score 1, n=73 (23.0%), score ≥2, n=40 (12.6%). There were significant differences in age and lymph nodes dissected between patients who received AC and those who did not receive AC (P<0.05) (Table 1).
Table 1
| Variables | Adjuvant chemotherapy | P overall | |
|---|---|---|---|
| No (n=104) | Yes (n=214) | ||
| Sex, n (%) | 0.344 | ||
| Female | 26 (25.0) | 66 (30.8) | |
| Male | 78 (75.0) | 148 (69.2) | |
| Age (years), mean (SD) | 64.4 (7.39) | 61.7 (7.30) | 0.002 |
| BMI (kg/m2), mean (SD) | 23.5 (3.18) | 23.7 (2.77) | 0.469 |
| Charlson Comorbidity Index, n (%) | 0.060 | ||
| ≥2 | 18 (17.3) | 22 (10.3) | |
| 0 | 58 (55.8) | 147 (68.7) | |
| 1 | 28 (26.9) | 45 (21.0) | |
| Tumor location, n (%) | 0.207 | ||
| Lower | 30 (28.8) | 51 (23.8) | |
| Middle | 65 (62.5) | 130 (60.7) | |
| Upper | 9 (8.7) | 33 (15.4) | |
| Neoadjuvant therapy, n (%) | 0.094 | ||
| Chemoradiotherapy | 8 (7.7) | 7 (3.3) | |
| Chemotherapy | 96 (92.3) | 207 (96.7) | |
| ECOG, n (%) | 0.335 | ||
| 0 | 3 (2.9) | 2 (0.9) | |
| 1 | 101 (97.1) | 212 (99.1) | |
| Minimally invasive surgery, n (%) | 0.067 | ||
| Hybrid | 2 (1.9) | 4 (1.9) | |
| No | 22 (21.2) | 25 (11.7) | |
| Yes | 80 (76.9) | 185 (86.4) | |
| Histological subtype, n (%) | 1.000 | ||
| Adenocarcinoma | 14 (13.5) | 28 (13.1) | |
| Other | 0 (0.00) | 1 (0.5) | |
| Squamous cell | 90 (86.5) | 185 (86.4) | |
| ypTNM, n (%) | 0.375 | ||
| 1 | 47 (45.2) | 80 (37.4) | |
| 2 | 14 (13.5) | 44 (20.6) | |
| 3 | 34 (32.7) | 69 (32.2) | |
| 4 | 9 (8.7) | 21 (9.8) | |
| ypT, n (%) | 0.589 | ||
| T0 | 7 (6.7) | 21 (9.8) | |
| T1 | 15 (14.4) | 32 (15.0) | |
| T2 | 30 (28.8) | 53 (24.8) | |
| T3 | 39 (37.5) | 88 (41.1) | |
| T4 | 7 (6.7) | 15 (7.01) | |
| Tis | 6 (5.8) | 5 (2.3) | |
| ypN, n (%) | 0.943 | ||
| N0 | 64 (61.5) | 129 (60.3) | |
| N1 | 24 (23.1) | 46 (21.5) | |
| N2 | 12 (11.5) | 28 (13.1) | |
| N3 | 4 (3.9) | 11 (5.1) | |
| Pathological response, n (%) | 1.000 | ||
| No | 91 (87.5) | 188 (87.9) | |
| pCR | 13 (12.5) | 26 (12.1) | |
| Lymph nodes dissected (n), mean (SD) | 23.8 (11.8) | 27.3 (14.6) | 0.022 |
| Recurrence, n (%) | 0.757 | ||
| 0 | 73 (70.2) | 145 (67.8) | |
| 1 | 31 (29.8) | 69 (32.2) | |
BMI, body mass index; ECOG, eastern cooperative oncology group; pCR, pathological complete remission; yp, post-neoadjuvant pathologic; TNM, tumor-node-metastasis; T, tumor; N, node; SD, standard deviation.
Treatment
All patients received neoadjuvant therapy, 15 (4.7%) received neoadjuvant chemoradiotherapy, and 303 (95.3%) received NAC. A total of 265 (83.3%) patients underwent minimally invasive surgery, 47 (14.8%) patients underwent open surgery, and 6 (1.9%) patients underwent a hybrid (Ivor Lewis) procedure. In patients who received AC, the average number of dissected lymph nodes was 27.3. For patients without AC, the average number of dissected lymph nodes was 23.8. There were 214 (67.3%) patients who received AC and 104 (32.7%) who did not. Furthermore, pCR (yT0, yTis) was achieved in 39 cases (12.3%) and residual lesions in 279 cases (87.7%) (Table 1).
Follow-up and survival
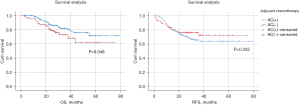
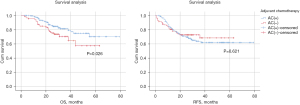
The study was followed up until June 2022, with a median follow-up of 28 months. The exact time of death for 2 of the 318 patients could not be obtained. Of the 318 patients included in this study, 100 (31.4%) had recurrence and 62 (19.5%) died during the study period. In the overall group, there was a significant difference in OS (P=0.048, Figure 2A) between patients who received AC and those who did not, but no significant difference in RFS (P=0.352, Figure 2B). The 1- and 3-year survival rates of patients who received AC in the overall group were 98.0% and 79.8%, respectively. The 1- and 3-year survival rates of patients who did not receive AC were 93.1% and 72.8%, respectively. In the subgroup analyses, excluding pCR patients, patients who received AC had significant differences in OS (P=0.026, Figure 3A) compared with those who did not, but not in RFS (P=0.621, Figure 3B). The 1- and 3-year survival rates of patients who received AC in the subgroup were 97.7% and 79.3%, respectively. The 1- and 3-year survival rates of patients who did not receive AC were 92.1% and 64.5%, respectively.
Univariate and multivariate analysis
In the univariate analysis in the overall group, sex, age, ypTNM, and ypN were significant prognostic factors of OS. In the multivariate analysis using sex, age, ypTNM, ypT, ypN, AC, and NAC as covariates, sex [hazard ratio (HR) 2.220, 95% confidence interval (CI): 1.096–4.497, P=0.027] and age (HR 2.213, 95% CI: 1.097–4.464, P=0.027) were independent prognostic factors for OS (Table 2). In the subgroup excluding pCR patients, univariate analysis showed that sex, age, ypTNM, ypN, and AC were significant prognostic factors of OS. In the multivariate analysis using sex, age, ypTNM, ypT, ypN, AC, and NAC as covariates, AC (HR 1.888, 95% CI: 1.079–3.306, P=0.026) and age (HR 2.348, 95% CI: 1.126–4.900, P=0.023) were independent prognostic factors for OS (Table 3).
Table 2
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | |||||
| Female | – | – | – | – | |
| Male | 2.413 (1.221–4.767) | 0.001 | 2.220 (1.096–4.497) | 0.027 | |
| Age | |||||
| <60 years | – | – | – | – | |
| ≥60 years | 2.417 (1.225–5.768) | 0.011 | 2.213 (1.097–4.464) | 0.027 | |
| BMI | 1.027 (0.944–1.117) | 0.540 | – | – | |
| ECOG | |||||
| 0 | – | – | – | – | |
| 1 | 20.485 (0.000–992,069.571) | 0.583 | – | – | |
| Charlson Comorbidity Index | |||||
| 0 | – | 0.875 | – | – | |
| 1 | 1.160 (0.638–2.111) | 0.626 | – | – | |
| ≥2 | 0.974 (0.435–2.182) | 0.949 | – | – | |
| Tumor location | |||||
| Upper | – | 0.331 | – | – | |
| Middle | 2.022 (0.796–5.139) | 0.139 | – | – | |
| Lower | 1.799 (0.651–4.974) | 0.258 | – | – | |
| Minimally invasive surgery | |||||
| Yes | – | 0.508 | – | – | |
| No | 1.311 (0.705–2.439) | 0.392 | – | – | |
| Hybrid | 1.880 (0.454–7.779) | 0.383 | – | – | |
| Histological subtype | |||||
| Adenocarcinoma | – | – | – | ||
| Squamous cell | 0.626 (0.325–1.207) | 0.162 | – | – | |
| Neoadjuvant therapy | |||||
| Chemoradiotherapy | – | – | – | – | |
| Chemotherapy | 3.594 (0.498–25.961) | 0.205 | 4.232 (0.553–32.379) | 0.165 | |
| ypTNM | |||||
| I | – | 0.002 | – | 0.469 | |
| II | 1.531 (0.642–3.652) | 0.337 | 1.186 (0.368–3.819) | 0.775 | |
| III | 2.766 (1.450–5.275) | 0.002 | 2.230 (0.318–15.630) | 0.420 | |
| IV | 3.867 (1.754–8.528) | 0.001 | 12.406 (0.486–316.671) | 0.128 | |
| ypT | |||||
| T0 | – | 0.098 | – | 0.509 | |
| T1 | 1.134 (0.207–6.204) | 0.885 | 1.138 (0.206–6.302) | 0.882 | |
| T2 | 3.054 (0.705–13.230) | 0.136 | 2.458 (0.554–10.918) | 0.237 | |
| T3 | 4.075 (0.972–17.086) | 0.055 | 2.266 (0.458–11.195) | 0.316 | |
| T4 | 4.281 (0.863–21.248) | 0.075 | 0.597 (0.040–8.917) | 0.708 | |
| Tis | 2.646 (0.372–18.850) | 0.331 | 1.472 (0.197–10.980) | 0.706 | |
| ypN | |||||
| N0 | – | 0.001 | – | 0.584 | |
| N1 | 2.017 (1.082–3.762) | 0.027 | 0.846 (0.140–5.100) | 0.855 | |
| N2 | 3.456 (1.755–6.802) | P<0.001 | 1.173 (0.180–7.647) | 0.867 | |
| N3 | 3.351 (1.3767–8.210) | 0.008 | 0.265 (0.012–5.929) | 0.402 | |
| Lymph nodes dissected | |||||
| N <15 | – | – | – | – | |
| N ≥15 | 0.730 (0.401–1.329) | 0.304 | – | – | |
| AC | |||||
| Yes | – | – | – | – | |
| No | 1.663 (0.996–2.775) | 0.052 | 1.689 (0.979–2.912) | 0.059 | |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; ECOG, eastern cooperative oncology group; AC, adjuvant chemotherapy; yp, post-neoadjuvant pathologic; TNM, tumor node metastasis; T, tumor; N, node
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Sex | |||||
| Female | – | – | – | – | |
| Male | 2.142 (1.078–4.256) | 0.030 | 2.045 (0.999–4.185) | 0.050 | |
| Age | |||||
| <60 years | – | – | – | – | |
| ≥60 years | 2.571 (1.259–5.247) | 0.010 | 2.348 (1.126–4.900) | 0.023 | |
| BMI | 1.034 (0.948–1.127) | 0.454 | |||
| Charlson Comorbidity Index | |||||
| 0 | – | 0.388 | – | – | |
| 1 | 1.527 (0.831–2.805) | 0.173 | – | – | |
| ≥2 | 1.053 (0.467–2.376) | 0.901 | – | – | |
| Tumor location | |||||
| Upper | – | 0..510 | – | – | |
| Middle | 1.737 (0.680–4.438) | 0.249 | – | – | |
| Lower | 1.693 (0.604–4.742) | 0.317 | – | – | |
| ECOG | 20.553 (0.001–650,254.438) | 0.567 | – | – | |
| Minimally invasive surgery | |||||
| Yes | – | – | – | – | |
| No | 1.628 (0.899–2.948) | 0.108 | – | – | |
| Hybrid | 1.880 (0.454–7.779) | 0.383 | – | – | |
| Histological subtype | |||||
| Adenocarcinoma | – | 0.222 | – | – | |
| Squamous cell | 0.557 (0.288–1.079) | 0.083 | – | – | |
| Neoadjuvant therapy | |||||
| Chemoradiotherapy | – | – | – | – | |
| Chemotherapy | 3.571 (0.494–25.826) | 0.207 | 4.512 (0.588–34.639) | 0.147 | |
| ypTNM | |||||
| I | – | 0.008 | – | 0.500 | |
| II | 1.421 (0.571–3.537) | 0.450 | 1.225 (0.362–4.146) | 0.774 | |
| III | 2.627 (1.298–5.319) | 0.007 | 2.195 (0.306–15.763) | 0.435 | |
| IV | 3.598 (1.558–8.305) | 0.003 | 11.307 (0.449–284.955) | 0.141 | |
| ypT | |||||
| T1 | – | 0.099 | – | 0.381 | |
| T2 | 2.683 (0.902–7.981) | 0.076 | 2.159 (0.704–6.621) | 0.178 | |
| T3 | 3.588 (1.258–10.229) | 0.017 | 2.008 (0.566–7.132) | 0.281 | |
| T4 | 3.794 (1.070–13.456) | 0.039 | 0.551 (0.045–6.749) | 0.641 | |
| ypN | |||||
| N0 | – | 0.004 | – | 0.694 | |
| N1 | 1.959 (1.032–3.718) | 0.040 | 0.932 (0.155–5.596) | 0.939 | |
| N2 | 3.208 (1.575–6.533) | 0.001 | 1.162 (0.178–7.588) | 0.876 | |
| N3 | 3.122 (1.258–7.749) | 0.014 | 0.299 (0.014–6.469) | 0.441 | |
| Lymph nodes dissected | |||||
| N <15 | – | – | – | – | |
| N ≥15 | 0.616 (0.336–1.127) | 0.116 | – | – | |
| AC | |||||
| Yes | – | – | – | – | |
| No | 1.804 (1.063–3.063) | 0.029 | 1.888 (1.079–3.306) | 0.026 | |
HR, hazard ratio; CI, confidence interval; BMI, body mass index; ECOG, eastern cooperative oncology group; AC, adjuvant chemotherapy; yp, post-neoadjuvant pathologic; TNM, tumor-node-metastasis; T, tumor; N, node.
Discussion
Surgical resection has always been the main treatment option for esophageal cancer. Despite advances in surgical modalities, long-term survival after surgery alone for locally advanced esophageal cancer remains low. In recent years, several randomized clinical trials have shown that multimodal treatment of esophageal cancer can improve survival outcomes (9). Neoadjuvant therapy has become the standard treatment mode for locally advanced esophageal cancer in some countries. The National Comprehensive Cancer Network (NCCN) guidelines recommend neoadjuvant chemoradiotherapy for patients with localized esophageal cancer. In contrast, guidelines in Japan recommend NAC for resectable stage II or III thoracic esophageal carcinoma, and for local residual tumor or local recurrence (4,10). Despite the interest in the use of AC, there is scarce evidence on its potential benefits.
Two large randomized trials, including NAC and AC after esophageal cancer surgery, demonstrated that patients treated with perioperative chemotherapy had better OS compared with surgery alone. In the MAGIC trial, 503 patients were randomized to compare OS with perioperative chemotherapy and surgery versus surgery alone. The perioperative chemotherapy group had better 5-year OS (36% vs. 23%, P=0.009) and progression-free survival (HR for progression, 0.66; 95% CI: 0.53–0.81, P<0.001) (11). In the FNCLCC and FFCD trials, 224 patients were randomly assigned to either perioperative chemotherapy and surgery (CS group; n=113) or surgery alone (S group; n=111). Compared with the S group, the CS group had a better OS (5-year rate: 38% vs. 24%; P=0.02) and DFS (5-year rate: 34% vs. 19%, P=0.003) (12). However, no large prospective study has investigated the benefit of AC after preoperative NAC in patients with esophageal cancer.
At present, it is believed that not all patients can benefit from chemotherapy, and there may be certain groups that benefit from chemotherapy. Postoperative chemotherapy may benefit two groups: those with persistent positive lymph nodes after NAC and those with a good pathological response to NAC (13). Matsuura and colleagues (14) investigated 113 patients, and 40 patients received AC [AC(+) group] while 73 patients did not [AC(−) group]. Among patients with ≥7 positive lymph nodes, the 2-year RFS rates of the AC(+) and AC(−) groups were 25.9% and 7.1%, respectively (P=0.04). Burt and colleagues (15) treated 335 (9.3%) of 3,592 patients with esophageal cancer (84.7% adenocarcinoma, 15.2% squamous cell carcinoma) with AC. AC did not significantly reduce the risk of death in patients with no residual disease (ypT0N0) or residual non-nodal disease (yp+N0). AC was associated with a 30% lower risk of death in the overall cohort among patients with residual nodal disease. A retrospective study on preoperative chemotherapy followed by surgery showed that patients receiving AC after induction therapy and esophagectomy had a survival benefit at all positive nodal stages (16). Bott and colleagues (17) compared 374 patients with clear resection margins. For patients receiving NAC (290, 76%), when AC was compared with no adjuvant therapy, the HR favored AC, but did not reach independent significance (OS HR 0.65, 95% CI: 0.40–1.06; P=0.087). NAC responders (Mandard score 1–3) appeared to be more likely to show a survival benefit from AC (HR 0.42, 95% CI: 0.15–1.11; P=1.081).
The combination perioperative approach is commonly used with modest improvements in OS compared with surgery alone, but with the cost of increased toxicity (18). Whether AC is needed for patients after neoadjuvant therapy and esophageal cancer surgery should be treated with caution. In a retrospective study of triple therapy (neoadjuvant therapy plus surgery plus AC) in patients with esophageal cancer, within the entire cohort, those who received postoperative chemotherapy had a 5-year OS benefit (OS 37.1% vs. 18.0%, P=0.024). This benefit was not observed in those who had a pathological complete response or had microscopic residual disease (19). In our center’s study, in the multivariate Cox regression analysis of the entire cohort, there was no significant difference between patients who received AC and those who did not (P=0.059). In the subgroup analysis of this study, patients with pCR were excluded. In the multivariate Cox regression analysis, AC (HR 1.888, 95% CI: 1.079–3.306) was an independent factor for patient prognosis. Patients receiving adjuvant therapy had a better OS. This suggests that adjuvant therapy may benefit patients with residual disease. In addition, age was also an independent prognostic factor in patients with esophageal cancer in the subgroup analysis, and OS was better in younger adults. This may be related to the tendency of heart, lung, and peripheral vascular conditions to worsen with age, resulting in increased postoperative complications (20).
There are some limitations to this study. This was a retrospective, small sample study, and the results were subject to bias. AC regimens vary, so it is impossible to determine which regimen is the best in terms of effectiveness. In addition, NAC combined with immunotherapy has now become a newer treatment method, which was not covered in this study.
Conclusions
In conclusion, not everyone can benefit from AC, and after neoadjuvant therapy, patients with residual disease who receive adjuvant therapy can achieve improved OS. Further prospective randomized controlled trials are needed to confirm these results.
Acknowledgments
Funding: This work was funded by the Henan Province Medical Science and Technology Research Project (No. LHGJ20200300).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1008/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1008/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1008/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Zhengzhou University (No. 2020-KY-277). The ethics committee approved the exemption from signing the informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Uhlenhopp DJ, Then EO, Sunkara T, et al. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol 2020;13:1010-21. [Crossref] [PubMed]
- Shah MA, Kennedy EB, Catenacci DV, et al. Treatment of Locally Advanced Esophageal Carcinoma: ASCO Guideline. J Clin Oncol 2020;38:2677-94. [Crossref] [PubMed]
- Wang Q, Peng L, Li T, et al. Postoperative Chemotherapy for Thoracic Pathological T3N0M0 Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 2020;27:1488-95. [Crossref] [PubMed]
- Ma Z, Yuan M, Bao Y, et al. Survival of Neoadjuvant and Adjuvant Therapy Compared With Surgery Alone for Resectable Esophageal Squamous Cell Carcinoma: A Systemic Review and Network Meta-Analysis. Front Oncol 2021;11:728185. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Atay SM, Blum M, Sepesi B. Adjuvant chemotherapy following trimodality therapy for esophageal carcinoma-Is the evidence sufficient? J Thorac Dis 2017;9:3626-9. [Crossref] [PubMed]
- Li X, Luan S, Yang Y, et al. Trimodal Therapy in Esophageal Squamous Cell Carcinoma: Role of Adjuvant Therapy Following Neoadjuvant Chemoradiation and Surgery. Cancers (Basel) 2022;14:3721. [Crossref] [PubMed]
- Kakeji Y, Oshikiri T, Takiguchi G, et al. Multimodality approaches to control esophageal cancer: development of chemoradiotherapy, chemotherapy, and immunotherapy. Esophagus 2021;18:25-32. [Crossref] [PubMed]
- Kato H, Nakajima M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg 2013;61:330-5. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Yan W, Zhao P, Fu H, et al. Survival After Induction Chemotherapy and Esophagectomy Is Not Improved by Adjuvant Chemotherapy. Ann Thorac Surg 2019;108:1505-13. [Crossref] [PubMed]
- Matsuura N, Yamasaki M, Yamashita K, et al. The role of adjuvant chemotherapy in esophageal cancer patients after neoadjuvant chemotherapy plus surgery. Esophagus 2021;18:559-65. [Crossref] [PubMed]
- Burt BM, Groth SS, Sada YH, et al. Utility of Adjuvant Chemotherapy After Neoadjuvant Chemoradiation and Esophagectomy for Esophageal Cancer. Ann Surg 2017;266:297-304. [Crossref] [PubMed]
- Samson P, Puri V, Lockhart AC, et al. Adjuvant chemotherapy for patients with pathologic node-positive esophageal cancer after induction chemotherapy is associated with improved survival. J Thorac Cardiovasc Surg 2018;156:1725-35. [Crossref] [PubMed]
- Bott RK, Beckmann K, Zylstra J, et al. Adjuvant therapy following neoadjuvant chemotherapy and surgery for oesophageal adenocarcinoma in patients with clear resection margins. Acta Oncol 2021;60:672-80. [Crossref] [PubMed]
- Athauda A, Nankivell M, Langley RE, et al. Impact of sex and age on chemotherapy efficacy, toxicity and survival in localised oesophagogastric cancer: A pooled analysis of 3265 individual patient data from four large randomised trials (OE02, OE05, MAGIC and ST03). Eur J Cancer 2020;137:45-56. [Crossref] [PubMed]
- Kim GJ, Koshy M, Hanlon AL, et al. The Benefit of Chemotherapy in Esophageal Cancer Patients With Residual Disease After Trimodality Therapy. Am J Clin Oncol 2016;39:136-41. [Crossref] [PubMed]
- Mantziari S, Teixeira Farinha H, Bouygues V, et al. Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis. Cancers (Basel) 2021;13:2104. [Crossref] [PubMed]
(English Language Editor: C. Betlazar-Maseh)



